Abstract
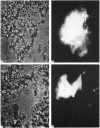
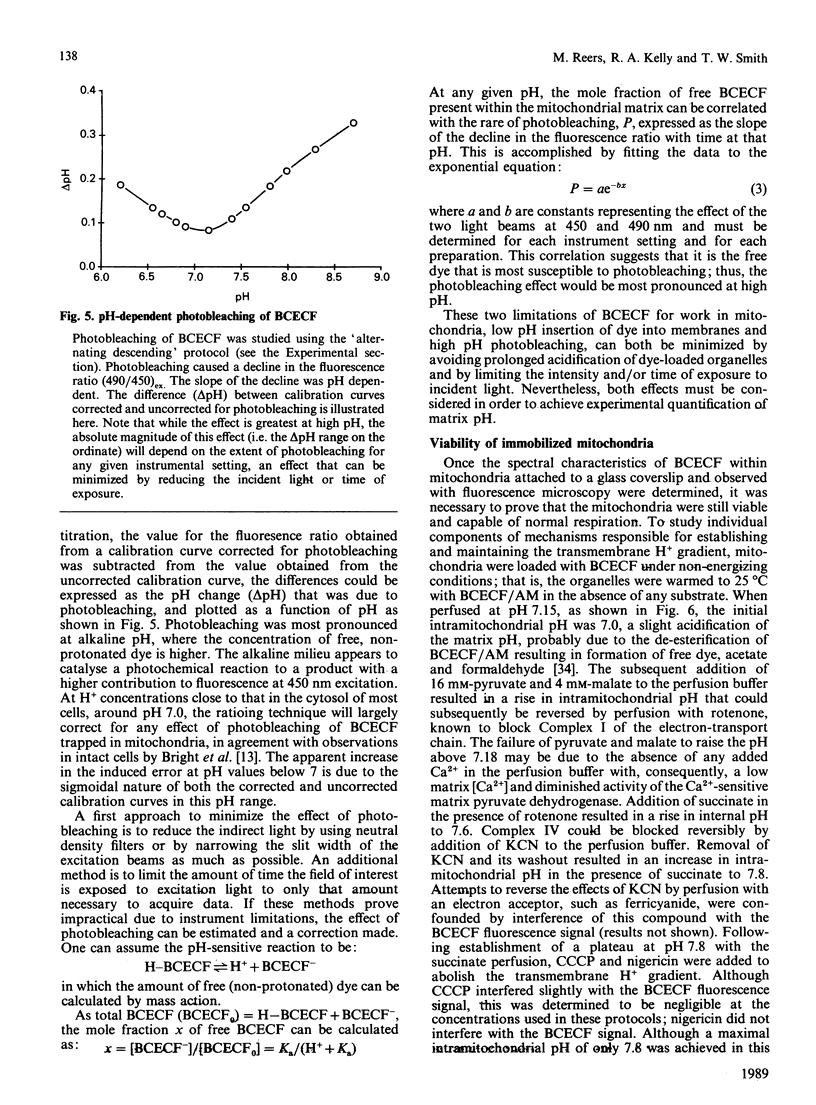
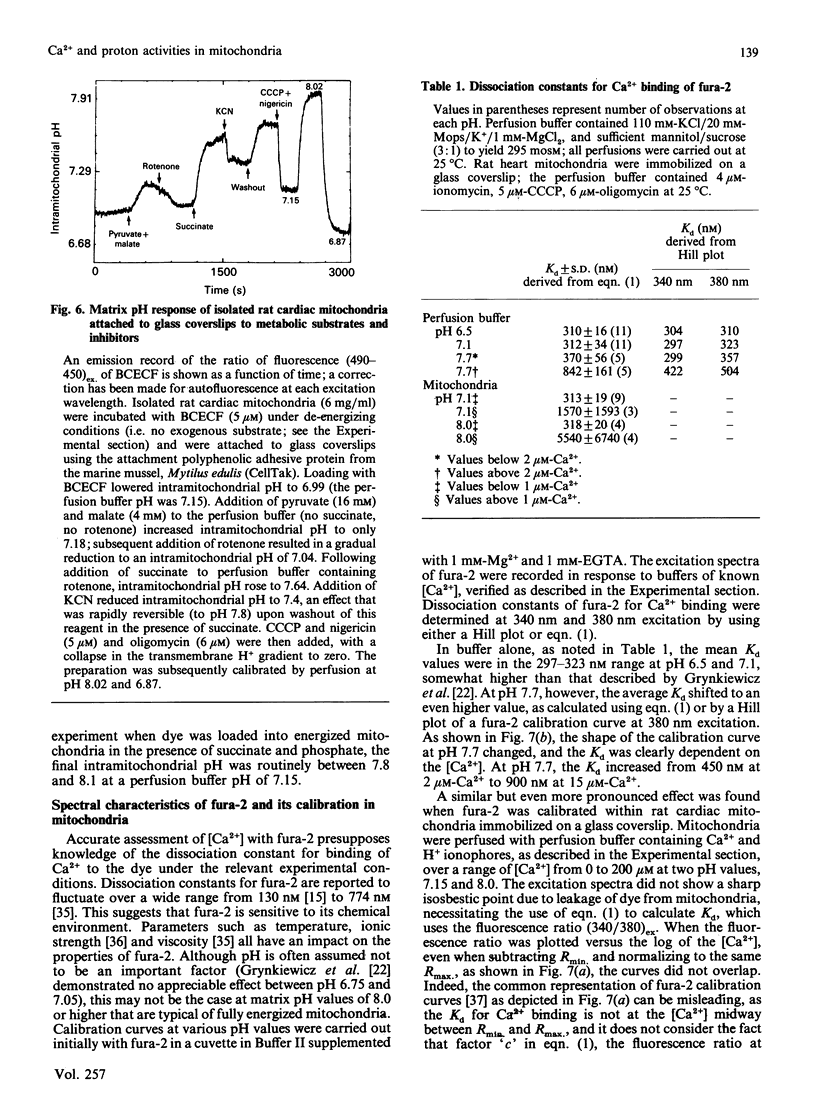
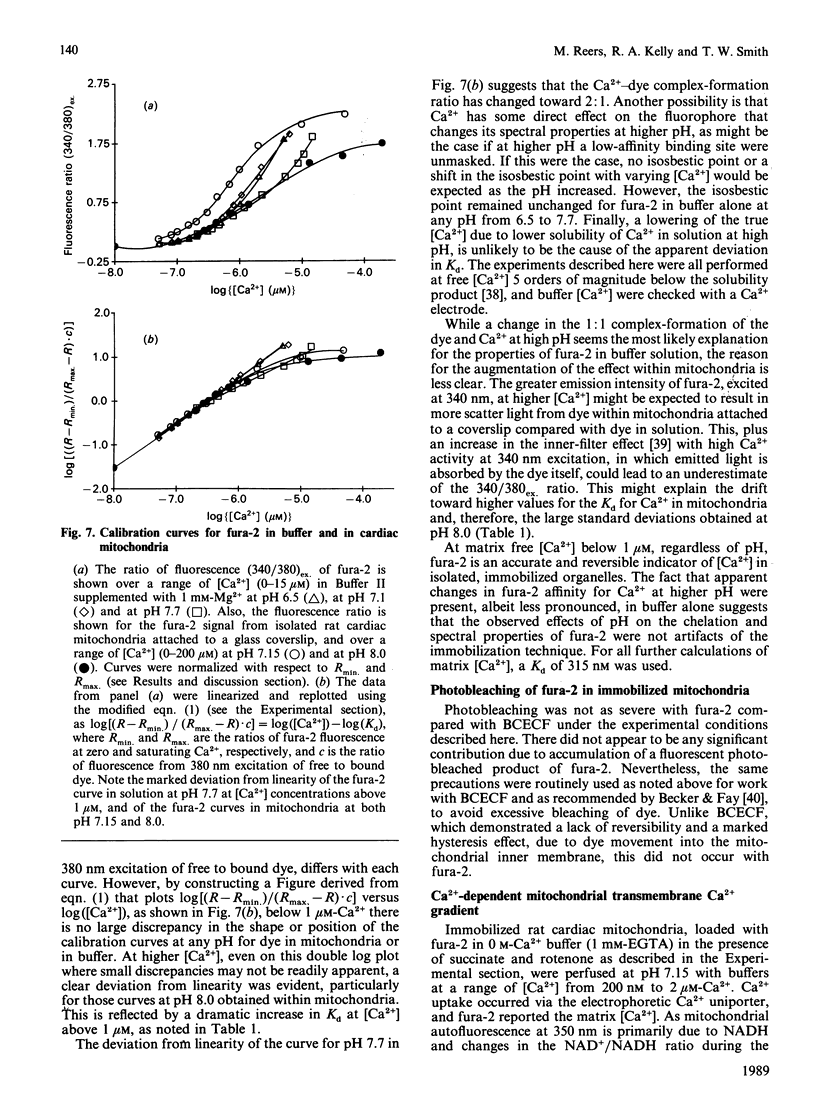
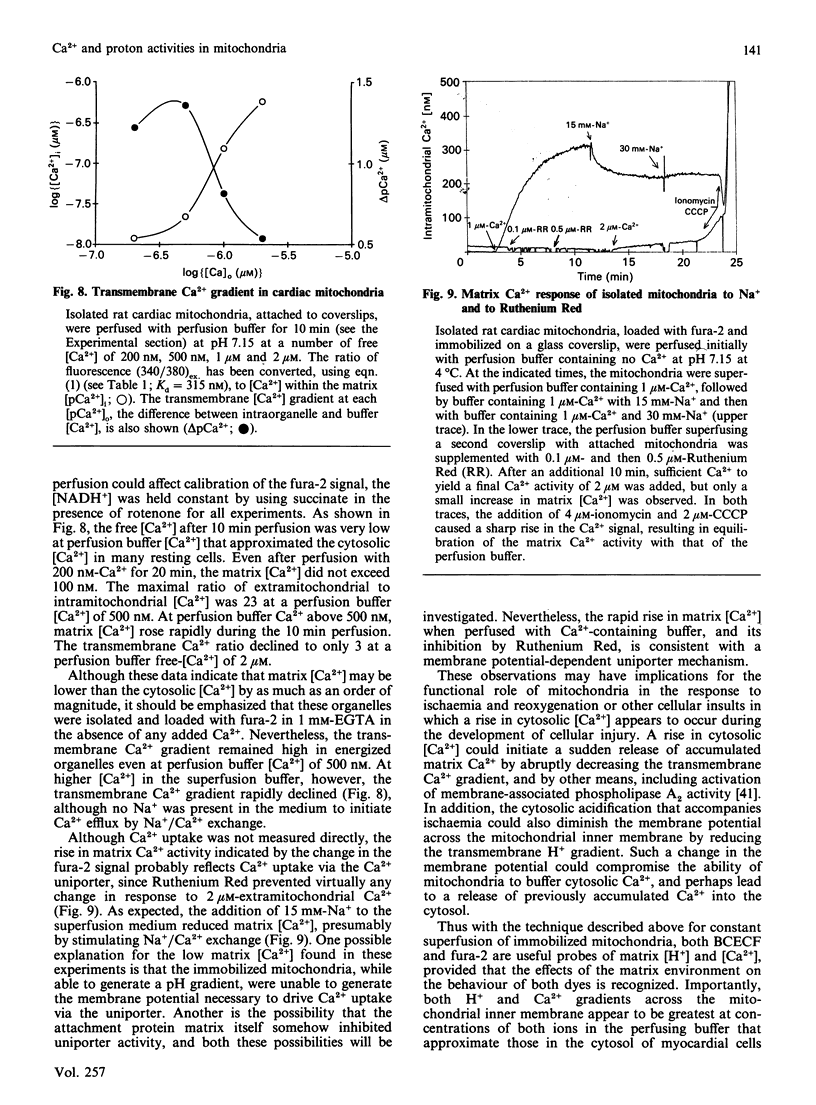
The ionic composition of the mitochondrial matrix, under both physiological and pathophysiological conditions, remains controversial. Although fura-2 and 2',7'-bis-(2-carboxyethyl)-5(6)-carboxyfluorescein (BCECF), fluorescent probes for [Ca2+] and [H+] respectively, have successfully been loaded into mitochondria [Lukács & Kapus (1987) Biochem. J. 248, 609-613; Davis, Altschuld, Jung & Brierley (1987) Biochem. Biophys. Res. Commun. 49, 40-45], the adaptation of fluorescence-ratio spectroscopy to the study of the matrix ion content poses unique problems. In this report, we describe a method for successfully attaching viable rat cardiac mitochondria to glass coverslips, allowing continuous superfusion of isolated organelles during fluorescence microscopy. This technique obviated the need to correct for the accumulation of ion-sensitive and -insensitive fluorescent species of dye both within the matrix and outside of mitochondria in suspension in a cuvette, a