Abstract
Background: Bullous pemphigoid (BP) is an autoimmune disease characterized by the appearance of very pruritic subepidermal blisters. It appears mostly in the elderly and is associated with multiple comorbidities, which makes its management and treatment difficult. The purpose of this systematic review is to compile current information on published cases of BP treated with omalizumab (omalizumab) and dupilumab (dupilumab) in order to obtain information on clinical efficacy and safety data available. Methods: A literature search of all cases of BP treated with omalizumab/dupilumab published in the literature up to January 2024 was performed using the Pubmed database. After an exhaustive search, a total of 61 studies encompassing 886 patients met the inclusion criteria and were included in the review. Results: The majority of patients with BP treated with omalizumab/dupilumab presented a significant improvement in symptomatology, being very safe drugs with minimal side effects. The main limitation of the presented review is the quality of the included studies, most of them being case series or individual cases. The development of studies with a higher level of scientific evidence in the near future would be of great interest. Conclusions: Both omalizumab and dupilumab appear to be effective options for treating BP in patients refractory to other pharmacological therapies. They are drugs with a good safety profile and the adverse reactions associated with their use are infrequent and generally mild.
Keywords: bullous pemphigoid, omalizumab, dupilumab
1. Introduction
Bullous pemphigoid (BP) is the most common autoimmune blistering disease in adults in developed countries [1]. It is characterized by the formation of autoantibodies against structural proteins of the dermal-epidermal junction and by the appearance of very itchy hives and subepidermal blisters [2].
Its incidence is about 0.2–3 new cases per 100,000 habitants [3], it appears more frequently in elderly patients (over 70 years of age) [4] and is associated with various comorbidities, such as neurological diseases, or other inflammatory diseases such as rheumatoid arthritis [5]. Probably in relation to the greater burden of comorbidities, as well as the clinical manifestations of the disease, the morbidity and mortality of patients with BP is 5 to 6 times higher compared to the general population adjusted for age and sex [1].
The pathogenesis of this disease is defined by an immunological component (IgG and IgE antibodies against hemidesmosomal proteins BP180 and BP230) and an inflammatory component (action of neutrophils and eosinophils that damage the dermo-epidermal junction). The deposit of antibodies in the basement membrane triggers an inflammatory response responsible for the clinical manifestations of the disease [1]. The ultimate cause is unknown, although exposure to certain drugs has been described as an etiological agent in some cases of BP [6].
The diagnosis of BP is based on the combination of clinical, histological, serological and immunofluorescence data. The suspected diagnosis must be clinical and requires a biopsy for histological and immunofluorescence study, as well as a serological evaluation [7,8].
Regarding current treatment of BP, it should be taken into account, that despite the availability of both topical and systemic treatments, such as corticosteroids and immunsupppresive drugs [9,10], the main limitation in the treatment of BP is the presence of side effects, which especially affect the typical patient group with BP, elderly patients and patients with multiple comorbidities.
Omalizumab is a humanized monoclonal antibody that selectively binds to immunoglobulin E. It is indicated for the treatment of severe allergic asthma, chronic spontaneous urticaria, and chronic rhysnosinusitis with nasal polyps. Various case reports have demonstrated the potential usefulness of omalizumab in BP, which could act by inhibiting the IgE-mediated inflammatory cascade, and is also a drug with an excellent safety profile [11]. On the other hand, Dupilumab is a drug that act son the α subunit of the interleukin 4 receptor (IL-4Rα) inhibiting IL-4 and IL-13 signaling. It is approved for the treatment of asthma, nodular prurigo, atopic dermatitis, and chronic rhinosinusitis with nasal polyposis. Currently, there are published cases in which a clinical improvement has been observed, with cessation of pruritus and reduction in blistering in patients with BP [12]. This improvement, as well as the absence of relevant adverse effects, makes dupilumab postulated as a treatment option for BP.
Given the recent evidence of the potential usefulness of omalizumab and dupilumab in the treatment of BP, as well as their excellent safety profile, it is of great interest to synthesize the available scientific evidence on their use in patients with BP, which is the objective of this systematic review.
2. Materials and Methods
2.1. Study Design and Objectives
A systematic review was carried out including all the reports on BP treated with the biological drugs omalizumab and dupilumab, with the objective to analyze the common clinical characteristics, systematize the evolution of the disease, collect effectiveness data, as well as available safety data.
2.2. Search Strategy
A bibliographic search of all cases published in the literature up to January 2024 was performed using the Pubmed database. The search command used was: ((pemphigoid) OR (bullous pemphigoid)) AND ((omalizumab) OR (dupilumab)). The PRISMA 2020 guidelines for systematic reviews were followed when carrying out this work.
2.3. Inclusion and Exclusion Criteria
The search was limited to: (A) Publications on patients with a clinical diagnosis of BP regardless of severity and presentation treated with omalizumab and dupilumab. (B) Any type of epidemiological study (clinical trials, cohort studies, case-control studies, cross-sectional studies and clinical case presentations). (C) Articles written in English and Spanish. Therefore, the following were excluded: (A) Those publications that did not evaluate patients with a diagnosis of BP treated with omalizumab and/or dupilumab. (B) Clinical guidelines, protocols and conference summaries. (C) Publications written in a language other than English and Spanish.
2.4. Selection of Studies
A first search was carried out in which the titles and abstracts were reviewed by two researchers (EGB and MSD) of all the studies obtained when applying the search command. Of all those studies that met the inclusion and exclusion criteria, the full text was reviewed, as well as their bibliographic references in search of additional sources. Articles that raised doubts about their inclusion or exclusion were subject to discussion with a third researcher (SAS) until a consensus was reached. Articles considered relevant were included in the present analysis.
2.5. Research Questions
The present systematic review attempted to answer the following questions.
What profile do patients with BP treated with biological drugs have?
How effective are omalizumab and dupilumab in the treatment of BP?
What is the safety and side effect profile of omalizumab and dupilumab in patients with BP?
2.6. Variables
To answer these questions, the variables evaluated were:
Clinical and sociodemographic variables related to the characteristics of BP in patients treated with omalizumab/dupilumab, as well as the existence of comorbidities and other autoimmune diseases.
Variables related to the therapeutic management carried out (treatment administered, dosage).
Variables related to the effectiveness of treatment with omalizumab and dupilumab in BP. The rate of complete response of patients under treatment in the assessed studies was collected.
Variables related to the safety of treatments.
2.7. Assessment of the Quality of the Scientific Evidence
The level of evidence of the studies included in the systematic review was evaluated according to the “Center for Evidence-Based Medicine” (CEBM). The levels of evidence were evaluated as follows:
1a: Evidence obtained from systematic reviews or meta-analysis of randomized controlled clinical trials.
1b: Evidence obtained from individual randomized controlled clinical trials.
2a: Evidence obtained from systematic reviews or meta-analysis of cohort studies.
2b: Evidence obtained from individual cohort studies.
3a: Evidence obtained from systematic reviews or meta-analysis of case-control studies.
3b: Evidence obtained from individual case-control studies.
4: Evidence obtained from case series.
5: Evidence obtained from expert opinions.
2.8. Statistical Analysis
Descriptive statistical techniques were used to evaluate the characteristics of the patients included in the evaluated publications. Continuous variables were expressed as mean and standard deviation. The qualitative variables were expressed based on their absolute and relative frequencies. Statistical analyzes were carried out with the JMP 9.0.1 program (SAS 105 Institute, Cary, NC, USA).
3. Results
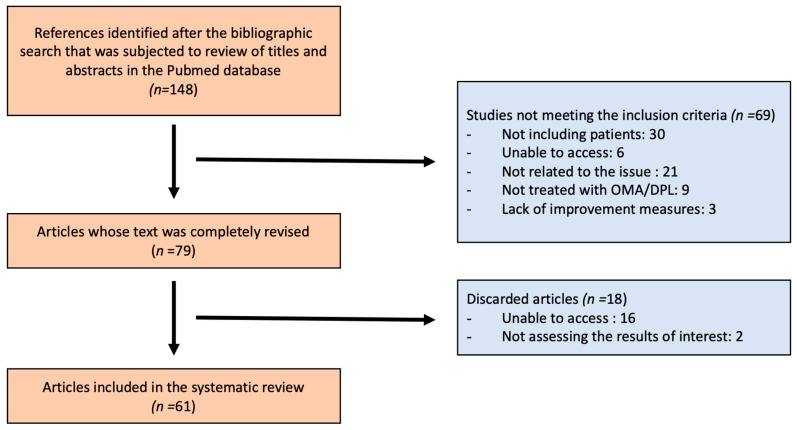
After an initial search, 148 articles were found. After reviewing the titles and abstracts of each of them, 69 were discarded for not meeting the inclusion criteria. Therefore, 79 articles were completely reviewed, of which 18 articles were finally discarded since 16 of them were not completely accessible and 2 of them did not assess the impact of omalizumab/dupilumab on BP. Therefore, 61 articles were included in the systematic review that included 886 patients (363 treated with omalizumab and 523 treated with dupilumab) (Figure 1). All the information included in the studies can be seen in Appendix A and Appendix B.
