Abstract
Background/Objectives: Mycoplasma genitalium (MG) infections and antibiotic resistance are increasing in prevalence while treatment options are limited. Limited data exist regarding MG resistance in Israel. Our aim was to study the prevalence of MG resistance in a sexually transmitted infection (STI) clinic in Israel. Methods: We performed a single-center retrospective study among patients attending an STI clinic during 2019–2020. MG isolates were tested to detect their resistance to azithromycin and fluoroquinolones (FQs) using commercial kits (Allplex™ MG & AziR Assay, Allplex™ MG & MoxiR Assay). We collected patient data regarding the risk factors for STIs and resistance. A multivariate logistic regression model was used to identify the risk factors for resistance. Results: Of the 142 patients who tested positive for MG, 50 (35.2%) and 22 (15.5%) had resistant mutations to azithromycin and FQ, respectively, and 13 (9.2%) showed resistance to both agents. In a multivariate logistic regression model, men who have sex with men (RR 7.01 95% CI 3.00–16.33) and past STIs (RR 2.33 95% CI 1.01–5.34) were independent risk factors for azithromycin resistance. Conclusions: We found a high prevalence of azithromycin resistance and, to a lesser degree, FQ resistance. These findings may help design the treatment guidelines and support routine resistance testing in high-risk populations.
Keywords: Mycoplasma genitalium, sexually transmitted infections, men who have sex with men, resistance, antibiotics
1. Introduction
Mycoplasma genitalium (MG) is an emerging pathogen in sexually transmitted infections (STIs). The most common presentation in men is urethritis, and it is associated with cervicitis and pelvic inflammatory disease (PID) in women [1]. Some reports have suggested an association between MG and preterm birth [2]. It may also present as an asymptomatic infection in all sexes. The prevalence of MG varies geographically and according to risk factors. In a national survey aiming to represent the general population in the US, the prevalence of MG was 1.8% for men and 1.7% for women in the years 2017–2018 [3]. In a prospective study of Israeli men (n = 259) tested at our sexual health facility between 2008 and 2010, MG had an overall prevalence of 6.6% and a prevalence of 11.9% in patients with symptoms [4]. An increase in the prevalence of MG over the years has been reported. In a large retrospective Danish study (n = 31,600), the detection rate of MG in nuclear acid amplification testing (NAAT) increased significantly over the years 2006–2010 from 7.9% to 10.3% for men, and from 2.4% to 3.8% for women [5]. Some of the reported increase in MG prevalence may be related to the spread of NAAT testing and advocating screening for MG in asymptomatic people with risk factors for STIs [5].
MG is a mollicute bacteria, i.e., it lacks a cell wall, meaning it is innately immune to cell-wall-targeting antibiotics, such as beta-lactams and vancomycin [6]. Only a few antibiotic classes have activity against MG. These include macrolides, fluoroquinolones, and tetracyclines. Azithromycin has an 85–95% cure rate in susceptible strains. However, substantial macrolide resistance is being reported worldwide due to mutations in the 23S rRNA gene [7,8,9]. In a large USA study, resistance to macrolides was detected in up to 50% of samples [10]. Data are emerging regarding resistance to the once-considered highly effective, second-line treatment with moxifloxacin, which is an agent of the fluoroquinolone (FQ) class. In an Australian study, 15% of samples had a mutation in the parC or gyrA genes associated with FQ resistance [7]. These mutations have been linked with microbiological treatment failure and persistent symptoms [11].
It has been postulated that the well-established empirical treatment of non-gonococcal urethritis with single doses of ceftriaxone and azithromycin may be selected for resistance to azithromycin in MG [12,13]. Due to widespread macrolide resistance, 2021 European guidelines now recommend macrolide resistance testing on all positive MG samples. Routine fluoroquinolone resistance testing is not recommended, though may be useful in patients with documented moxifloxacin treatment failure [12]. These guidelines recommend extended macrolide treatment in wild type MG or when resistance testing is unavailable. Fluoroquinolones are recommended in macrolide-resistant cases [12]. Studies from a Melbourne clinic have reported a high rate of microbial cure with doxycycline as the first agent to reduce microbial load, followed by a resistance test guided by azithromycin or moxifloxacin [14,15]. Based on these and other studies, CDC 2021 guidelines currently recommend resistance-guided therapy: sequential doxycycline therapy followed by azithromycin if sensitive or moxifloxacin if resistant to macrolides or if macrolide testing is unavailable [16]. Although MG is prevalent in Israel [17], routine resistance testing for MG is not available. Therefore, there is limited information regarding the resistance patterns of MG in Israel, particularly in high-risk populations. In this study, we tested for macrolide and FQ resistance in patients at Levinski Clinic, a free-of-charge sexual health center in Tel Aviv, Israel, which offers routine STI NAAT testing. The Levinski Clinic in Tel Aviv caters to the greater vicinity of the Tel Aviv area and is the largest sexual health center in Israel. The majority of STIs in the Tel Aviv area are diagnosed by this clinic.