particular problem with fura-2. By using this technique for superfusion of immobilized mitochondria, we found the pKa of BCECF for H+ at 25 degrees C shifted from 6.8 in buffer to 7.2 in rat cardiac mitochondria, with a marked hysteresis effect noted for intramitochondrial BCECF calibration curves. At higher pH, photobleaching of BCECF was enhanced. The dissociation constant (Kd) of fura-2 for Ca2+ was found to be 315 nM at 25 degrees C, pH 8.0, but only at [Ca2+] below 1 microM. At matrix [Ca2+] greater than 1 microM, the Kd shifted into the micromolar range, an effect that appeared to be pH-dependent. Importantly, the matrix [Ca2+] was determined to be between 10 and 100 nM at perfusion buffer [Ca2+] below 500 nM, but rose rapidly at the higher extramitochondrial [Ca2+] reported to occur in ischaemic cardiac myocytes. Importantly, mitochondrial transmembrane H+ and Ca2+ gradients both appeared to be maximal at perfusion buffer [H+] and [Ca2+] that approximate those of the cytosol of many resting cells.
Full text
PDF


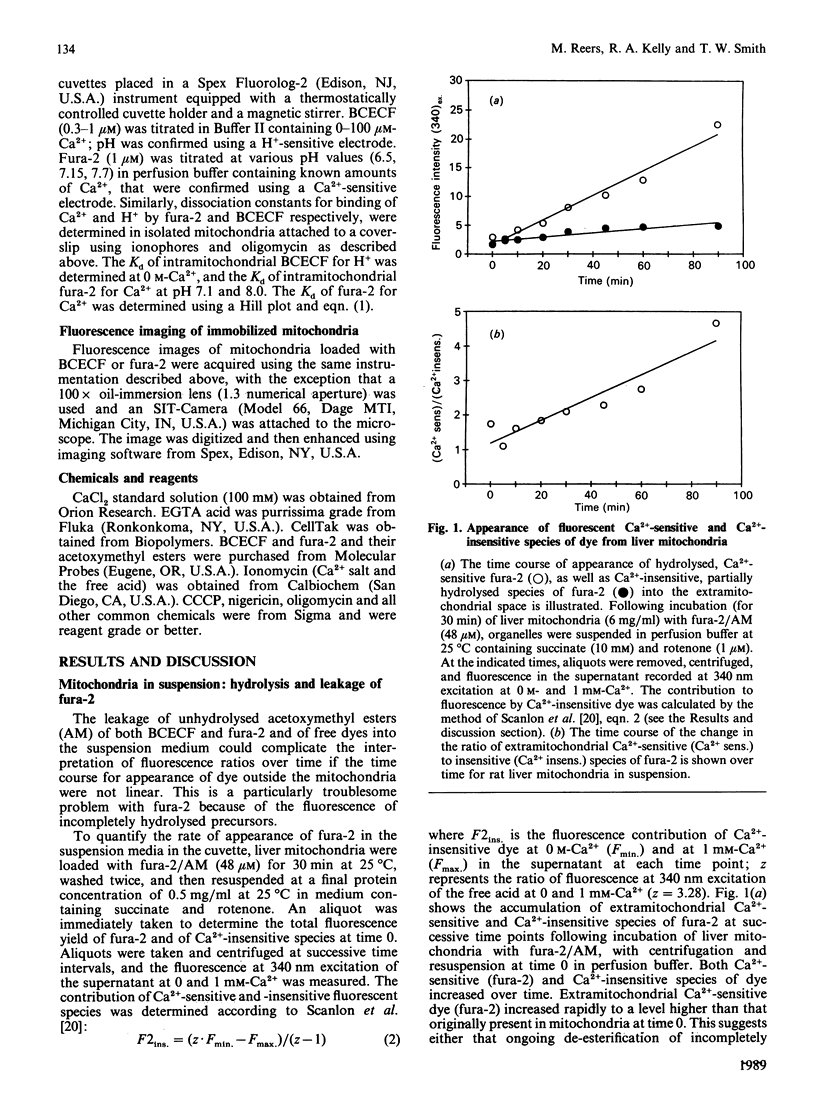

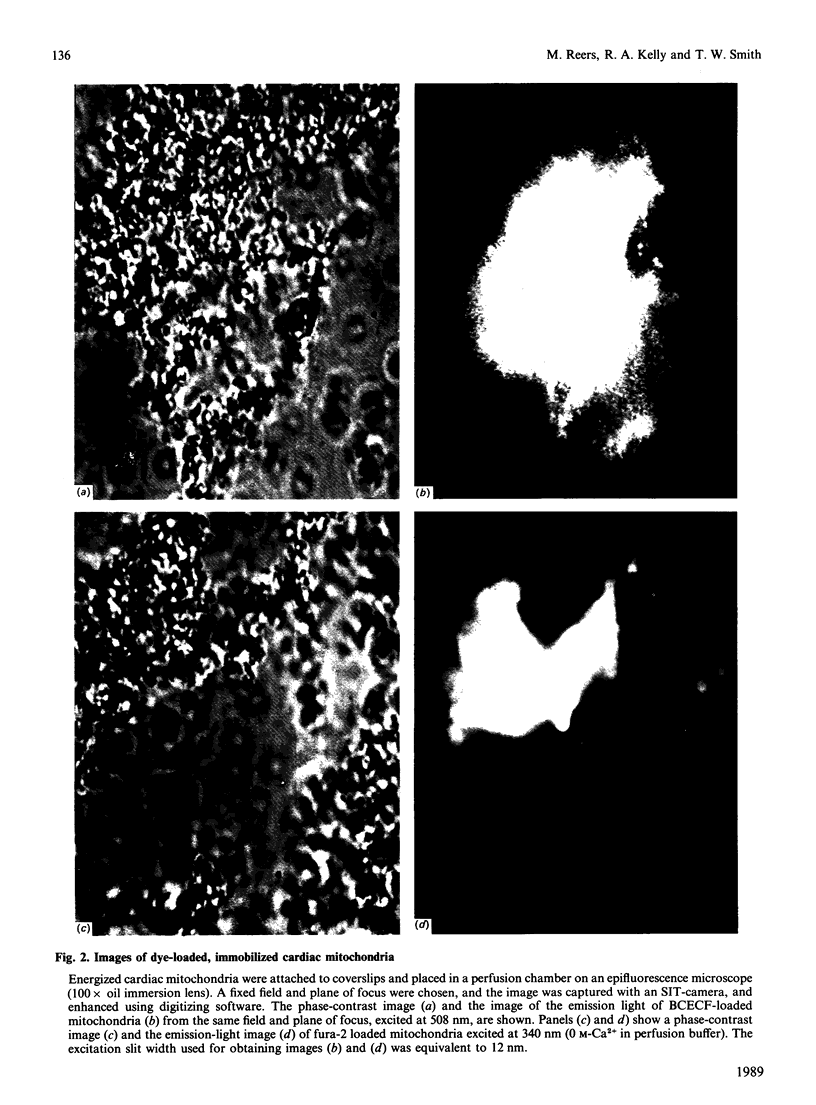
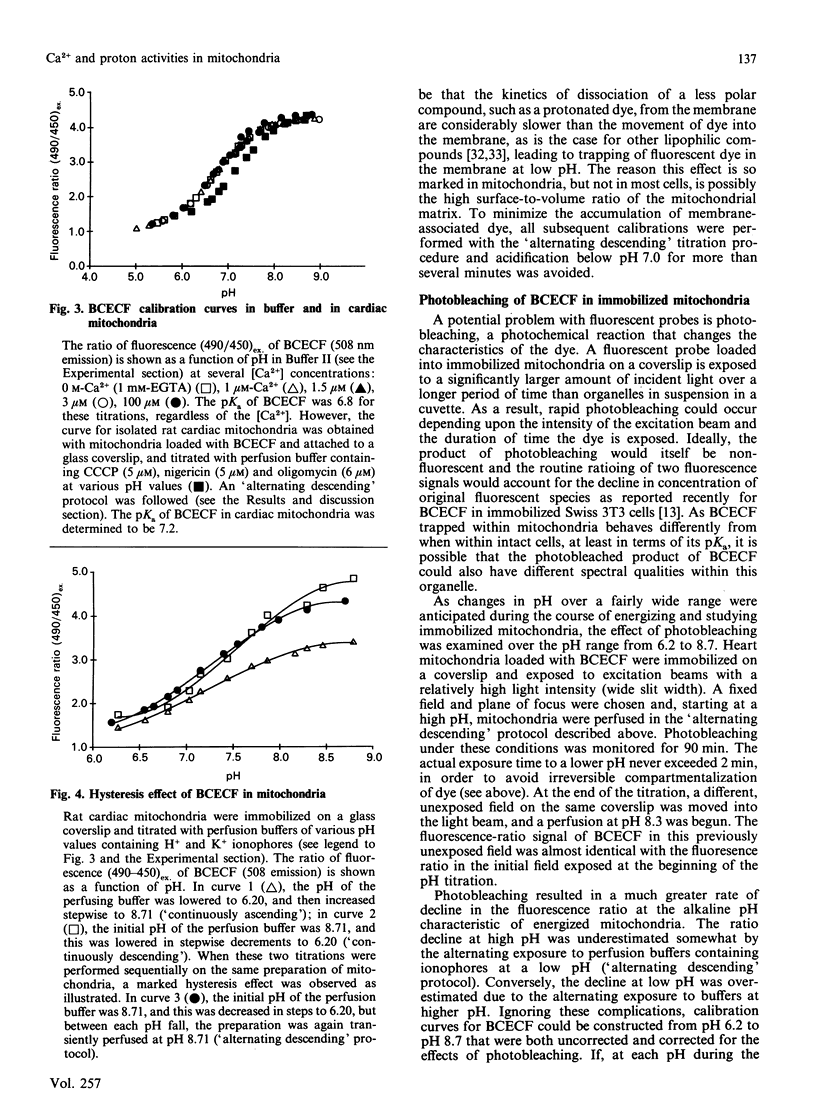