Figure 1.
Search strategy. OMA (omalizumab); DPL (dupilumab).
3.1. Sociodemographic and Clinical Characteristics of Patients with BP Treated with Omalizumab and Dupilumab
A total of 363 patients with BP who were treated with omalizumab off-label were included in the review. The average age of the patients was 66.7 years. Of the studies that included the sex of the patients, the majority were women (44 vs. 36 men) although not all studies reviewed specified the age/sex of the patients. The majority of patients had multiple comorbidities associated with BP (diabetes mellitus, high blood pressure, osteoporosis, obesity, heart disease, chronic kidney disease, other associated autoimmune diseases). Furthermore, several cases developed BP as a consequence of treatment for other pathologies (such as dipeptidyl peptidase 4 inhibitors for diabetes treatment [13]; BP due to oncological treatment with anti-HER-2 drugs) [14]. The majority of patients had received treatment with several first-line drugs for BP prior to the initiation of treatment with omalizumab (corticosteroids, methotrexate), which had not achieved improvement in the disease.
Regarding patients with BP treated with dupilumab, a total of 523 off-label patients were included. The average age was 68.2 years, of which 59 were women and 174 men (although not all studies specified the age/sex of the patient). The majority of patients presented comorbidities typically associated with BP (cancer, immunosuppression, diabetes, heart failure, osteoporosis). In addition, several patients developed BP after starting treatment for another pathology they had (BP triggered by nivolumab for treatment of lung metastases due to melanoma [15], BP induced by pembrolizumab to treat cervical cancer [16]). Most of them were treated with other first-line drugs (corticosteroids or immunosupppresants) which did not achieve control of the disease.
3.2. Effectiveness of BP Treatment with Omalizumab/Dupilumab
Regarding the effectiveness of BP treatment with omalizumab and dupilumab, the following data were found (Table 1). Regarding treatment with omalizumab, the majority of patients with BP who were treated with 300 milligrams (mg) of omalizumab achieved complete remission of the disease (76.13%), achieving the disappearance of the characteristic skin lesions, as well as the pruritus. The time from the start of treatment to the improvement of the lesions varied among the cases presented, from two weeks to six months, with no recurrences after suspending omalizumab in most cases. Treatment duration was variable between case reports. In some cases, omalizumab was associated with rituximab, achieving remission of the disease more quickly [17]. Likewise, in many of these patients an improvement in the disease was observed, assessable by various scales such as the visual analogue scale (VAS). Along with this, the levels of IgE, eosinophils and antibodies also decreased anti-BP180, BP230.
Table 1.
Overview of data regarding omalizumab and dupilumab treatment for Bullous Pemphigoid.
| Omalizumab | Dupilumab | |
|---|---|---|
| Number of patients treated | 363 | 523 |
| Approved indications for the drug | Severe allergic asthma, chronic spontaneous urticaria, chronic risnosinusitis with nasal polyps | Asthma, nodular prurigo, atopic dermatitis, chronic rhinosinusitis with nasal polyposis, eosinophilic esophagitis, hand dermatitis |
| Route of administration and dosage used in the studies | 300 mg/450 mg/600 mg subcutaneously every 2–4 weeks | 600 mg subcutaneously initially followed by 300 mg subcutaneously every 1–2 weeks |
| Efficacy data of omalizumab and dupilumab to treat BP | Complete response: 76.13%, improvement in BP between 2 weeks and 6 months after starting treatment | Complete response: 70.39%, healing achieved between 2 weeks and 6 months after starting treatment |
| Safety data | Less than 1% of patients presented adverse events, the most frequent were intense pruritus that resolved with dupilumab and dermatitis at the injection site that resolved spontaneously. | Less than 1% of patients presented adverse events, the most frequent were dermatitis at the injection site that resolved spontaneously and eosinophilia that resolved by adding IS. |
Regarding patients with BP who were treated with dupilumab (600 mg induction + 300 mg maintenance), the majority (70.39%) achieved remission of the disease, with control of symptoms and resolution of blistering lesions, this being objective both by scales (EVA, bullous pemphigoid disease area index (BPDAI)) as well as laboratory data where many of them achieved a reduction in Th2 lymphocytes andantibodiesanti-BP180, BP230. The time to achieve improvement ranged from two weeks to six months, after which dupilumab was suspended, maintaining complete remission in most cases. The association of dupilumab with CTC achieved better BP control in certain cases [18,19].
3.3. Side Effects of Treatment with Omalizumab/Dupilumab
The majority of patients treated with omalizumab did not experience any adverse effects. Some adverse effects that were observed were: dermatitis at the injection site that resolved spontaneously, thrombocytopenia in two patients (one of them did not need to stop AOM and another of them who had multiple comorbidities died), intense pruritus that resolved by adding dupilumab and exacerbation of skin lesions in a patient who required discontinuation of omalizumab. The majority of patients tolerated the treatment adequately, with adverse effects being infrequent and mostly mild.
Most patients treated with dupilumab did not experience any adverse effects. Some presented eosinophilia (in two patients, it was resolved by adding immunosuppresive drugs), thrombosis on two occasions, dermatitis at the injection site that did not require suspending dupilumab, two patients developed pneumonia in relation to their comorbidities that did not require suspending dupilumab and were cured with antibiotics. As in the case of omalizumab, most of these adverse effects were mild and transient.
4. Discussion
Omalizumab and dupilumab are two biological drugs widely used in dermatology for the treatment of pathologies such as chronic spontaneous urticaria and atopic dermatitis [1]. In light of the results of the present systematic review, they could be useful in the management of BP, given their effectiveness and safety data.
Regarding BP, both treatments, omalizumab and dupilumab could act on the pathogenesis of the disease. On the one hand, omalizumab acts as an anti-IgE drug, blocking anti-hemidesmosomal IgE antibodies which are involved in the development of the inflammatory reaction. On the other hand, dupilumab blocks IL-4 and IL-13 action, therefore inhibiting the Th2 pathway which is overexpressed in BP lesions [1].
The patients analyzed in the present systematic review who have received treatment with omalizumab and dupilumab are mostly elderly patients, with multiple comorbidities [4]. This study population resembles the patient profile commonly seen in real clinical practice [5], which adds value to the results obtained, facilitating the translation of the results of the review to medical practice. However, it should be taken into account that patients treated with omalizumab and dupilumab are mainly refractory to other treatments, which is why these drugs were administered off-label. The evaluation of the effectiveness and safety of the early treatment with biologic drugs for BP patients could be of interest to avoid the use of immunosuppressive drugs in patients with an increased comorbidity and mortality.
Regarding effectiveness data, omalizumab and dupilumab could be considered effective drugs for BP, with complete response rates of 76.13% and 70.39% in the reviewed literature. These data are better than response rates seen in other studies for drugs such as methotrexate [20] or oral corticosteroids [21]. On the other hand, the dosage of omalizumab (300 mg subcutaneous every four weeks) and dupilumab (600 mg subcutaneous induction + 300 mg subcutaneous every one to two weeks) could favor greater adherence to treatment in elderly patients. Directly observed treatment (administered by nursing or qualified personnel) may even be useful [18,22]. Although exact complete response rates are not totally comparable due to the lack of standardized criteria among the included studies, future information on the clinical characteristics which could act as biomarkers of response to these drugs are necessary. The predominant implication of IgE antibodies or Th2 inflammatory response in each patient could make a difference in terms of clinical response.
On the other hand, it must be taken into account that the main limitation found in routine clinical practice for the treatment of BP is the comorbidity that patients present, in addition to their advanced age. This fact greatly limits the use of drugs that could be effective, such as systemic corticosteroids or immunosuppressive drugs, but which have an unfavorable side effect profile. Side effects found in the literature reviewed for omalizumab include injection site dermatitis, persistent pruritus, exacerbation of skin lesions, and thrombopenia. In the case of dupilumab, the most frequently described side effects are eosinophilia, infections and dermatitis at the injection site. In both cases, the profile of side effects is not very serious, which represents a comparative advantage with respect to the rest of the systemic treatments commonly used.