2. Patients and Methods
2.1. Sample Population
This study was approved by the Edith Wolfson Medical Center institutional review board (0116-20-WOMC). This retrospective study was performed on samples from patients who approached the Levinsky clinic between the years 2019 and 2020. The Levinsky clinic is a sexual health clinic located in Tel Aviv, the largest city in Israel. All people attending this clinic are tested for STIs. Most people attending this clinic are at high risk for STIs. The basic screening for symptomatic and asymptomatic individuals includes serological testing for human immunodeficiency syndrome (HIV), syphilis, and nucleic acid amplification tests (NAATs) of urine samples for Neisseria gonorrhoeae, Chlamydia trachomatis, and MG. In addition, rectal, throat, vaginal, and urethral specimens are collected for testing according to the patient’s reported sexual practices and symptoms.
In this study, we included symptomatic and asymptomatic individuals who attended the Levinsky clinic between 1 January 2019 and 31 December 2020, and tested positive for MG by means of an NAAT from any site.
2.2. STI Testing
Patients underwent testing for STIs as part of the routine practice of the Levinsky clinic. Urine, rectal, throat, vaginal, and urethral specimens were collected by swabs. Most attendees performed self-swabbing at the clinic, and if that was not possible, a physician or a nurse at the clinic took the sample. The samples were immediately placed in a universal transport medium (Copan, Murrieta, CA, USA) and were transferred at room temperature to the microbiology laboratory at the Edith Wolfson Medical Center. DNA for analyses was extracted using the NUCLISENS easyMAG system (Biomerieux, Boston, MA, USA) according to the manufacturer’s instructions. Thereafter, the DNA was frozen at −80 °C for further analysis. The presence of C. trachomatis, N. gonorrhoeae, and MG was assessed by a multiplex polymerase chain reaction (PCR) (AllplexTM CT/NG/MG/TV Assay, Seegene Inc., Seol, Republic of Korea), which conforms to the European diagnostic standards for in vitro diagnostics (CE-IVD), certified for use in Europe and the USA, according to the manufacturer’s instructions. All tests included both a negative control and a positive control for each pathogen.
2.3. Antibiotic Resistance Screening
The analysis of antimicrobial resistance to azithromycin and FQs was performed after collecting all the samples. DNA was thawed, and the presence of resistance to azithromycin and FQ in MG isolates was analyzed by PCR using commercial kits. Allplex™ MG & AziR Assay (Seegene Inc., Seol, Republic of Korea) was used to detect resistance mutations to azithromycin (A2059T, A2058T, A2058C, A2058G, A2059C, and A2059G) according to the manufacturer’s instructions. Allplex™ MG & MoxiR Assay (Seegene, Inc., Seol, Republic of Korea) was used to detect resistance mutations to FQ (A247C, G248A, G248T, G259A, G259C, G259T) according to the manufacturer’s instructions. The PCR reactions were run on the CFX96™ Real-Time PCR Detection System (Bio-Rad Laboratories, Hercules, CA, USA) and analyzed using Seegene Viewer for Real-Time Instruments software V3.23.000 (Seegene Inc., Seol, Republic of Korea).
2.4. Statistical Analysis
To describe the population, we used mean and medians for continuous variables and numbers and percentages for dichotomous variables. Variables were compared using Student’s t-test, Mann–Whitney’s U test, Pearson’s chi-squared test, ANOVA, and Fisher’s exact test as appropriate. All statistical tests were 2-sided. p-value < 0.05 was defined as statistically significant for analyses. Risk factors for resistance were assessed using logistic regression. Independent variables found to be significantly associated with the dependent variable in bivariate analysis were entered into backwards, conditional multivariate logistic regression analysis, with results presented as the odds ratio (OR) with a 95% confidence interval (CI). Data were analyzed using SPSS Statistics for Windows, Version 26.0 (Armonk, NY, USA: IBM Corp., 2020).
