Abstract
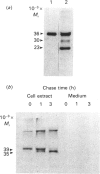
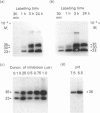
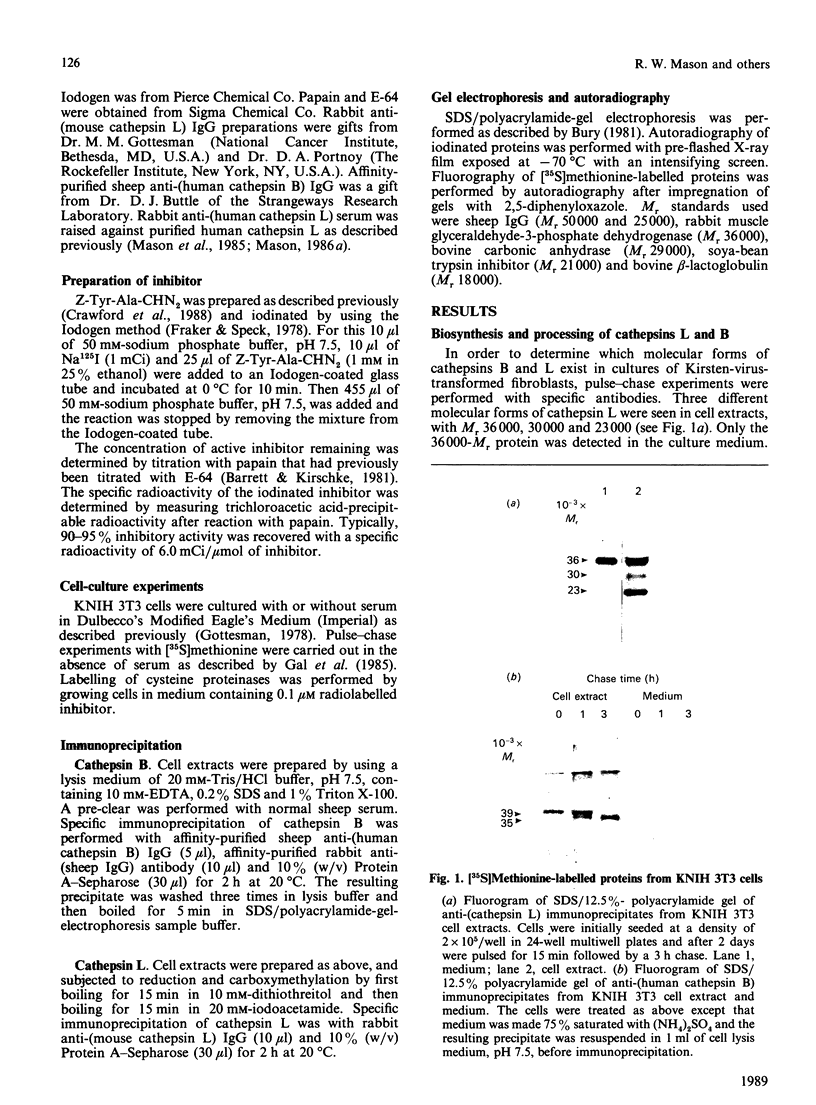
The major active forms of cathepsins B and L were identified in Kirsten-virus-transformed mouse fibroblasts by the use of a specific radiolabelled inhibitor, benzyloxycarbonyl-Tyr(-125I)-Ala-CHN2. No other proteins were labelled, demonstrating the specificity of this inhibitor for cysteine proteinases. Cathepsins B and L were distinguished by the use of specific antibodies. One active form of cathepsin B, Mr 33,000-35,000, and two active forms of cathepsin L, Mr 30,000 and 23,000, were identified. The intracellular precursors of these proteins had higher Mr values of 39,000 and 36,000 for cathepsins B and L respectively, as shown by pulse-chase experiments with [35S]methionine-labelled proteins. These did not react with the inhibitor under our culture conditions. The precursor of cathepsin L was secreted whereas the precursor of cathepsin B was not, demonstrating that secretions of the two enzymes are regulated differently. In contrast with results found previously for the purified protein [Mason, Gal & Gottesman (1987) Biochem. J. 248, 449-454], the secreted precursor form of cathepsin L did not react with the inhibitor either, indicating that it is not active and therefore, as such, cannot be directly involved in tumour invasion. The secreted protein did react with the inhibitor when incubated at pH 3.0, showing that the protein can be activated, although this did not occur under our culture conditions.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baici A., Knöpfel M. Cysteine proteinases produced by cultured rabbit V2 carcinoma cells and rabbit skin fibroblasts. Int J Cancer. 1986 Nov 15;38(5):753–761. doi: 10.1002/ijc.2910380520. [DOI] [PubMed] [Google Scholar]
- Barrett A. J., Kirschke H. Cathepsin B, Cathepsin H, and cathepsin L. Methods Enzymol. 1981;80(Pt 100):535–561. doi: 10.1016/s0076-6879(81)80043-2. [DOI] [PubMed] [Google Scholar]
- Chan S. J., San Segundo B., McCormick M. B., Steiner D. F. Nucleotide and predicted amino acid sequences of cloned human and mouse preprocathepsin B cDNAs. Proc Natl Acad Sci U S A. 1986 Oct;83(20):7721–7725. doi: 10.1073/pnas.83.20.7721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang J. C., Lesser M., Yoo O. H., Orlowski M. Increased cathepsin B-like activity in alveolar macrophages and bronchoalveolar lavage fluid from smokers. Am Rev Respir Dis. 1986 Sep;134(3):538–541. doi: 10.1164/arrd.1986.134.3.538. [DOI] [PubMed] [Google Scholar]
- Crawford C., Mason R. W., Wikstrom P., Shaw E. The design of peptidyldiazomethane inhibitors to distinguish between the cysteine proteinases calpain II, cathepsin L and cathepsin B. Biochem J. 1988 Aug 1;253(3):751–758. doi: 10.1042/bj2530751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean R. T., Barrett A. J. Lysosomes. Essays Biochem. 1976;12:1–40. [PubMed] [Google Scholar]
- Delaissé J. M., Eeckhout Y., Vaes G. In vivo and in vitro evidence for the involvement of cysteine proteinases in bone resorption. Biochem Biophys Res Commun. 1984 Dec 14;125(2):441–447. doi: 10.1016/0006-291x(84)90560-6. [DOI] [PubMed] [Google Scholar]
- Denhardt D. T., Greenberg A. H., Egan S. E., Hamilton R. T., Wright J. A. Cysteine proteinase cathepsin L expression correlates closely with the metastatic potential of H-ras-transformed murine fibroblasts. Oncogene. 1987;2(1):55–59. [PubMed] [Google Scholar]