There are other biological drugs that could be useful for the treatment of BP, such as RTX an anti-CD20 drug. Although there is data on its effectiveness (with response rates of 70.5%) [22,23], Its side effect profile, which includes the possibility of serious infections and oncological processes, would a priori not make it optimal for the management of patients with BP with multiple comorbidities, which is why its data have not been the objective of this systematic review.
The main limitation of the review presented is the quality of the studies included in it, most of them being case series or individual cases. The development of studies with a higher level of scientific evidence in the near future would be of great interest for adequate knowledge of the degree of effectiveness of omalizumab and dupilumab in BP. Moreover, the lack of standardized criteria for defining clinical response in BP treatment could make it difficult to compare the effectiveness between both treatments.
5. Conclusions
BP patients with treated with omalizumab and dupilumab show a profile of sociodemographic and clinical characteristics that could be comparable to that of patients with BP treated in routine clinical practice, although these are patients refractory to other systemic treatments, including corticosteroids and immunosupppresive agents. Both omalizumab and dupilumab appear to be effective options for the treatment of BP in patients refractory to other pharmacological therapies. Moreover, omalizumab and dupilumab are drugs with a good safety profile for use in patients with BP, with adverse reactions associated with their use being rare and generally mild.
Appendix A
Table A1.
Summary of the Studies Including Patients Treated with Omalizumab.
| Study | Type of Study | Number of Patients | Dose Used | Previous Treatments | Results Obtained | Side Effects | Observations | Level of Evidence |
|---|---|---|---|---|---|---|---|---|
| “Cao P et al. (2022)” [23] | Systematic review 75 studies included | 211 patients with BP, 53 treated with omalizumab, 122 with RTX and 36 with dupilumab | NE, average treatment duration 6.6 months | CTC, MTX, MMF, AZA, CLP, CFF | Complete BP remission in 67.9% of patients (36/56) and partial remission in20.8% (11/53) | Recurrence 5.7% (3/53) Death due to thrombocytopenia(1.9%, 1/53) |
The rest of the patients included in the study (122) were treated with RTX, giving a higher number of recurrences, AEs and mortality | 2a |
| “Oren-Shabtai M et al. (2023)” [22] | Presentation of 9 cases | 9, 3 of them treated with omalizumab, 7 with RTX and 1 with dupilumab, average age 60.4 years | 300 mg every 4 weeks | CTC, BIO, RTX | 78% clinical improvement, 55% complete remission at 3 months | None | omalizumab achieves greater IgE reduction. Multiple comorbidities: Parkinson’s, dementia, DM, HF, hypothyroidism, IR | 4 |
| “D’Aguanno K et al. (2022)” [24] | Systematic review of 22 articles | 56 | 300 mg every 4 weeks. One patient received a single dose of 450 mg | CTC | 87.5% respond to treatment at 13 weeks (55.4% complete remission, 32.1% partial) | None | NP | 2a |
| “Seyed J et al. (2020)” [25] | Presentation of 1 case | 1, M 70 years | 300 mg every 4 weeks | CTC, DAP, MTX, MMF | Decrease in BP-100, EVA scale went from 9/10 to 2/10 after 2 months. Complete remission after 3 months | Persistent pruritus that was resolved by adding 600 mg dupilumab + 300 mg dupilumab | Complete remission with omalizumab + dupilumab after trying various treatments. Pcte with metabolic ds | 4 |
| “Vassallo C et al. (2022)” [26] | Retrospective study of 222 patients | 222, 5 BP-dependent CTC patients with IC were selected for other treatments. Average age: 77.4 years. 3M, 2F | 300 mg every 4 weeks, treatment duration: average 9.2 months | CTC, IS, AH | Resolution of skin lesions in all patients and reduction of pruritus. Reduction of IgE and anti-BP180, BP230 and eosinophils | NE | Patients with several comorbidities: DM2, hepatitis, hip replacement, vitiligo, osteoporosis | 4 |
| “Kremer N et al. (2019)” [11] | Systematic review of 35 publications | 84 PCs (62 receive RTX and 22 omalizumab) | NE | CTC | Complete remission 85% with RTX and 84% with omalizumab | 24% in treatment with RTX and 20% with omalizumab | Fewer recurrences with RTX than with omalizumab | 2a |
| “Velin M et al. (2022)” [27] | Retrospective study | 112 (19 met inclusion criteria), mean age 76 years | 300 mg every 4 weeks SB, average of 9 months of treatment | CTC, MTX | 60% complete remission, 20% partial remission | One case with poor skin tolerance (burning sensation) for treatment with dupilumab (lasted 1 month with treatment) | Of the 19 patients, 12 received MTX, 7 omalizumab and 8 dupilumab | 4 |
| “Kwon IJ et al. (2023)” [17] | Retrospective study | 49 (25 RTX only, 17 RTX + omalizumab) | 13 patients: 300 mg omalizumab twice (every 4 weeks) and 4 patients 300 mg omalizumab once | CTC, RTX | RTX + omalizumab improvement in 15 days vs. 67.5 days if only RTX. Control only RTX 92% vs. RTX + omalizumab 100% | None | 0% mortality RTX + omalizumab, 16% if RTX monotherapy | 4 |
| “Yu KK et al. (2022)” [28] | Open, uncontrolled study | 6, F, average age 72.8 years | 300 mg every 2–4 weeks (6 cycles) | CTC, AZA, MC | Benefit in 5/6 patients with reduction in pruritus, eosinophils, blisters at 2 weeks | None | All failed previous treatment with CTC | 4 |
| “Seyed J et al. (2019)” [29] | Presentation of 2 cases | 2, between 60–65 years | 300 mg every 2 weeks for 1 month | CTC, AZA, TC | After 1 month of treatment with AOM, blisters and itching disappear | None | NP | 4 |
| “Alexandre M et al. (2022)” [30] | Retrospective study | 13, 5M, 8F, average age 66 years | 300 mg/450 mg/600 mg | CTC, MMF, DOX, MTX | Improvement in pruritus, hives, blisters, complete remission 85% at 3 months | 2 pneumonias, 1 kidney failure in very frail elderly patients (no clear correlation with omalizumab use) | 7/13 had mucosal involvement | 4 |
| “James T et al. (2019)” [31] | Presentation of 1 case | 1, M 72 years | NE | CTC, IS, IVIg, RTX | Resolution of blisters, IgG decrease | NE | Multiple comorbidities: DM2, CKD grade IV | 4 |
| “Ewy S et al. (2019)” [32] | Presentation of 1 case | 1, F 74 years | 300 mg every 4 weeks | NE | Reduction of pain and skin lesions | Injection site dermatitis that resolved spontaneously within 2 days | NP | 4 |
| “Navarro-Triviño FJ et al. (2021)” [33] | Presentation of 1 case | 1M, 70 years | 300 mg subcutaneously every 3 weeks | CTC, AZA | Blisters disappear after 3 months | None | Analytical findings and refusal of patient to be treated with RTX due to PML risk | 4 |
| “Balakirski G et al. (2016)” [34] | Presentation of 2 cases | 2, F 40 years old, M 63 years old | 1. 300 mg every 3 weeks 2. 300 mg every 3 weeks |
1. CTC, AZA 2. CTC |
1 and 2: Pruritus improvement after 5 days of treatment with omalizumab | None | 1. omalizumab was started at 300 mg every 4 weeks, but due to increased blisters, 300 mg was started every 3 weeks. 2. omalizumab was discontinued due to other health problems, reappearance of blisters, continued CTC |
4 |
| “Garrido PM et al. (2020)” [13] | Presentation of 1 case | 1, F 76 years | 300 mg every 4 weeks | CTC, DOX, AZA, IVIg | Pruritus disappears after 3 days, complete resolution of skin lesions after 2 months | None | BP triggered by treatment with DDP-4 (vildagliptin) for DM treatment | 4 |
| “From D et al. (2021)” [35] | Presentation of 6 cases | 6 (4F, 2M), average age 64.5 years | 300 mg every 4 weeks | NE, but CTC contraindicated due to comorbidities | NE | NE | Patients treated with AOM since due to their comorbidities they are IC other treatments | 4 |
| “Gönül MZ et al. (2016)” [36] | Presentation of 1 case | 1, M 70 years | 300 mg every 4 weeks, total of 11 doses | CTC, TC, DAP | Disappearance of blisters, IgE decrease a week after treatment | Thrombocytopenia that did not require discontinuation of omalizumab | NP | 4 |