3. Results
This study included 142 patients who tested positive for MG. Patients’ characteristics are detailed in Table 1. Twenty-four patients had two positive samples, and two patients had three positive samples from multiple anatomical locations. Eight patients had samples from multiple dates, and the latter dates were considered to be tests of cure. These samples were removed from the primary analysis, which determined the prevalence of antimicrobial resistance. The mean age was 31.7 years, and 35.2% were females. This study included 38 (26.8%) symptomatic patients. As can be seen in Table 1, 46.5% had a past history of an STI, and 13 patients (9.2%) had received past antibiotic treatment. Men who have sex with men (MSM) comprised 46.5% of this cohort, and 80.3% of subjects had sexual relationships in the past with more than 20 partners. About a fourth (28.8%) had an additional concomitant STI, where C. trachomatis was the most common (18.2%).
Table 1.
Patient characteristics.
| Characteristic | N (%) |
|---|---|
| Number of patients | 142 |
| Mean age | 31.7 (SD 9.3) |
| Gender—female | 50 (35.2%) |
| Symptomatic | 38 (26.8%) |
| History of STI | 66 (46.5%) |
| Previous antibiotic treatment | 13 (9.2%) |
| MSM | 64 (45.1%) |
| More than 20 lifetime sexual partners | 114 (80.3%) |
| More than 20 sexual partners in past three months | 37 (26.1%) |
| Reported receiving money for sex | 27 (19%) |
| Reported substance use | 58 (40.8%) |
| HIV positive | 7 (4.9%) |
| Concomitant STI | 41 (28.8%) |
| Concomitant Chlamydia trachomatis | 26 (18.2%) |
| Concomitant Neisseria gonorrhoeae | 15 (10.5%) |
Resistance
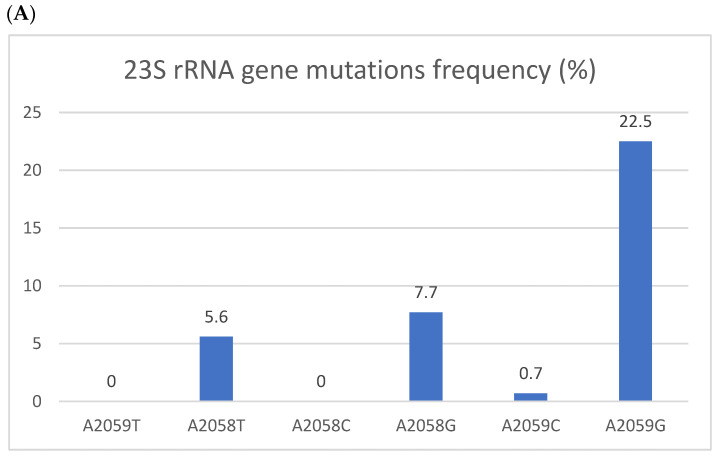
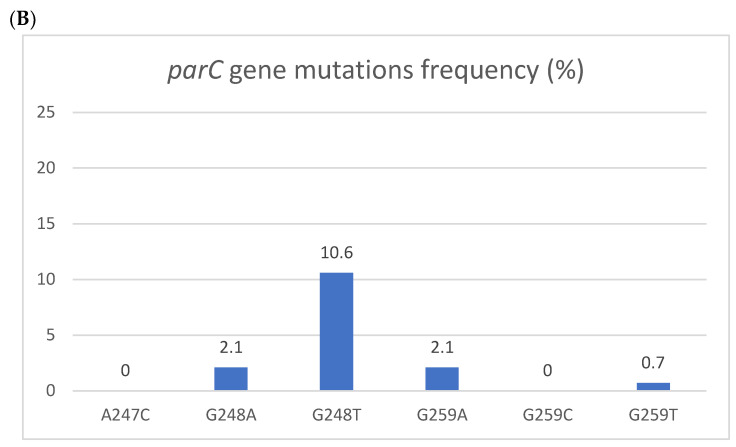
Antibiotic resistance was common. In total, 50 of the 142 patients (35.2%) had at least one azithromycin-resistant mutation, and 22 (15.5%) had an FQ-resistant mutation. Thirteen patients (9.2%) showed dual resistance mutations to both agents. Mutation frequencies are depicted in Figure 1. In a bivariate analysis (Table 2), male gender, a past STI, MSM, rectal infection, having more than one site involved with MG, receiving money for sex, and substance use were associated with azithromycin resistance mutations. Having fewer sexual partners was associated with wild-type MG. However, in a multivariate logistic regression model (Table 2), only MSM (RR 7.011 95% CI 3.009–16.336) and a past STI (RR 2.333 95% CI 1.018–5.347) were independent risk factors for azithromycin resistance. In our cohort, 59.4% of MSM and 15.4% of patients who were not MSM had an azithromycin-resistant mutation (p < 0.001). Eleven of the thirteen patients with resistance mutations to both azithromycin and FQ were MSM. A similar multivariate analysis on FQ resistance was not conducted due to the low number of resistant samples.