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aarsman A. J., Neys F., Van den Bosch H. A simple and versatile affinity column for phospholipase A2. Biochim Biophys Acta. 1984 Mar 7;792(3):363–366. doi: 10.1016/0005-2760(84)90205-4. [DOI] [PubMed] [Google Scholar]
- Arslan P., Di Virgilio F., Beltrame M., Tsien R. Y., Pozzan T. Cytosolic Ca2+ homeostasis in Ehrlich and Yoshida carcinomas. A new, membrane-permeant chelator of heavy metals reveals that these ascites tumor cell lines have normal cytosolic free Ca2+. J Biol Chem. 1985 Mar 10;260(5):2719–2727. [PubMed] [Google Scholar]
- Aw T. Y., Andersson B. S., Jones D. P. Mitochondrial transmembrane ion distribution during anoxia. Am J Physiol. 1987 Apr;252(4 Pt 1):C356–C361. doi: 10.1152/ajpcell.1987.252.4.C356. [DOI] [PubMed] [Google Scholar]
- Aw T. Y., Andersson B. S., Jones D. P. Suppression of mitochondrial respiratory function after short-term anoxia. Am J Physiol. 1987 Apr;252(4 Pt 1):C362–C368. doi: 10.1152/ajpcell.1987.252.4.C362. [DOI] [PubMed] [Google Scholar]
- Bar L. K., Barenholz Y., Thompson T. E. Dependence on phospholipid composition of the fraction of cholesterol undergoing spontaneous exchange between small unilamellar vesicles. Biochemistry. 1987 Aug 25;26(17):5460–5465. doi: 10.1021/bi00391a037. [DOI] [PubMed] [Google Scholar]