| “Lonowski S et al. (2020)” [37] | Retrospective study | 11, average age 78 years | 10 treatments with 300 mg omalizumab every 4 weeks. One treatment with 375 mg every 2 weeks | CTC, RTX, AZA | 6/11 complete answer 3/11 partial answer 2/11 no response |
1 of them exacerbation of skin lesions that required discontinuation of omalizumab 1 of them had infection, died and had treatment with CTC 9/11 no adverse effects |
The exacerbation of lesions with AOM was an AE that had not previously appeared and is the result of future research. | 4 |
| “Menzinger S et al. (2018)” [38] | Presentation of a case | 1, F 76 years | 300 mg every 4 weeks | CTC | Disappearance of the disease after 8 weeks of treatment, itching improves after 2 days | None | Multiple comorbidities (CKD dementia, ischemic heart disease…) | 4 |
| “Liu J et al. (2022)” [39] | Presentation of a case | 1, M 76 years | 300 mg every 4 weeks | AH, CTC | After 3 days itching improved, no new blisters appeared | NE | Multiple comorbidities that CI tto IS | 4 |
| “Yalcin AD et al. (2014)” [40] | Presentation of a case | 1, M 28 years | 300 mg, 13 doses in total | CTC, CFF | Complete remission | NE | Young person (28 years old) | 4 |
| “London VA et al. (2012)” [41] | Presentation of 1 case | 1, F 70 years | 300 mg every 4 weeks | CTC, AZA, MMF, CFF | Disappearance of blisters, decrease in anti BP180, disappearance of findings in IF | None | NP | 4 |
| “Barrios DM et al. (2021)” [14] | Retrospective study | 34, 50% F mean age 67.5 a (9 of them due to BP) due to QT drug effects | NE | Combination with ipilimumab, atezolizumab, durvalumab | omalizumab improves pruritus and BP | 2/9 did not respond to omalizumab) | Treatment with AOM for complications QT treatment in solid tumors | 4 |
| “Sinha S et al. (2020)” [42] | Presentation of a case | 1, F 44 years | 450 mg | CTC, RTX, AZA | Disappearance of blisters | NE | Obesity | 4 |
| “Dufour C et al. (2012)” [43] | Presentation of 1 case | 1M 5 months | 100 mg, every 2 weeks for 3 months | CTC, AZA | Reduction of urticarial lesions and blisters. Complete resolution after 10 months of treatment | NE | BP in a 5-month-old baby | 4 |
| “Fairley JA et al. (2009)” [44] | Presentation of a case | 1, F 70 years | 300 mg every 2 weeks | CTC, AZA, MC | Partial remission, decrease in eosinophils and anti BP180 | NE | NP | 4 |
| “From A et al. (2021)” [45] | Presentation of 3 cases | 1 of them with BP, M 65 years old | 300 mg every 4 weeks | CTC, MMF | Complete remission after 3 months of treatment | NE | NP | 4 |
| “Chebani R et al. (2024)” [46] | Retrospective study | 100, average age 77 years | 300 mg | NE | Complete remission 77% at 3 months | NE | More significant improvement if high levels of anti BP180 | 4 |
AF: family history, HBsAg: hepatitis B surface antigen, AH: antihistamines, ATB: antibiotic, AZA: azathioprine, BIO: biological, BPDAI: bullous pemphigoid disease area index, CFF: cyclophosphamide, CH: colchicine, IC: contraindication, CLP: cyclosporine, CMV: cytomegalovirus, CTC: corticosteroids, DAP: dapsone, DM: diabetes mellitus, DOX: doxycycline, dupilumab dupilumab, CKD: chronic kidney disease, VAS: visual analog pain scale, F: woman, AF: atrial fibrillation, HTN: arterial hypertension, IC: heart failure, IDPP4: dipeptidyl peptidase 4 inhibitors, IF: immunofluorescence, Ig: immunoglobulins, IVIg: intravenous Ig, IM: immunomodulators, IR: renal failure, IS: immunosuppressants, PML: progressive multifocal leukoencephalopathy, M: male, MC: minocycline, MG: milligrams, MMF: mycophenolate mofetil, MTP: methylprednisolone, MTX: methotrexate, No.: number, NE: not specified, NP: not applicable, NT: nicotinamide, AOM: omalizumab, PA: bullous pemphigoid, PCTE: patient, QT: chemotherapy, PROM: premature rupture of membranes, RTX: rituximab, SB: subcutaneous, SD: syndrome, SEM: week, OS: week of gestation, TBC: tuberculosis, TC: tetracyclines, PET: pulmonary thromboembolism, TTO: treatment, HIV: human immunodeficiency virus.
Appendix B
Table A2.
Summary of the Studies Including Patients Treated with Dupilumab.
| Study | Type of Study | Number of Patients | Dose Used | Previous Treatments | Results Obtained | Side Effects | Observations | Level of Evidence |
|---|---|---|---|---|---|---|---|---|
| “Cao P et al. (2022)” [23] | Systematic review 75 studies included | 211 in total, 36 treated with dupilumab, 53 with omalizumab and 122 with RTX | NE | CTC, MTX, MMF, AZA, CLP, CFF | Total remission 66.7% (24/36) Partial remission 19.4% (7/36) at 4.5 months |
Recurrence 5.6% (2/36). No adverse effects | The rest of the patients included in the study (122) were treated with RTX, giving a higher number of recurrences, AEs and mortality | 2a |
| “Russo R et al. (2022)” [47] | Literary review of 9 articles | 30 (16 M, 14 F), average age 69.85 years | NE | CTC, I.S. | Decrease in Th2 lymphocytes and improvement in pruritus | None, no interaction with other drugs | Some comorbidities: TB, melanoma, cancer, obesity, DM… | 2a |
| “Zhao L et al. (2023)” [48] | Retrospective cohort study | 146, average age 73 years, 86% M | 300 mg every 2 weeks after an initial dose of 600 mg | NE | 127 (87%) BP control in 1 month | Injection site injuries did not require suspending dupilumab | 3 pctes pneumonia that improved with ATB without needing to suspend dupilumab (pneumonia associated with comorbidities) | 2b4 |
| “Zhang Y et al. (2021)” [49] | Retrospective study | 24, average age 64.50 years (8 treatment with dupilumab + AZA + MTP and 16 AZA + MTP) | 600 mg initially, followed by 300 mg weekly | MTP, AZA | Complete remission 62.5%, partial remission 12.5% | Eosinophilia, recurrence 12.5% | Add dupilumab to MTP + AZA + effective than without dupilumab | 4 |
| “Liang J et al. (2023)” [50] | Number of cases | 9, average age 68 years | NE | CTC | Total remission: 74.6% Partial remission 11.1% |
NE | NP | 4 |
| “Abdat R et al. (2020)” [12] | Case series from 5 academic centers | 13, average age 76.8 years | NE | NE | Total remission: 53.8%, response to treatment 92.3% | None | NP | 4 |
| “Yan T et al. (2023)” [18] | Retrospective cohorts | 40 (20 treated with dupilumab and another 20 with dupilumab + CTC) | 600 mg initially followed by 300 mg weekly | CTC | Of the 20 treated only with dupilumab: 12 had complete remission, 8 had partial remission after 6 months of treatment. | NE | dupilumab improves AP symptoms, but fails to reduce BP180 levels | 2b |
| “Oren-Shabtai M et al. (2023)” [22] | Series of 9 cases | 9, 1 of them treated with dupilumab, 3 with omalizumab and 7 with RTX average age 60.4 years | 600 mg initially, followed by 300 mg weekly | CTC, BIO, RTX | 78% clinical improvement, 55% complete remission | None | NP | 4 |
| “Learned C et al. (2023)” [51] | Retrospective study | 17 (10 M and 7 F), average age 72.2 years | 300 mg weekly | MMF, DOX, CTC IVIg | 14 patients had complete remission, 2 had partial remission and 1 had significant improvement. | None | The patients included had tried 4 lines of treatment prior to dupilumab | 4 |
| “Seyed J et al. (2020)” [25] | Description of a case | 1, M 70 years | 600 mg initially, followed by 300 mg weekly | CTC, DAP, MTX, MMF, omalizumab | Disappearance of pruritus, VAS scale 0/10, complete remission in AOM association | NE | Complete remission with omalizumab + dupilumab after trying various treatments. Pcte with metabolic ds | 4 |
| “Velin M et al. (2022)” [27] | Retrospective study | 112 (19 met inclusion criteria) | 300 mg every 2 weeks | CTC, MTX | 60% complete remission, 20% partial remission | Only 1 percent in treatment with dupilumab skin burning sensation, only lasted 1 month with dupilumab | Of the 19 patients, 12 received MTX, 7 omalizumab and 8 dupilumab | 4 |