Figure 1.
Resistant mutations to azithromycin (A) and fluoroquinolones (B). Resistant mutations to azithromycin (A) reflected by the 223S rRNA gene mutation and fluoroquinolones (B) reflected by parC gene mutations. The Y axis denotes the percentage of patients, and the numbers above the bars are percentages. Some isolates had more than one mutation.
Table 2.
Multivariate logistic regression (n = 142) assessing risk factors for azithromycin resistance.
| Bivariate Analysis | Multivariate Analysis | ||
|---|---|---|---|
| p Value | RR * (95% CI) | p Value | |
| More than one site involved | 0.05 | ||
| Urinary infection | 0.544 | ||
| Rectal infection | 0.038 | ||
| Throat infection | 0.534 | ||
| Concomitant Chlamydia trachomatis | 0.889 | ||
| Concomitant Neisseria gonorrhoeae | 0.945 | ||
| Male gender | <0.001 | ||
| Symptoms | 0.584 | ||
| Past STI * | <0.001 | 2.333 (1.018–5.347) | 0.45 |
| Previous antibiotic treatment | 0.386 | ||
| MSM * | <0.001 | 7.011 (3.009–16.336) | <0.001 |
| 20 or more lifetime sexual partners | <0.045 | ||
| 5 or less sexual partners in the last three months | 0.001 | 0.685 (0.494–0.950) | 0.24 |
| Reported receiving money for sex | 0.014 | ||
| Substance use | 0.046 | ||
| HIV * status | 0.356 | ||
* Abbreviations: RR—relative risk; STI—sexually transmitted infections; MSM—men who have sex with men; and HIV—human immunodeficiency virus.
Test of cure (TOC) data were available for 48 patients, and 10/48 (20.8%) were positive for MG. Of the positive patients on TOC, one originally had wild-type MG, seven had azithromycin resistance mutations, one had FQ resistance, and one patient had dual resistance. Nine of the ten patients with a positive TOC were MSM. Resistant mutations for azithromycin were detected in 10/38 (26.3%) patients who were negative on TOC, of which five had dual resistance. Two patients had the parC mutation only.
4. Discussion
In this single-center cohort, we discovered substantial MG resistance to azithromycin (35.2%) and, to a lesser degree, to FQ (9.2%). These results are similar to those reported in other countries [11,12,18,19,20]. In a systematic review and meta-analysis, the prevalence of azithromycin resistance was 35.5%. Prevalence increased from 10% before 2010 to an average of 51% in 2016–2017. FQ resistance remained stable over that time at 7.7% [18]. These resistances have been linked to treatment failure [11,19] and highlight the importance of resistance-guided therapy, as is now recommended [12,20].
Azithromycin inhibits protein synthesis by binding to the A2058 and A2059 residues of 23S rRNA or close to the peptidyl transferase site (V region) of the 50S ribosomal subunit. Binding results either in the inhibition of transfer of the transfer RNA (tRNA) from the aminoacyl site to the V region, or the dissociation of tRNA at this site. Resistance occurs by target modification through the single nucleotide polymorphism (SNP) and, therefore, is easily acquired [21,22,23,24,25]. In our cohort, multiple patient characteristics were associated with azithromycin resistance in the bivariate analysis, but only MSM patients were at higher risk for azithromycin resistance in the multivariate analysis. A high prevalence of azithromycin resistance has been reported elsewhere in this population. In a report for a clinic in Sydney, 75% of MSM patients with a positive MG test had an azithromycin resistance mutation [26]. In a French study, the overall rate of MG resistance (prevalent and incident cases) to azithromycin was 67.6% [27].
