Abstract
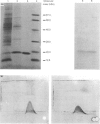
Cytochrome b5 from rat liver microsomes (microsomal fractions) was purified in its native form. The procedure described has great capacity, is fast, and the final product is pure as judged from SDS/polyacrylamide-gel electrophoresis. Antibodies to cytochrome b5 are obtained after administration of the antigen inserted into small unilamellar lipid vesicles.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abe K., Kimura S., Kizawa R., Anan F. K., Sugita Y. Amino acid sequences of cytochrome b5 from human, porcine, and bovine erythrocytes and comparison with liver microsomal cytochrome b5. J Biochem. 1985 Jun;97(6):1659–1668. doi: 10.1093/oxfordjournals.jbchem.a135224. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Burchell A. A simple method for purification of rat hepatic microsomal cytochrome b5. Biochem J. 1985 Feb 15;226(1):339–341. doi: 10.1042/bj2260339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elhammer A., Peterson E., Dallner G. Distribution and transport of apo- and holocytochrome b5 in the endoplasmic reticulum of rat liver. Biochim Biophys Acta. 1983 Apr 21;730(1):76–84. doi: 10.1016/0005-2736(83)90319-x. [DOI] [PubMed] [Google Scholar]
- Gerlier D., Bakouche O., Dore J. F. Liposomes as a tool to study the role of membrane presentation in the immunogenicity of a MuLV-related tumor antigen. J Immunol. 1983 Jul;131(1):485–490. [PubMed] [Google Scholar]
- Haglid K. G., Stavrou D. Water-soluble and pentanol-extractable proteins in human brain normal tissue and human brain tumours, with special reference to S-100 protein. J Neurochem. 1973 Jun;20(6):1523–1532. doi: 10.1111/j.1471-4159.1973.tb00270.x. [DOI] [PubMed] [Google Scholar]
- Harboe N., Ingild A. Immunization, isolation of immunoglobulins, estimation of antibody titre. Scand J Immunol Suppl. 1973;1:161–164. doi: 10.1111/j.1365-3083.1973.tb03798.x. [DOI] [PubMed] [Google Scholar]
- Kyhse-Andersen J. Electroblotting of multiple gels: a simple apparatus without buffer tank for rapid transfer of proteins from polyacrylamide to nitrocellulose. J Biochem Biophys Methods. 1984 Dec;10(3-4):203–209. doi: 10.1016/0165-022x(84)90040-x. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- OZOLS J., STRITTMATTER P. THE INTERACTION OF PORPHYRINS AND METALLOPORHYRINS WITH APOCYTOCHROME BETA-5. J Biol Chem. 1964 Apr;239:1018–1023. [PubMed] [Google Scholar]
- Okada Y., Frey A. B., Guenthner T. M., Oesch F., Sabatini D. D., Kreibich G. Studies on the biosynthesis of microsomal membrane proteins. Site of synthesis and mode of insertion of cytochrome b5, cytochrome b5 reductase, cytochrome P-450 reductase and epoxide hydrolase. Eur J Biochem. 1982 Feb;122(2):393–402. doi: 10.1111/j.1432-1033.1982.tb05894.x. [DOI] [PubMed] [Google Scholar]
- Ozols J., Heinemann F. S. Chemical structure of rat liver cytochrome b5. Isolation of peptides by high-pressure liquid chromatography. Biochim Biophys Acta. 1982 May 21;704(1):163–173. doi: 10.1016/0167-4838(82)90143-1. [DOI] [PubMed] [Google Scholar]
- Takagaki Y., Radhakrishnan R., Wirtz K. W., Khorana H. G. The membrane-embedded segment of cytochrome b5 as studied by cross-linking with photoactivatable phospholipids. II. The nontransferable form. J Biol Chem. 1983 Aug 10;258(15):9136–9142. [PubMed] [Google Scholar]