| “Hu L et al. (2023)” [52] | Retrospective study | 11, average age 76 years, 4M, 7F | 600 mg followed by 300 mg every 2 weeks | I.S., M.C. | In 2 weeks 10/11 patients control the disease | None | NP | 4 |
| “Qi W et al. (2023)” [53] | Compare 2 groups | 27 (9 received MTP + dupilumab), 18 only MTP, mean age 72 years | NE | MTP | Improvement of the disease in patients treated with MTP + dupilumab | None with dupilumab | NP | 4 |
| “Klepper EM et al. (2021)” [54] | Case report | 1, F 79 years | 600 mg initially, followed by 300 mg weekly | CTC, DAP, DOX | After 1 month of treatment with dupilumab, 100% reduction in itching | NE | dupilumab indicated in people over 6 years of age | 4 |
| “Yang J et al. (2022)” [55] | Retrospective cohort study | 40 (20 MTP only, 20 MTP + dupilumab) | 600 mg initially, followed by 300 mg weekly | MTP | Greater control of BP, pruritus and quality of life in MTP + dupilumab | Eosinophilia, thrombosis in 2 patients (1 from each group), PE, gastritis, pneumonia, herpes zoster | NP | 4 |
| “Foerster Y et al. (2023)” [56] | Report of 3 patients, only 1 treatment with dupilumab | 3 patients with BP + HIV-1, patients treated with dupilumab M aged 60 years | 600 mg initially, then 300 mg every 2 weeks | CTC, AZA, DAP, DOX + antiretroviral treatment | Disappearance of itching and blisters | NE | Of the 3 exposed cases, only 1 was treated with dupilumab | 4 |
| “Zhang X et al. (2023)” [57] | Retrospective study | 7 | 600 mg initially, followed by 300 mg weekly for 16 weeks | CTC, OMZ, tofacinib, CLP | 6/7 complete remission 1/7 partial improvement | None | NP | 4 |
| “Sanfilippo E et al. (2023)” [58] | Report of 1 case | 1, M 80 years | NE | CTC | Improvement of itching and disappearance of blisters | NE | Background: AF, HF, T2DM, HTN, prostate cancer, stroke | 4 |
| “Takamura S et al. (2022)” [59] | Presentation of 1 case | 1, F 72 years | NE | NE | Improves pruritus, blisters and anti-BP180 negativity | NE | NP | 4 |
| “Wang Q et al. (2023)” [60] | Presentation of 1 case | 1, M 60 years | 600 mg initially, followed by 300 mg weekly | CTC, MTX | Improvement of itching in 3 days and blisters in 2 weeks, disappearance of Ig in 6 weeks | NE | Tto for 10 weeks after induction: CTC + dupilumab without AP recurrences | 4 |
| “Wang SH et al. (2023)” [61] | Number of cases | 10 (7M, 3F) mean age 72.7 | Initially 600 mg, then 300 mg every 2 weeks | MTP, MC, AH, IVIg | 90% improvement in pruritus, complete remission 70%, average duration 8.3 weeks | Eosinophilia in 2 cases that was resolved with IS | Multiple comorbidities: DM, allergic rhinitis, osteoporosis, CMV infection, pneumocystis pneumonia | 4 |
| “Wang M et al. (2022)” [62] | Presentation of 2 cases | 2 | 1. 300 mg dupilumab twice 2. 300 mg dupilumab twice |
1. MTP + MTX 2. MTP |
1. pruritus improvement in 2 weeks 2. lesion remission in 2 weeks |
None | dupilumab prevents complications from other treatments such as RTX (infections and heart disease) | 4 |
| “Bruni M et al. (2022)” [15] | Case study | 1, M 76 years | 300 mg dupilumab | MTP, DOX | Complete remission in 6 months | NE | BP triggered by nivolumab for treatment of lung metastases due to melanoma |
4 |
| “Liu JH et al. (2023)” [63] | Case study | 1, M 73 years old | 600 mg subcutaneously, followed by 300 mg subcutaneously | DOX, CTC | Disappearance of PA lesions and psoriasis after 16 days of treatment with dupilumab. No relapses | NE | Effective treatment with dupilumab for psoriasis + BP | 4 |
| “Manzo Margiotta F et al. (2023)” [64] | Presentation of a case | 1, M 74 years | 600 mg followed by 300 mg every 2 weeks | CTC, DOX, NT, DAP | Resolution of blisters and itching after 16 weeks of treatment | None | NP | 4 |
| “Valenti M et al. (2022)” [65] | Case report | 1 | 600 mg followed by 300 mg every 2 weeks | MTP, AZA, DAP, CH | After 3 months anti BP230 normal levels | NE | NP | 4 |
| “Savoldy MA et al. (2022)” [66] | Case study | 1, M 78 years old | 300 mg every 2 weeks | CTC, DOX, IS | Improvement after 6 weeks | None | AP triggered after COVID-19 vaccination | 4 |
| “Zhou AE et al. (2022)” [67] | Presentation of a case | 1, F 17 years old | 300 mg | CTC, RTX, IVIg | Complete resolution after 4 weeks, improvement after 2 weeks of starting dupilumab | NE, no relapses | Young woman (17 years old) with BP, not AF | 4 |
| “Pop SR et al. (2022)” [16] | Presentation of a case | 1, F 59 years | 300 mg dupilumab + CTC treatment |
CTC, DOX, NT, DAP, MMF | Improvement of blisters | NE, CTC could be suspended without regrowth, leaving only dupilumab | BP induced by pembrolizumab for cervical cancer treatment. | 4 |
| “Riqueleme- Mc Loughlin et al. (2021)” [68] | Presentation of a case | 1, F 37 years | 600 mg at 30 weeks, followed by 300 mg at 2 weeks | CTC | Itching and blisters improve, fetus birth without incidents | PROM at 34.4 weeks, birth by cesarean section | Case of gestational AP + frequent in 2nd and 3rd trimester | 4 |
| “Zhang Y et al. (2021)” [69] | Presentation of a case | 1, F 61 years | 600 mg followed by 300 mg | CTC, AZA | Disappearance of itching after a month, no formation of new blisters | NE | NP | 4 |
| “Kaye A et al. (2018)” [70] | Presentation of a case | 1, M 80 years | 600 mg followed by 300 mg | CTC | Full resolution at 3 m and normalization levels BP180 and BP230 | NE | TB infection and HBsAg + contraindicated immunosuppressive treatment | 4 |
| “Jendoubi F et al. (2022)” [71] | Presentation of a case | 1, F 76 years | 600 mg followed by 300 mg every 2 weeks | CTC | Complete resolution of pruritus and blisters, without recurrence after 6 months | None | Pcte with nodular pemphigus (BP variant) | 4 |
| “Fournier C et al. (2023)” [72] | Presentation of 3 cases | 1, M 74 years old | 600 mg followed by 300 mg | CTC | Complete remission | NE | Development of BP following treatment with nivolumab for melanoma | 4 |
| “Huand D et al. (2023)” [19] | Retrospective cohort study | 36 patients, 20 receive MTP, 16 dupilumab +MTP, average age 71 years | 600 mg of dupilumab followed by 300 mg athenext week | NE | Pruritus decrease and BPDAI scale improvement + effective with MTP + dupilumab at 2 weeks | 2 cases dermatitis at injection site, 3 transient hyperglycemia, 4 hypereosinophilia | MTP + dupilumab group, MTP is suspended and 300 mg 2/wk dupilumab is continued as monotherapy. | 4 |
| “Chen J et al. (2023)” [73] | Presentation of 2 cases | 2, M, 66 and 79 years | 600 mg initially, followed by 300 mg weekly for 16 weeks | NE | Clinical improvement without relapses | One of them had erythema at the injection site, which resolved itself. | Comorbidities: DM, asthma, HTN | 4 |
AF: family history, HBsAg: hepatitis B surface antigen, AH: antihistamines, ATB: antibiotic, AZA: azathioprine, BIO: biological, BPDAI: bullous pemphigoid disease area index, CFF: cyclophosphamide, CH: colchicine, IC: contraindication, CLP: cyclosporine, CMV: cytomegalovirus, CTC: corticosteroids, DAP: dapsone, DM: diabetes mellitus, DOX: doxycycline, dupilumab dupilumab, CKD: chronic kidney disease, VAS: visual analog pain scale, F: woman, AF: atrial fibrillation, HTN: arterial hypertension, IC: heart failure, IDPP4: dipeptidyl peptidase 4 inhibitors, IF: immunofluorescence, Ig: immunoglobulins, IVIg: intravenous Ig, IM: immunomodulators, IR: renal failure, IS: immunosuppressants, PML: progressive multifocal leukoencephalopathy, M: male, MC: minocycline, MG: milligrams, MMF: mycophenolate mofetil, MTP: methylprednisolone, MTX: methotrexate, No.: number, NE: not specified, NP: not applicable, NT: nicotinamide, AOM: omalizumab, BP: bullous pemphigoid, PCTE: patient, QT: chemotherapy, PROM: premature rupture of membranes, RTX: rituximab, SB: subcutaneous, SD: syndrome, SEM: week, OS: week of gestation, TBC: tuberculosis, TC: tetracyclines, PET: pulmonary thromboembolism, TTO: treatment, HIV: human immunodeficiency virus.