Quinolones rapidly inhibit bacterial DNA synthesis, an event that is followed by rapid bacterial cell death. They inhibit the enzymatic activities of two members of the topoisomerase class of enzymes—DNA gyrase and topoisomerase IV. Reports have shown that moxifloxacin resistance results from mutations in the quinolone resistance-determining region of either the parC gene (topoisomerase IV) or the gyrA gene (DNA gyrase) [28,29,30]. The most prevalent parC mutation detected in our cohort was G248T, which confers to the amino acid change S83I. This SNP affecting the serine amino acid at position 83 is the most common SNP. In Australia and in other reports, the prevalence of this mutation increased significantly over the years 2012–2020 [19]. This SNP is associated with treatment failure [11], and it has been suggested that its increasing prevalence indicates that the treatment may be selected for this variation [18]. Unfortunately, most of our patients did not return for a TOC, and we do not have enough data to reflect the relationship between resistance development over time and treatment outcome.
There are no commercial tests available that target gyrA mutations, and therefore, we did not test for gyrA mutations. Our understanding of the molecular pathways for FQ resistance in MG is still evolving. Earlier studies showed an uncertain role of gyrA in FQ resistance, perhaps due to the small sample size and a relatively low prevalence of these mutations [29,31]. In a recent 2023 large Australian cohort, gyrA SNPs were less common than parC changes and were associated with treatment failure, which showed a synergistic effect when combined with a corresponding parC mutation [11]. If gyrA testing becomes more widespread, we may be able to gain a further understanding of the role of these mutations on resistance.
In recent years, in attempts to decrease the rise in STIs in high-risk populations, screening for STIs has become common. It is assumed that screening can be effective in reducing the prevalence of all STIs. A recent review by Kenyon et al. challenges this assumption regarding N. gonorrhoeae, C. trachomatis, and MG [32]. There is little evidence that screening for MG indeed reduces the prevalence of this pathogen. In addition, there is growing evidence regarding the harm of screening asymptomatic individuals related to increased antibiotic consumption and increased resistance to MG, as well as to N. gonorrhoeae and C. trachomatis. Increased antibiotic consumption has a deleterious effect on the microbiome. Screening may also negatively affect the well-being of the patients [32]. As a result, some agencies have recommended testing only symptomatic patients [12,33]. Our results highlight the problem of antibiotic resistance in MG isolates in high-risk patients and emphasize the complexity regarding whether patients indeed benefit from screening.
Limitations to our study include selection bias and information bias. The healthcare system in Israel is universal and single-payer, i.e., all citizens have access to free primary care, including sexual health services. The Levinski sexual health clinic is unique in that it provides free and anonymous care to all. It caters to people with health insurance who require anonymous sexual health services, to non-citizens who do not have health insurance, including asylum seekers, as well as to other populations with reduced access to healthcare services, such as people with substance abuse issues and sex workers. This bias was reflected in our results, where, for example, 19% reported receiving money for sex and 45% were MSM. Thus, our findings may not be representative of the entire population of Israel and are more comparable to results reported by other sexual health clinics elsewhere. Information regarding patient history was gathered before testing. Patients were offered anonymity, which aided in collecting personal information that patients might have been hesitant to share otherwise. However, even in an anonymous setting, patients may elect to keep information private and not share certain details with the provider, which may influence subgroup analysis. We were also unable to corroborate the demographic data with an insurance provider, as is often performed in studies with an identified or a de-identified population.
In summary, in our high-risk cohort, we found a high prevalence of in vitro antibiotic resistance of MG mostly to azithromycin and, to a lesser extent to FQ, with MSM being a risk factor for resistance. These findings are alarming due to the limited treatment options available for MG. Future directions include assessing trends in resistance through the years and assessing prevalence and resistance in the general population in Israel. These findings may help design screening programs and treatment guidelines in Israel and support the routine use of resistance testing in high-risk populations.