- Becker P. L., Fay F. S. Photobleaching of fura-2 and its effect on determination of calcium concentrations. Am J Physiol. 1987 Oct;253(4 Pt 1):C613–C618. doi: 10.1152/ajpcell.1987.253.4.C613. [DOI] [PubMed] [Google Scholar]
- Bers D. M. A simple method for the accurate determination of free [Ca] in Ca-EGTA solutions. Am J Physiol. 1982 May;242(5):C404–C408. doi: 10.1152/ajpcell.1982.242.5.C404. [DOI] [PubMed] [Google Scholar]
- Bright G. R., Fisher G. W., Rogowska J., Taylor D. L. Fluorescence ratio imaging microscopy: temporal and spatial measurements of cytoplasmic pH. J Cell Biol. 1987 Apr;104(4):1019–1033. doi: 10.1083/jcb.104.4.1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broekemeier K. M., Schmid P. C., Schmid H. H., Pfeiffer D. R. Effects of phospholipase A2 inhibitors on ruthenium red-induced Ca2+ release from mitochondria. J Biol Chem. 1985 Jan 10;260(1):105–113. [PubMed] [Google Scholar]
- Brown R. E., Thompson T. E. Spontaneous transfer of ganglioside GM1 between phospholipid vesicles. Biochemistry. 1987 Aug 25;26(17):5454–5460. doi: 10.1021/bi00391a036. [DOI] [PubMed] [Google Scholar]