Author Contributions
Conceptualization E.G.-B., M.S.-D. and S.A.-S.; methodology, E.G.-B., D.M.-B. and M.S.-D.; formal analysis, E.G.-B. and D.M.-B.; data curation M.S.-D. and E.G.-B.; writing—original draft preparation, M.S.-D. and E.G.-B.; writing—review and editing, D.M.-B. and S.A.-S.; supervision, S.A.-S. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in the study are included in the article, further inquiries can be directed to the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
Funding Statement
This research received no external funding.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Fuertes de Vega I., Iranzo-Fernández P., Mascaró-Galy J.M. Bullous pemphigoid: Clinical practice guidelines. Actas Dermosifiliogr. 2014;105:328–346. doi: 10.1016/j.ad.2012.10.022. [DOI] [PubMed] [Google Scholar]
- 2.Miyamoto D., Santi C.G., Aoki V., Maruta C.W. Bullous pemphigoid. An. Bras. Dermatol. 2019;94:133–146. doi: 10.1590/abd1806-4841.20199007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Khandpur S., Verma P. Bullous pemphigoid. Indian J. Dermatol. Venereol. Leprol. 2011;77:450–455. doi: 10.4103/0378-6323.82398. [DOI] [PubMed] [Google Scholar]
- 4.Borradori L., Van Beek N., Feliciani C., Tedbirt B., Antiga E., Bergman R., Böckle B.C., Caproni M., Caux F., Chandran N., et al. Updated S2 K guidelines for the management of bullous pemphigoid initiated by the European Academy of Dermatology and Venereology (EADV) J. Eur. Acad. Dermatol. Venereol. 2022;36:1689–1704. doi: 10.1111/jdv.18220. [DOI] [PubMed] [Google Scholar]
- 5.Huttelmaier J., Benoit S., Goebeler M. Comorbidity in bullous pemphigoid: Up-date and clinical implications. Front. Immunol. 2023;14:1196999. doi: 10.3389/fimmu.2023.1196999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bastuji-Garin S., Joly P., Picard-Dahan C., Bernard P., Vaillant L., Pauwels C., Salagnac V., Lok C., Roujeau J.-C. Drugs associated with bullous pemphigoid. A case-control study. Arch. Dermatol. 1996;132:272–276. doi: 10.1001/archderm.1996.03890270044006. [DOI] [PubMed] [Google Scholar]
- 7.Schmidt E., della Torre R., Borradori L. Clinical features and practical diagnosis of bullous pemphigoid. Dermatol. Clin. 2011;29:427–438. doi: 10.1016/j.det.2011.03.010. [DOI] [PubMed] [Google Scholar]
- 8.Bernard P., Antonicelli F. Bullous Pemphigoid: A Review of its Diagnosis, Associations and Treatment. Am. J. Clin. Dermatol. 2017;18:513–528. doi: 10.1007/s40257-017-0264-2. [DOI] [PubMed] [Google Scholar]
- 9.Fivenson D.P., Breneman D.L., Rosen G.B., Hersh C.S., Cardone S., Mutasim D. Nicotinamide and tetracycline therapy of bullous pemphigoid. Arch. Dermatol. 1994;130:753–758. doi: 10.1001/archderm.1994.01690060083010. [DOI] [PubMed] [Google Scholar]
- 10.Heilborn J.D., Ståhle-Bäckdahl M., Albertioni F., Vassilaki I., Peterson C., Stephansson E. Low-dose oral pulse methotrexate as monotherapy in elderly patients with bullous pemphigoid. Pt 1J. Am. Acad. Dermatol. 1999;40:741–749. doi: 10.1016/S0190-9622(99)70156-8. [DOI] [PubMed] [Google Scholar]
- 11.Kremer N., Snast I., Cohen E.S., Hodak E., Mimouni D., Lapidoth M., Mazor S., Levi A. Rituximab and Omalizumab for the Treatment of Bullous Pemphigoid: A Systematic Review of the Literature. Am. J. Clin. Dermatol. 2019;20:209–216. doi: 10.1007/s40257-018-0401-6. [DOI] [PubMed] [Google Scholar]
- 12.Abdat R., Waldman R.A., de Bedout V., Czernik A., Mcleod M., King B., Gordon S., Ahmed R., Nichols A., Rothe M., et al. Dupilumab as a novel therapy for bullous pemphigoid: A multicenter case series. J. Am. Acad. Dermatol. 2020;83:46–52. doi: 10.1016/j.jaad.2020.01.089. [DOI] [PubMed] [Google Scholar]
- 13.Garrido P.M., Alexandre M.I., Travassos A.R., Filipe P. Dipeptidyl-peptidase IV inhibitor-associated bullous pemphigoid efficiently treated with omalizumab. Dermatol. Ther. 2020;33:e14160. doi: 10.1111/dth.14160. [DOI] [PubMed] [Google Scholar]
- 14.Barrios D.M., Phillips G.S., Geisler A.N., Trelles S.R., Markova A., Noor S.J., Quigley E., Haliasos H., Moy A., Schram A., et al. IgE blockade with omalizumab reduces pruritus related to immune checkpoint inhibitors and anti-HER2 therapies. Ann. Oncol. 2021;32:736–745. doi: 10.1016/j.annonc.2021.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bruni M., Moar A., Schena D., Girolomoni G. A case of nivolumab-induced bullous pemphigoid successfully treated with dupilumab. Dermatol. Online J. 2022;28:2. doi: 10.5070/D328257396. [DOI] [PubMed] [Google Scholar]
- 16.Pop S.R., Strock D., Smith R.J. Dupilumab for the treatment of pembrolizumab-induced bullous pemphigoid: A case report. Dermatol. Ther. 2022;35:e15623. doi: 10.1111/dth.15623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kwon I.J., Kim T., Yoo D.S., Min S., Kim S.C., Kim J.H. Clinical effect of omalizumab as an adjuvant treatment to rituximab in patient with refractory bullous pemphigoid. J. Dermatol. 2023;50:705–709. doi: 10.1111/1346-8138.16678. [DOI] [PubMed] [Google Scholar]
- 18.Yan T., Xie Y., Liu Y., Shan Y., Wu X., Wang J., Zuo Y.-G., Zhang Z. Dupilumab effectively and rapidly treats bullous pemphigoid by inhibiting the activities of multiple cell types. Front. Immunol. 2023;14:1194088. doi: 10.3389/fimmu.2023.1194088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Huang D., Zhang Y., Yu Y., Jiang Y., Kong L., Ding Y., Shi Y., Gao Y. Long-term efficacy and safety of dupilumab for severe bullous pemphigoid: A prospective cohort study. Int. Immunopharmacol. 2023;125:111157. doi: 10.1016/j.intimp.2023.111157. [DOI] [PubMed] [Google Scholar]
- 20.Delaumenie S., Assikar S., Prudhomme R., Matei I., Souyri N., Dalmay F., Bedane C. Methotrexate is safe and efficient as long-term treatment for bullous pemphigoid. Eur. J. Dermatol. 2019;29:217–218. doi: 10.1684/ejd.2019.3501. [DOI] [PubMed] [Google Scholar]
- 21.Hébert V., Hébert V., Bastos S., Bastos S., Drenovska K., Drenovska K., Meijer J., Meijer J., Ingen-Housz-Oro S., Ingen-Housz-Oro S., et al. International multicentre observational study to assess the efficacy and safety of a 0·5 mg kg−1 per day starting dose of oral corticosteroids to treat bullous pemphigoid. Br. J. Dermatol. 2021;185:1232–1239. doi: 10.1111/bjd.20593. [DOI] [PubMed] [Google Scholar]
- 22.Oren-Shabtai M., Mimouni D., Nosrati A., Atzmony L., Kaplan B., Barzilai A., Baum S. Biological treatment for bullous pemphigoid. Front. Immunol. 2023;14:1157250. doi: 10.3389/fimmu.2023.1157250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cao P., Xu W., Zhang L. Rituximab, Omalizumab, and Dupilumab Treatment Outcomes in Bullous Pemphigoid: A Systematic Review. Front. Immunol. 2022;13:928621. doi: 10.3389/fimmu.2022.928621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.D’aguanno K., Gabrielli S., Ouchene L., Muntyanu A., Ben-Shoshan M., Zhang X., Iannattone L., Netchiporouk E. Omalizumab for the Treatment of Bullous Pemphigoid: A Systematic Review of Efficacy and Safety. J. Cutan. Med. Surg. 2022;26:404–413. doi: 10.1177/12034754221089267. [DOI] [PubMed] [Google Scholar]