Author Contributions
A.H.—design, writing, summarizing patients’ files, and laboratory work; O.Y.—design, reviewing; Y.G.—design, reviewing; R.S.—design, reviewing; O.S.—analytical laboratory work, and reviewing; Y.S.—laboratory work, reviewing; Y.W.—laboratory work, reviewing; Y.M.—design, funding, statistical analysis, and reviewing. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
This study was approved by Wolfson Medical Center, IRB committee approval number 0116-20-WOMC on 29 June 2020.
Informed Consent Statement
Patient consent was waived by the IRB committee due to the retrospective nature of the study and the anonymity of the participants.
Data Availability Statement
Data is available from the authors upon request.
Conflicts of Interest
All authors have no conflict of interests.
Funding Statement
This study was funded by the internal funds of the Infectious Disease Unit, Wolfson Medical Center, Holon, Israel.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Lis R., Rowhani-Rahbar A., Manhart L.E. Mycoplasma genitalium Infection and Female Reproductive Tract Disease: A Meta-analysis. Clin. Infect. Dis. 2015;61:418–426. doi: 10.1093/cid/civ312. [DOI] [PubMed] [Google Scholar]
- 2.Frenzer C., Egli-Gany D., Vallely L.M., Vallely A.J., Low N. Adverse pregnancy and perinatal outcomes associated with Mycoplasma genitalium: Systematic review and meta-analysis. Sex. Transm. Infect. 2022;98:222–227. doi: 10.1136/sextrans-2021-055352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Torrone E.A., Kruszon-Moran D., Philips C., Morris M.R., Bowden K.E., Papp J., Bachmann L.H., Weinstock H., Kersh E.N. Prevalence of Urogenital Mycoplasma genitalium Infection, United States, 2017 to 2018. Sex. Transm. Dis. 2021;48:e160–e162. doi: 10.1097/OLQ.0000000000001394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gottesman T., Yossepowitch O., Samra Z., Rosenberg S., Dan M. Prevalence of Mycoplasma genitalium in men with urethritis and in high risk asymptomatic males in Tel Aviv: A prospective study. Int. J. STD AIDS. 2017;28:127–132. doi: 10.1177/0956462416630675. [DOI] [PubMed] [Google Scholar]
- 5.Salado-Rasmussen K., Jensen J.S. Mycoplasma genitalium Testing Pattern and Macrolide Resistance: A Danish Nationwide Retrospective Survey. Clin. Infect. Dis. 2014;59:24–30. doi: 10.1093/cid/ciu217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tully J.G., Taylor-Robinson D., Cole R.M., Rose D.L. A newly discovered mycoplasma in the human urogenital tract. Lancet. 1981;1:1288–1291. doi: 10.1016/s0140-6736(81)92461-2. [DOI] [PubMed] [Google Scholar]
- 7.Tagg K.A., Jeoffreys N.J., Couldwell D.L., Donald J.A., Gilbert G.L. Fluoroquinolone and Macrolide Resistance-Associated Mutations in Mycoplasma genitalium. J. Clin. Microbiol. 2013;51:2245–2249. doi: 10.1128/JCM.00495-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Couldwell D.L., Tagg K.A., Jeoffreys N.J., Gilbert G.L. Failure of moxifloxacin treatment in Mycoplasma genitalium infections due to macrolide and fluoroquinolone resistance. Int. J. STD AIDS. 2013;24:822–828. doi: 10.1177/0956462413502008. [DOI] [PubMed] [Google Scholar]