- Docherty K., Hutton J. C., Steiner D. F. Cathepsin B-related proteases in the insulin secretory granule. J Biol Chem. 1984 May 25;259(10):6041–6044. [PubMed] [Google Scholar]
- Fraker P. J., Speck J. C., Jr Protein and cell membrane iodinations with a sparingly soluble chloroamide, 1,3,4,6-tetrachloro-3a,6a-diphrenylglycoluril. Biochem Biophys Res Commun. 1978 Feb 28;80(4):849–857. doi: 10.1016/0006-291x(78)91322-0. [DOI] [PubMed] [Google Scholar]
- Gal S., Willingham M. C., Gottesman M. M. Processing and lysosomal localization of a glycoprotein whose secretion is transformation stimulated. J Cell Biol. 1985 Feb;100(2):535–544. doi: 10.1083/jcb.100.2.535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottesman M. M. Transformation-dependent secretion of a low molecular weight protein by murine fibroblasts. Proc Natl Acad Sci U S A. 1978 Jun;75(6):2767–2771. doi: 10.1073/pnas.75.6.2767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanewinkel H., Glössl J., Kresse H. Biosynthesis of cathepsin B in cultured normal and I-cell fibroblasts. J Biol Chem. 1987 Sep 5;262(25):12351–12355. [PubMed] [Google Scholar]
- Mason R. W., Gal S., Gottesman M. M. The identification of the major excreted protein (MEP) from a transformed mouse fibroblast cell line as a catalytically active precursor form of cathepsin L. Biochem J. 1987 Dec 1;248(2):449–454. doi: 10.1042/bj2480449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason R. W., Green G. D., Barrett A. J. Human liver cathepsin L. Biochem J. 1985 Feb 15;226(1):233–241. doi: 10.1042/bj2260233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason R. W. Species variants of cathepsin L and their immunological identification. Biochem J. 1986 Nov 15;240(1):285–288. doi: 10.1042/bj2400285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason R. W. Species variations amongst lysosomal cysteine proteinases. Biomed Biochim Acta. 1986;45(11-12):1433–1440. [PubMed] [Google Scholar]
- Mason R. W., Walker J. E., Northrop F. D. The N-terminal amino acid sequences of the heavy and light chains of human cathepsin L. Relationship to a cDNA clone for a major cysteine proteinase from a mouse macrophage cell line. Biochem J. 1986 Dec 1;240(2):373–377. doi: 10.1042/bj2400373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mort J. S., Recklies A. D. Interrelationship of active and latent secreted human cathepsin B precursors. Biochem J. 1986 Jan 1;233(1):57–63. doi: 10.1042/bj2330057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimura Y., Kato K. Intracellular transport and processing of lysosomal cathepsin B. Biochem Biophys Res Commun. 1987 Oct 14;148(1):254–259. doi: 10.1016/0006-291x(87)91103-x. [DOI] [PubMed] [Google Scholar]
- Poole A. R., Mort J. S. Biochemical and immunological studies of lysosomal and related proteinases in health and disease. J Histochem Cytochem. 1981 Mar;29(3A):494–502. doi: 10.1177/29.3.494. [DOI] [PubMed] [Google Scholar]
- Shaw E., Dean R. T. The inhibition of macrophage protein turnover by a selective inhibitor of thiol proteinases. Biochem J. 1980 Feb 15;186(2):385–390. doi: 10.1042/bj1860385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sloane B. F., Honn K. V. Cysteine proteinases and metastasis. Cancer Metastasis Rev. 1984;3(3):249–263. doi: 10.1007/BF00048388. [DOI] [PubMed] [Google Scholar]
- Watanabe H., Green G. D., Shaw E. A comparison of the behavior of chymotrypsin and cathepsin B towards peptidyl diazomethyl ketones. Biochem Biophys Res Commun. 1979 Aug 28;89(4):1354–1360. doi: 10.1016/0006-291x(79)92158-2. [DOI] [PubMed] [Google Scholar]