- 25.Jafari S.M.S., Feldmeyer L., Bossart S., Simon D., Schlapbach C., Borradori L. Case Report: Combination of Omalizumab and Dupilumab for Recalcitrant Bullous Pemphigoid. Front. Immunol. 2021;11:611549. doi: 10.3389/fimmu.2020.611549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vassallo C., Somenzi A., De Amici M., Barruscotti S., Brazzelli V. Omalizumab as a corticosteroid-sparing agent in the treatment of bullous pemphigoid. Dermatol. Ther. 2022;35:e15946. doi: 10.1111/dth.15946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Velin M., Dugourd P.-M., Sanchez A., Bahadoran P., Montaudié H., Passeron T. Efficacy and safety of methotrexate, omalizumab and dupilumab for bullous pemphigoid in patients resistant or contraindicated to oral steroids. A monocentric real-life study. J. Eur. Acad. Dermatol. Venereol. 2022;36:e539–e542. doi: 10.1111/jdv.17999. [DOI] [PubMed] [Google Scholar]
- 28.Yu K.K., Crew A.B., Messingham K.A.N., Fairley J.A., Woodley D.T. Omalizumab therapy for bullous pemphigoid. J. Am. Acad. Dermatol. 2014;71:468–474. doi: 10.1016/j.jaad.2014.04.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jafari S.M.S., Gadaldi K., Feldmeyer L., Yawalkar N., Borradori L., Schlapbach C. Effects of Omalizumab on FcεRI and IgE Expression in Lesional Skin of Bullous Pemphigoid. Front. Immunol. 2019;10:1919. doi: 10.3389/fimmu.2019.01919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Alexandre M., Bohelay G., Gille T., Le Roux-Villet C., Soued I., Morin F., Caux F., Grootenboer-Mignot S., Prost-Squarcioni C. Rapid Disease Control in First-Line Therapy-Resistant Mucous Membrane Pemphigoid and Bullous Pemphigoid with Omalizumab as Add-On Therapy: A Case Series of 13 Patients. Front. Immunol. 2022;13:874108. doi: 10.3389/fimmu.2022.874108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.James T., Salman S., Stevenson B., Bundell C., Kelly G., Nolan D., John M. IgE blockade in autoimmunity: Omalizumab induced remission of bullous pemphigoid. Clin. Immunol. 2019;198:54–56. doi: 10.1016/j.clim.2018.12.015. [DOI] [PubMed] [Google Scholar]
- 32.Ewy S., Pham H., Quan K., Su B., Tachdjian R. Successful Omalizumab Therapy for Bullous Pemphigoid Despite Transient Reaction. J. Drugs Dermatol. JDD. 2019;18:947–949. [PubMed] [Google Scholar]
- 33.Navarro-Triviño F.J., Llamas-Molina J.M., Ayen-Rodriguez A., Cancela-Díez B., Ruiz-Villaverde R. Dramatic improvement of bullous pemphigoid with omalizumab in an elderly patient. Eur. J. Hosp. Pharm. 2021;28:350–352. doi: 10.1136/ejhpharm-2020-002418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Balakirski G., Alkhateeb A., Merk H.F., Leverkus M., Megahed M. Successful treatment of bullous pemphigoid with omalizumab as corticosteroid-sparing agent: Report of two cases and review of literature. J. Eur. Acad. Dermatol. Venereol. 2016;30:1778–1782. doi: 10.1111/jdv.13758. [DOI] [PubMed] [Google Scholar]
- 35.De D., Kaushik A., Handa S., Mahajan R., Schmidt E. Omalizumab: An underutilized treatment option in bullous pemphigoid patients with co-morbidities. J. Eur. Acad. Dermatol. Venereol. 2021;35:e469–e472. doi: 10.1111/jdv.17229. [DOI] [PubMed] [Google Scholar]
- 36.Gönül M., Keseroglu H.O., Ergin C., Özcan I., Erdem Ö. Bullous pemphigoid successfully treated with omalizumab. Indian J. Dermatol. Venereol. Leprol. 2016;82:577–579. doi: 10.4103/0378-6323.183628. [DOI] [PubMed] [Google Scholar]
- 37.Lonowski S., Sachsman S., Patel N., Truong A., Holland V. Increasing evidence for omalizumab in the treatment of bullous pemphigoid. JAAD Case Rep. 2020;6:228–233. doi: 10.1016/j.jdcr.2020.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Menzinger S., Kaya G., Schmidt E., Fontao L., Laffitte E. Biological and Clinical Response to Omalizumab in a Patient with Bullous Pemphigoid. Acta Derm. Venereol. 2018;98:284–286. doi: 10.2340/00015555-2845. [DOI] [PubMed] [Google Scholar]
- 39.Liu J., Xiang T., Wang W., Bu Z. Case Report: Omalizumab Successfully Treated Recalcitrant Bullous Pemphigoid in an Elderly Patient with Multiple Comorbidities. Clin. Cosmet. Investig. Dermatol. 2022;15:1391–1396. doi: 10.2147/CCID.S373682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yalcin A.D., Genc G.E., Celik B., Gumuslu S. Anti-IgE monoclonal antibody (omalizumab) is effective in treating bullous pemphigoid and its effects on soluble CD200. Clin. Lab. 2014;60:523–524. doi: 10.7754/Clin.Lab.2013.130642. [DOI] [PubMed] [Google Scholar]
- 41.London V.A., Kim G.H., Fairley J.A., Woodley D.T. Successful treatment of bullous pemphigoid with omalizumab. Arch. Dermatol. 2012;148:1241–1243. doi: 10.1001/archdermatol.2012.1604. [DOI] [PubMed] [Google Scholar]
- 42.Sardana K., Sinha S., Agrawal D., Kulhari A., Malhotra P. Complete Remission in a Patient with Treatment Refractory Bullous Pemphigoid after a Single Dose of Omalizumab. Indian Dermatol. Online J. 2020;11:607–611. doi: 10.4103/idoj.IDOJ_438_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dufour C., Souillet A.L., Chaneliere C., Jouen F., Bodemer C., Jullien D., Cambazard F., Joly P., Reix P. Successful management of severe infant bullous pemphigoid with omalizumab. Br. J. Dermatol. 2012;166:1140–1142. doi: 10.1111/j.1365-2133.2011.10748.x. [DOI] [PubMed] [Google Scholar]
- 44.Fairley J.A., Baum C.L., Brandt D.S., Messingham K.A.N. Pathogenicity of IgE in autoimmunity: Successful treatment of bullous pemphigoid with omalizumab. J. Allergy Clin. Immunol. 2009;123:704–705. doi: 10.1016/j.jaci.2008.11.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.De A., Chowdhury B., Khemka M., Sarda A., Das S. Biologics Beyond Boundaries: Innovative Use of Biologics in Dermatology. Indian J. Dermatol. 2021;66:314–317. doi: 10.4103/ijd.IJD_128_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chebani R., Lombart F., Chaby G., Dadban A., Debarbieux S., Viguier M.-A., Ingen-Housz-Oro S., Pham-Ledard A., Bedane C.R., Picard-Dahan C., et al. Omalizumab in the treatment of bullous pemphigoid resistant to first-line therapy: A French national multicentre retrospective study of 100 patients. Br. J. Dermatol. 2024;190:258–265. doi: 10.1093/bjd/ljad369. [DOI] [PubMed] [Google Scholar]