- 9.Bradshaw C.S., Chen M.Y., Fairley C.K. Persistence of Mycoplasma genitalium Following Azithromycin Therapy. PLoS ONE. 2008;3:e3618. doi: 10.1371/journal.pone.0003618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Getman D., Jiang A., O’Donnell M., Cohen S. Mycoplasma genitalium Prevalence, Coinfection, and Macrolide Antibiotic Resistance Frequency in a Multicenter Clinical Study Cohort in the United States. J. Clin. Microbiol. 2016;54:2278–2283. doi: 10.1128/JCM.01053-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Murray G.L., Bodiyabadu K., Danielewski J., Garland S.M., Machalek D.A., Fairley C.K., Jensen J.S., Williamson D.A., Tan L.Y., Mokany E., et al. Moxifloxacin and Sitafloxacin Treatment Failure in Mycoplasma genitalium Infection: Association with parC Mutation G248T (S83I) and Concurrent gyrA Mutations. J. Infect. Dis. 2020;221:1017–1024. doi: 10.1093/infdis/jiz550. [DOI] [PubMed] [Google Scholar]
- 12.Jensen J.S., Cusini M., Gomberg M., Moi H., Wilson J., Unemo M. 2021 European guideline on the management of Mycoplasma genitalium infections. J. Eur. Acad. Dermatol. Venereol. 2022;36:641–650. doi: 10.1111/jdv.17972. [DOI] [PubMed] [Google Scholar]
- 13.Lau A., Bradshaw C.S., Lewis D., Fairley C.K., Chen M.Y., Kong F.Y.S., Hocking J.S. The Efficacy of Azithromycin for the Treatment of Genital Mycoplasma genitalium: A Systematic Review and Meta-analysis. [(accessed on 17 August 2024)];Clin. Infect. Dis. 2015 61:1389–1399. doi: 10.1093/cid/civ644. Available online: https://pubmed.ncbi.nlm.nih.gov/26240201/ [DOI] [PubMed] [Google Scholar]
- 14.Read T.R.H., Fairley C.K., Murray G.L., Jensen J.S., Danielewski J., Worthington K., Doyle M., Mokany E., Tan L., Chow E.P., et al. Outcomes of Resistance-guided Sequential Treatment of Mycoplasma genitalium Infections: A Prospective Evaluation. Clin. Infect. Dis. 2019;68:554–560. doi: 10.1093/cid/ciy477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Durukan D., Read T.R.H., Murray G., Doyle M., Chow E.P.F., Vodstrcil L.A., Fairley C.K., Aguirre I., Mokany E., Tan L.Y., et al. Resistance-Guided Antimicrobial Therapy Using Doxycycline–Moxifloxacin and Doxycycline–2.5 g Azithromycin for the Treatment of Mycoplasma genitalium Infection: Efficacy and Tolerability. Clin. Infect. Dis. 2020;71:1461–1468. doi: 10.1093/cid/ciz1031. [DOI] [PubMed] [Google Scholar]
- 16.Workowski K.A., Bachmann L.H., Chan P.A., Johnston C.M., Muzny C.A., Park I., Reno H., Zenilman J.M., Bolan G.A. Sexually Transmitted Infections Treatment Guidelines, 2021. MMWR Recomm. Rep. 2021;70:1–187. doi: 10.15585/mmwr.rr7004a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kridin K., Ingram B., Becker D., Shiloah N., Azrad M., Habib S., Peretz A. Sexually Transmitted Diseases in Northern Israel: Insights From a Large Referral Laboratory. J. Low. Genit. Tract. Dis. 2023;27:51–55. doi: 10.1097/LGT.0000000000000717. [DOI] [PubMed] [Google Scholar]
- 18.Machalek D.A., Tao Y., Shilling H., Jensen J.S., Unemo M., Murray G., Chow E.P., Low N., Garland S.M., Vodstrcil L.A., et al. Prevalence of mutations associated with resistance to macrolides and fluoroquinolones in Mycoplasma genitalium: A systematic review and meta-analysis. Lancet Infect. Dis. 2020;20:1302–1314. doi: 10.1016/S1473-3099(20)30154-7. [DOI] [PubMed] [Google Scholar]
- 19.Murray G.L., Bodiyabadu K., Vodstrcil L.A., Machalek D.A., Danielewski J., Plummer E.L., Garland S.M., Whiley D.M., Sweeney E.L., Bradshaw C.S. parC Variants in Mycoplasma genitalium: Trends over Time and Association with Moxifloxacin Failure. Antimicrob. Agents Chemother. 2022;66:e00278–22. doi: 10.1128/aac.00278-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mycoplasma genitalium—STI Treatment Guidelines [Internet] [(accessed on 17 August 2024)]; Available online: https://www.cdc.gov/std/treatment-guidelines/mycoplasmagenitalium.htm.