- Davis M. H., Altschuld R. A., Jung D. W., Brierley G. P. Estimation of intramitochondrial pCa and pH by fura-2 and 2,7 biscarboxyethyl-5(6)-carboxyfluorescein (BCECF) fluorescence. Biochem Biophys Res Commun. 1987 Nov 30;149(1):40–45. doi: 10.1016/0006-291x(87)91602-0. [DOI] [PubMed] [Google Scholar]
- Denton R. M., McCormack J. G. Ca2+ transport by mammalian mitochondria and its role in hormone action. Am J Physiol. 1985 Dec;249(6 Pt 1):E543–E554. doi: 10.1152/ajpendo.1985.249.6.E543. [DOI] [PubMed] [Google Scholar]
- Drummond I. A., McClure S. A., Poenie M., Tsien R. Y., Steinhardt R. A. Large changes in intracellular pH and calcium observed during heat shock are not responsible for the induction of heat shock proteins in Drosophila melanogaster. Mol Cell Biol. 1986 May;6(5):1767–1775. doi: 10.1128/mcb.6.5.1767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabiato A., Fabiato F. Calculator programs for computing the composition of the solutions containing multiple metals and ligands used for experiments in skinned muscle cells. J Physiol (Paris) 1979;75(5):463–505. [PubMed] [Google Scholar]