- 47.Russo R., Capurro N., Cozzani E., Parodi A. Use of Dupilumab in Bullous Pemphigoid: Where Are We Now? J. Clin. Med. 2022;11:3367. doi: 10.3390/jcm11123367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhao L., Wang Q., Liang G., Zhou Y., Yiu N., Yang B., Zhang G., Li W., Feng S., Shang P., et al. Evaluation of Dupilumab in Patients with Bullous Pemphigoid. JAMA Dermatol. 2023;159:953–960. doi: 10.1001/jamadermatol.2023.2428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhang Y., Xu Q., Chen L., Chen J., Zhang J., Zou Y., Gong T., Ji C. Efficacy and Safety of Dupilumab in Moderate-to-Severe Bullous Pemphigoid. Front. Immunol. 2021;12:738907. doi: 10.3389/fimmu.2021.738907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Liang J., Abulikemu K., Maolidan, Hu F., Zhao J., Qiu Y., Wang Q., Sang Y., Hong Y., Kang X. Nine cases of refractory bullous pemphigoid treated with dupilumab and literature review. Int. Immunopharmacol. 2023;116:109788. doi: 10.1016/j.intimp.2023.109788. [DOI] [PubMed] [Google Scholar]
- 51.Learned C., Cohen S.R., Cunningham K., Alsukait S., Santiago S., Lu J., Rothe M., Nichols A., Rosmarin D. Long-term treatment outcomes and safety of dupilumab as a therapy for bullous pemphigoid: A multicenter retrospective review. J. Am. Acad. Dermatol. 2023;89:378–382. doi: 10.1016/j.jaad.2023.03.036. [DOI] [PubMed] [Google Scholar]
- 52.Hu L., Huang R., Jiang F., You S., Wu Q. Concomitant use of dupilumab with glucocorticoid in bullous pemphigoid reduces disease severity: A preliminary study. Immun. Inflamm. Dis. 2023;11:e924. doi: 10.1002/iid3.924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Qi W., Rushan X. The efficacy and safety of dupilumab combined with methylprednisolone in the treatment of bullous pemphigoid in China. Int. Immunopharmacol. 2023;118:110050. doi: 10.1016/j.intimp.2023.110050. [DOI] [PubMed] [Google Scholar]
- 54.Klepper E.M., Robinson H.N. Dupilumab for the treatment of nivolumab-induced bullous pemphigoid: A case report and review of the literature. Dermatol. Online J. 2021;27:9. doi: 10.5070/D327955136. [DOI] [PubMed] [Google Scholar]
- 55.Yang J., Gao H., Zhang Z., Tang C., Chen Z., Wang L., Yang F., Chen S., He S., Liu S., et al. Dupilumab combined with low-dose systemic steroid therapy improves efficacy and safety for bullous pemphigoid. Dermatol. Ther. 2022;35:e15648. doi: 10.1111/dth.15648. [DOI] [PubMed] [Google Scholar]
- 56.Foerster Y., Sollfrank L., Rechtien L., Harrer T., Berking C., Sticherling M. Case report: Bullous pemphigoid in HIV-1-positive patients: Interplay or coincidence? A case series and review of the literature. Front. Immunol. 2023;14:1179294. doi: 10.3389/fimmu.2023.1179294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhang X., Man X., Tang Z., Dai R., Shen Y. Dupilumab as a novel therapy for bullous pemphigoid. Int. J. Dermatol. 2023;62:e263–e266. doi: 10.1111/ijd.16525. [DOI] [PubMed] [Google Scholar]
- 58.Sanfilippo E., Lopez A.G., Saardi K.M. Erythrodermic Bullous Pemphigoid in Skin of Color Treated with Dupilumab. J. Drugs Dermatol. 2023;22:685–686. doi: 10.36849/JDD.7196. [DOI] [PubMed] [Google Scholar]
- 59.Takamura S., Teraki Y. Treatment of bullous pemphigoid with dupilumab: Dupilumab exerts its effect by primarily suppressing T-helper 2 cytokines. J. Dermatol. 2022;49:845–850. doi: 10.1111/1346-8138.16428. [DOI] [PubMed] [Google Scholar]
- 60.Wang Q., Ruan Y., Guo F., Zhu H., Pan M. Effect of Dupilumab on Generalized Verrucosis in Refractory Bullous Pemphigoid. Acta Derm.-Venereol. 2023;103:adv12324. doi: 10.2340/actadv.v103.12324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wang S.-H., Shan Y., Li S.-Z., Zuo Y.-G. Anti-interleukin 4 receptor α antibody for the treatment of Chinese bullous pemphigoid patients with diverse comorbidities and a 1-year follow-up: A monocentric real-world study. Front. Immunol. 2023;14:1165106. doi: 10.3389/fimmu.2023.1165106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wang M., Wang J., Shi B. Case report: Dupilumab for the treatment of bullous pemphigoid. Dermatol. Ther. 2022;35:e15541. doi: 10.1111/dth.15541. [DOI] [PubMed] [Google Scholar]
- 63.Liu J.-H., Gao Q., Ma W.-Y., Cheng Z.-L., Luo N.-N., Hao P.-S. Successful Treatment of Psoriasis Combined with Bullous Pemphigoid with Dupilumab: A Case Report. Clin. Cosmet. Investig. Dermatol. 2023;16:1583–1587. doi: 10.2147/CCID.S415019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Margiotta F.M., Fidanzi C., Bevilacqua M., Janowska A., Romanelli M., Manni E. Effectiveness and safety of Dupilumab for the treatment at 104 weeks of recalcitrant bullous pemphigoid. Skin Res. Technol. 2023;29:e13488. doi: 10.1111/srt.13488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Valenti M., De Giacomo P., Lavecchia A., Valenti G. A severe case of IgA bullous pemphigoid successfully treated with dupilumab. Dermatol. Ther. 2022;35:e15890. doi: 10.1111/dth.15890. [DOI] [PubMed] [Google Scholar]
- 66.Savoldy M.A., Tadicherla T., Moureiden Z., Ayoubi N., Baldwin B.T. The Successful Treatment of COVID-19-Induced Bullous Pemphigoid with Dupilumab. Cureus. 2022;14:e30541. doi: 10.7759/cureus.30541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zhou A.E., Shao K., Ferenczi K., Adalsteinsson J.A. Recalcitrant bullous pemphigoid responsive to dupilumab in an adolescent patient. JAAD Case Rep. 2022;29:149–151. doi: 10.1016/j.jdcr.2022.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Loughlin C.R., Mascaró J.M. Treatment of pemphigoid gestationis with dupilumab. Clin. Exp. Dermatol. 2021;46:1578–1579. doi: 10.1111/ced.14765. [DOI] [PubMed] [Google Scholar]
- 69.Zhang Y., Zhang J., Chen J., Lin M., Gong T., Cheng B., Ji C. Dupilumab successfully treated refractory bullous pemphigoid with early clinical manifestations imitating atopic dermatitis: A case letter. Australas. J. Dermatol. 2021;62:525–527. doi: 10.1111/ajd.13692. [DOI] [PubMed] [Google Scholar]
- 70.Kaye A., Gordon S.C., Deverapalli S.C., Her M.J., Rosmarin D. Dupilumab for the Treatment of Recalcitrant Bullous Pemphigoid. JAMA Dermatol. 2018;154:1225–1226. doi: 10.1001/jamadermatol.2018.2526. [DOI] [PubMed] [Google Scholar]
- 71.Jendoubi F., Bost C., Tournier E., Paul C., Konstantinou M.P. Severe pemphigoid nodularis successfully treated with dupilumab. Dermatol. Ther. 2022;35:e15727. doi: 10.1111/dth.15727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Fournier C., Hirsch I., Spreafico A., Butler M.O., Dhani N., Sauder M.B. Dupilumab as a treatment for cutaneous immune-related adverse events induced by immune checkpoint inhibitors: A case series and review of the literature. SAGE Open Med. Case Rep. 2023;11:1–4. doi: 10.1177/2050313X231195462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Chen J., Huang H., Deng L., Wu S. Dupilumab as monotherapy for bullous pemphigoid with multiple underlying diseases: A report of two cases. Indian J. Dermatol. Venereol. Leprol. 2023;89:888–890. doi: 10.25259/IJDVL_1149_2022. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The original contributions presented in the study are included in the article, further inquiries can be directed to the corresponding author.