Abstract
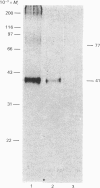
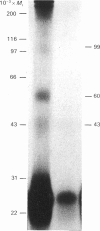
The structure of prolactin (PRL) receptor in the rabbit mammary gland was examined using a receptor-specific monoclonal antibody (MAb). The PRL receptor preparation used was purified by making use of a PRL-affinity column. MAb inhibited the binding of PRL to the receptor, in a dose-dependent manner and completely at a high concentration. Using the receptor directly labelled by 125I, the preparation was incubated with MAbs and the immune complex was collected by Pansorbin and examined by SDS/polyacrylamide-gel electrophoresis. The autoradiography showed that three species with apparent Mr values of 77,000, 41,000 and 25,000 specifically reacted with MAbs. The pattern changed little in the presence or absence of dithiothreitol. Western blot analysis showed that two species (Mr 77,000 and 41,000) reacted with MAb. Affinity labelling of the receptor with labelled PRL revealed three bands with Mr values of 96,000, 60,000 and 43,000 on SDS gels. The high-Mr complex (Mr greater than 200,000) was always present at the top of the gel. These results show that the mammary gland contains at least three PRL-binding subunits. The differences in Mr before and after PRL binding were close to the Mr of PRL. This would suggest that each PRL binding subunit reacts with one PRL molecule.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ashkenazi A., Madar Z., Gertler A. Partial purification and characterization of bovine mammary gland prolactin receptor. Mol Cell Endocrinol. 1987 Mar;50(1-2):79–87. doi: 10.1016/0303-7207(87)90079-7. [DOI] [PubMed] [Google Scholar]
- Bonifacino J. S., Dufau M. L. Lactogen receptors in rat Leydig cells: analysis of their structure with bifunctional cross-linking reagents. Endocrinology. 1985 Apr;116(4):1610–1614. doi: 10.1210/endo-116-4-1610. [DOI] [PubMed] [Google Scholar]
- Bonifacino J. S., Dufau M. L. Structure of the ovarian lactogen receptors. Analysis with bifunctional cross-linking reagents. J Biol Chem. 1984 Apr 10;259(7):4542–4549. [PubMed] [Google Scholar]
- Borst D. W., Sayare M. Photoactivated cross-linking of prolactin to hepatic membrane binding sites. Biochem Biophys Res Commun. 1982 Mar 15;105(1):194–201. doi: 10.1016/s0006-291x(82)80030-2. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Djiane J., Dusanter-Fourt I., Katoh M., Kelly P. A. Biological activities of binding site specific monoclonal antibodies to prolactin receptors of rabbit mammary gland. J Biol Chem. 1985 Sep 25;260(21):11430–11435. [PubMed] [Google Scholar]
- Djiane J., Houdebine L. M., Kelly P. A. Prolactin-like activity of anti-prolactin receptor antibodies on casein and DNA synthesis in the mammary gland. Proc Natl Acad Sci U S A. 1981 Dec;78(12):7445–7448. doi: 10.1073/pnas.78.12.7445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haeuptle M. T., Aubert M. L., Djiane J., Kraehenbuhl J. P. Binding sites for lactogenic and somatogenic hormones from rabbit mammary gland and liver. J Biol Chem. 1983 Jan 10;258(1):305–314. [PubMed] [Google Scholar]
- Hughes J. P., Simpson J. S., Friesen H. G. Analysis of growth hormone and lactogenic binding sites cross-linked to iodinated human growth hormone. Endocrinology. 1983 Jun;112(6):1980–1985. doi: 10.1210/endo-112-6-1980. [DOI] [PubMed] [Google Scholar]
- Katoh M., Djiane J., Kelly P. A. Monoclonal antibodies against rabbit mammary prolactin receptors. Specific antibodies to the hormone binding domain. J Biol Chem. 1985 Sep 25;260(21):11422–11429. [PubMed] [Google Scholar]
- Katoh M., Djiane J., Kelly P. A. Prolactin-binding components in rabbit mammary gland: characterization by partial purification and affinity labeling. Endocrinology. 1985 Jun;116(6):2612–2620. doi: 10.1210/endo-116-6-2612. [DOI] [PubMed] [Google Scholar]
- Katoh M., Raguet S., Zachwieja J., Djiane J., Kelly P. A. Hepatic prolactin receptors in the rat: characterization using monoclonal antireceptor antibodies. Endocrinology. 1987 Feb;120(2):739–749. doi: 10.1210/endo-120-2-739. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Mahajan P. B., Ebner K. E. Effect of oxidizing agents on rabbit mammary prolactin receptors. Mol Cell Endocrinol. 1986 Jun;46(1):37–42. doi: 10.1016/0303-7207(86)90067-5. [DOI] [PubMed] [Google Scholar]
- Mitani M., Dufau M. L. Purification and characterization of prolactin receptors from rat ovary. J Biol Chem. 1986 Jan 25;261(3):1309–1315. [PubMed] [Google Scholar]
- Necessary P. C., Humphrey P. A., Mahajan P. B., Ebner K. E. Purification of rabbit mammary prolactin receptor by acidic elution from a prolactin affinity column. J Biol Chem. 1984 Jun 10;259(11):6942–6946. [PubMed] [Google Scholar]
- Sakai S., Ike F., Kohmoto K., Johke T. Separation of rabbit mammary-gland prolactin receptors by ion-exchange chromatography, h.p.l.c.-gel filtration and ultracentrifugation. Biochem J. 1986 Aug 1;237(3):647–653. doi: 10.1042/bj2370647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakai S., Ike F. Two separate receptors for prolactin in the rabbit mammary gland. Endocrinol Jpn. 1987 Dec;34(6):863–870. doi: 10.1507/endocrj1954.34.863. [DOI] [PubMed] [Google Scholar]
- Sakai S., Katoh M., Berthon P., Kelly P. A. Characterization of prolactin receptors in pig mammary gland. Biochem J. 1984 Dec 15;224(3):911–922. doi: 10.1042/bj2240911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakai S., Kohmoto K., Johke T. A receptor site for prolactin in lactating mouse mammary tissues. Endocrinol Jpn. 1975 Oct;22(5):379–387. doi: 10.1507/endocrj1954.22.379. [DOI] [PubMed] [Google Scholar]
- Sakai S., Murakami H. Binding of prolactin and monoclonal antibody to prolactin receptors immobilized on a nitrocellulose membrane filter. Anal Biochem. 1987 Dec;167(2):406–410. doi: 10.1016/0003-2697(87)90184-9. [DOI] [PubMed] [Google Scholar]
- Shiu R. P., Friesen H. G. Blockade of prolactin action by an antiserum to its receptors. Science. 1976 Apr 16;192(4236):259–261. doi: 10.1126/science.176727. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waters M. J., Lusins S., Friesen H. G. Immunological and physicochemical evidence for tissue specific prolactin receptors in the rabbit. Endocrinology. 1984 Jul;115(1):1–10. doi: 10.1210/endo-115-1-1. [DOI] [PubMed] [Google Scholar]
- Yamada K., Donner D. B. Structures of the somatotropin receptor and prolactin receptor on rat hepatocytes characterized by affinity labelling. Biochem J. 1984 Jun 1;220(2):361–369. doi: 10.1042/bj2200361. [DOI] [PMC free article] [PubMed] [Google Scholar]