- 21.Jensen J.S., Bradshaw C.S., Tabrizi S.N., Fairley C.K., Hamasuna R. Azithromycin treatment failure in Mycoplasma genitalium -positive patients with nongonococcal urethritis is associated with induced macrolide resistance. Clin. Infect. Dis. 2008;47:1546–1553. doi: 10.1086/593188. [DOI] [PubMed] [Google Scholar]
- 22.Parnham M.J., Erakovic Haber V., Giamarellos-Bourboulis E.J., Perletti G., Verleden G.M., Vos R. Azithromycin: Mechanisms of action and their relevance for clinical applications. Pharmacol. Ther. 2014;143:225–245. doi: 10.1016/j.pharmthera.2014.03.003. [DOI] [PubMed] [Google Scholar]
- 23.Poehlsgaard J., Douthwaite S. The bacterial ribosome as a target for antibiotics. Nat. Rev. Microbiol. 2005;3:870–881. doi: 10.1038/nrmicro1265. [DOI] [PubMed] [Google Scholar]
- 24.Kanoh S., Rubin B.K. Mechanisms of action and clinical application of macrolides as immunomodulatory medications. Clin. Microbiol. Rev. 2010;23:590–615. doi: 10.1128/CMR.00078-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sandri A., Carelli M., Visentin A., Savoldi A., De Grandi G., Mirandola M., Lleo M.M., Signoretto C., Cordioli M. Mycoplasma genitalium antibiotic resistance-associated mutations in genital and extragenital samples from men-who-have-sex-with-men attending a STI clinic in Verona, Italy. Front. Cell. Infect. Microbiol. 2023;13:1155451. doi: 10.3389/fcimb.2023.1155451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bradley I., Varma R., Knight V., Iliakis D., McNally L., Jalocon D., McNally L., Jalocon D., Jeoffreys N., Chen S., et al. Prevalence of rectal Mycoplasma genitalium and macrolide resistance in men who have sex with men attending Sydney Sexual Health Centre. Sex. Health. 2020;17:114–120. doi: 10.1071/SH18221. [DOI] [PubMed] [Google Scholar]
- 27.Berçot B., Charreau I., Rousseau C., Delaugerre C., Chidiac C., Pialoux G., Capitant C., Bourgeois-Nicolaos N., Raffi F., Pereyre S., et al. High Prevalence and High Rate of Antibiotic Resistance of Mycoplasma genitalium Infections in Men Who Have Sex With Men: A Substudy of the ANRS IPERGAY Pre-exposure Prophylaxis Trial. Clin. Infect. Dis. 2021;73:e2127–e2133. doi: 10.1093/cid/ciaa1832. [DOI] [PubMed] [Google Scholar]
- 28.Couldwell D.L., Lewis D.A. Mycoplasma genitalium infection: Current treatment options, therapeutic failure, and resistance-associated mutations. Infect. Drug Resist. 2015;8:147–161. doi: 10.2147/IDR.S48813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hamasuna R., Le P.T., Kutsuna S., Furubayashi K., Matsumoto M., Ohmagari N., Fujimoto N., Matsumoto T., Jensen J.S. Mutations in ParC and GyrA of moxifloxacin-resistant and susceptible Mycoplasma genitalium strains. PLoS ONE. 2018;13:e0198355. doi: 10.1371/journal.pone.0198355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.van der Schalk T.E., Braam J.F., Kusters J.G. Molecular basis of antimicrobial resistance in Mycoplasma genitalium. Int. J. Antimicrob. Agents. 2020;55:105911. doi: 10.1016/j.ijantimicag.2020.105911. [DOI] [PubMed] [Google Scholar]
- 31.Murray G.L., Bradshaw C.S., Bissessor M., Danielewski J., Garland S.M., Jensen J.S., Fairley C.K., Tabrizi S.N. Increasing Macrolide and Fluoroquinolone Resistance in Mycoplasma genitalium. Emerg. Infect. Dis. 2017;23:809–812. doi: 10.3201/eid2305.161745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kenyon C., Herrmann B., Hughes G., de Vries H.J.C. Management of asymptomatic sexually transmitted infections in Europe: Towards a differentiated, evidence-based approach. Lancet Reg. Health Eur. 2023;34:100743. doi: 10.1016/j.lanepe.2023.100743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wood G.E., Bradshaw C.S., Manhart L.E. Update in Epidemiology and Management of Mycoplasma genitalium Infections. Infect. Dis. Clin. N. Am. 2023;37:311–333. doi: 10.1016/j.idc.2023.02.009. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data is available from the authors upon request.