- Grynkiewicz G., Poenie M., Tsien R. Y. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985 Mar 25;260(6):3440–3450. [PubMed] [Google Scholar]
- Hansford R. G. Relation between mitochondrial calcium transport and control of energy metabolism. Rev Physiol Biochem Pharmacol. 1985;102:1–72. doi: 10.1007/BFb0034084. [DOI] [PubMed] [Google Scholar]
- Lukács G. L., Kapus A. Measurement of the matrix free Ca2+ concentration in heart mitochondria by entrapped fura-2 and quin2. Biochem J. 1987 Dec 1;248(2):609–613. doi: 10.1042/bj2480609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malgaroli A., Milani D., Meldolesi J., Pozzan T. Fura-2 measurement of cytosolic free Ca2+ in monolayers and suspensions of various types of animal cells. J Cell Biol. 1987 Nov;105(5):2145–2155. doi: 10.1083/jcb.105.5.2145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy J. G., Marsh J. D., Smith T. W. The role of calcium in ischemic myocardial injury. Circulation. 1987 Jun;75(6 Pt 2):V15–V24. [PubMed] [Google Scholar]
- Palmer J. W., Tandler B., Hoppel C. L. Biochemical properties of subsarcolemmal and interfibrillar mitochondria isolated from rat cardiac muscle. J Biol Chem. 1977 Dec 10;252(23):8731–8739. [PubMed] [Google Scholar]
- Poenie M., Alderton J., Steinhardt R., Tsien R. Calcium rises abruptly and briefly throughout the cell at the onset of anaphase. Science. 1986 Aug 22;233(4766):886–889. doi: 10.1126/science.3755550. [DOI] [PubMed] [Google Scholar]
- Poenie M., Alderton J., Tsien R. Y., Steinhardt R. A. Changes of free calcium levels with stages of the cell division cycle. Nature. 1985 May 9;315(6015):147–149. doi: 10.1038/315147a0. [DOI] [PubMed] [Google Scholar]
- Reers M., Pfeiffer D. R. Inhibition of mitochondrial phospholipase A2 by mono- and dilysocardiolipin. Biochemistry. 1987 Dec 15;26(25):8038–8041. doi: 10.1021/bi00399a002. [DOI] [PubMed] [Google Scholar]
- Riley W. W., Jr, Pfeiffer D. R. The effect of Ca2+ and acyl coenzyme A:lysophospholipid acyltransferase inhibitors on permeability properties of the liver mitochondrial inner membrane. J Biol Chem. 1986 Oct 25;261(30):14018–14024. [PubMed] [Google Scholar]
- Rink T. J., Tsien R. Y., Pozzan T. Cytoplasmic pH and free Mg2+ in lymphocytes. J Cell Biol. 1982 Oct;95(1):189–196. doi: 10.1083/jcb.95.1.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scanlon M., Williams D. A., Fay F. S. A Ca2+-insensitive form of fura-2 associated with polymorphonuclear leukocytes. Assessment and accurate Ca2+ measurement. J Biol Chem. 1987 May 5;262(13):6308–6312. [PubMed] [Google Scholar]
- Steinberg S. F., Bilezikian J. P., Al-Awqati Q. Fura-2 fluorescence is localized to mitochondria in endothelial cells. Am J Physiol. 1987 Nov;253(5 Pt 1):C744–C747. doi: 10.1152/ajpcell.1987.253.5.C744. [DOI] [PubMed] [Google Scholar]
- Thomas J. A. Intracellularly trapped pH indicators. Soc Gen Physiol Ser. 1986;40:311–325. [PubMed] [Google Scholar]
- Tsien R. Y. A non-disruptive technique for loading calcium buffers and indicators into cells. Nature. 1981 Apr 9;290(5806):527–528. doi: 10.1038/290527a0. [DOI] [PubMed] [Google Scholar]
- Williams D. A., Fogarty K. E., Tsien R. Y., Fay F. S. Calcium gradients in single smooth muscle cells revealed by the digital imaging microscope using Fura-2. Nature. 1985 Dec 12;318(6046):558–561. doi: 10.1038/318558a0. [DOI] [PubMed] [Google Scholar]
- van Adelsberg J., Al-Awqati Q. Regulation of cell pH by Ca+2-mediated exocytotic insertion of H+-ATPases. J Cell Biol. 1986 May;102(5):1638–1645. doi: 10.1083/jcb.102.5.1638. [DOI] [PMC free article] [PubMed] [Google Scholar]