Abstract
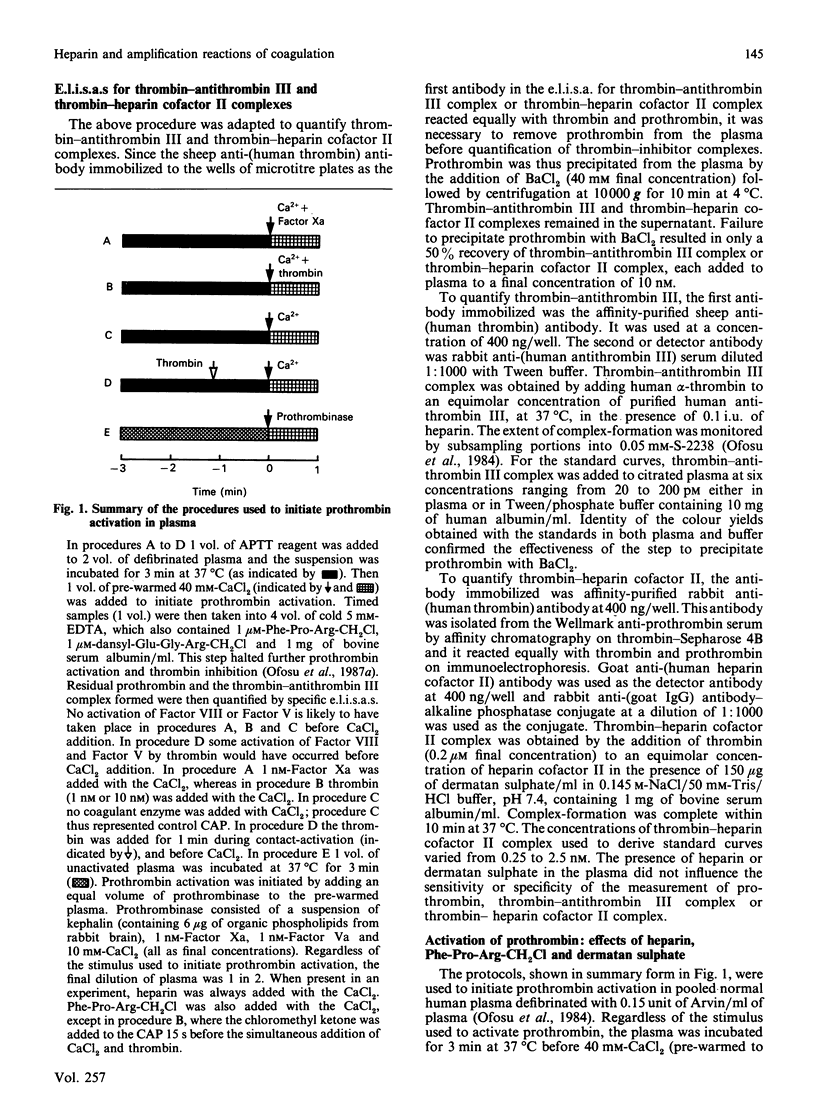
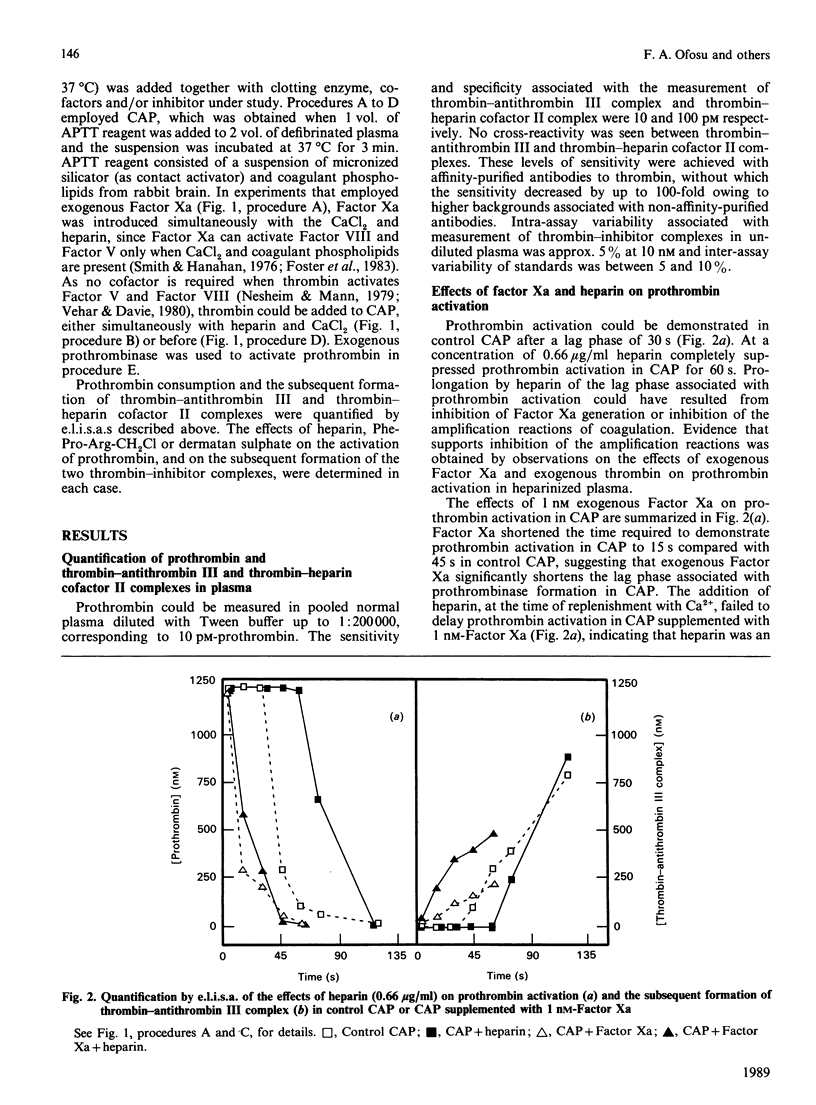
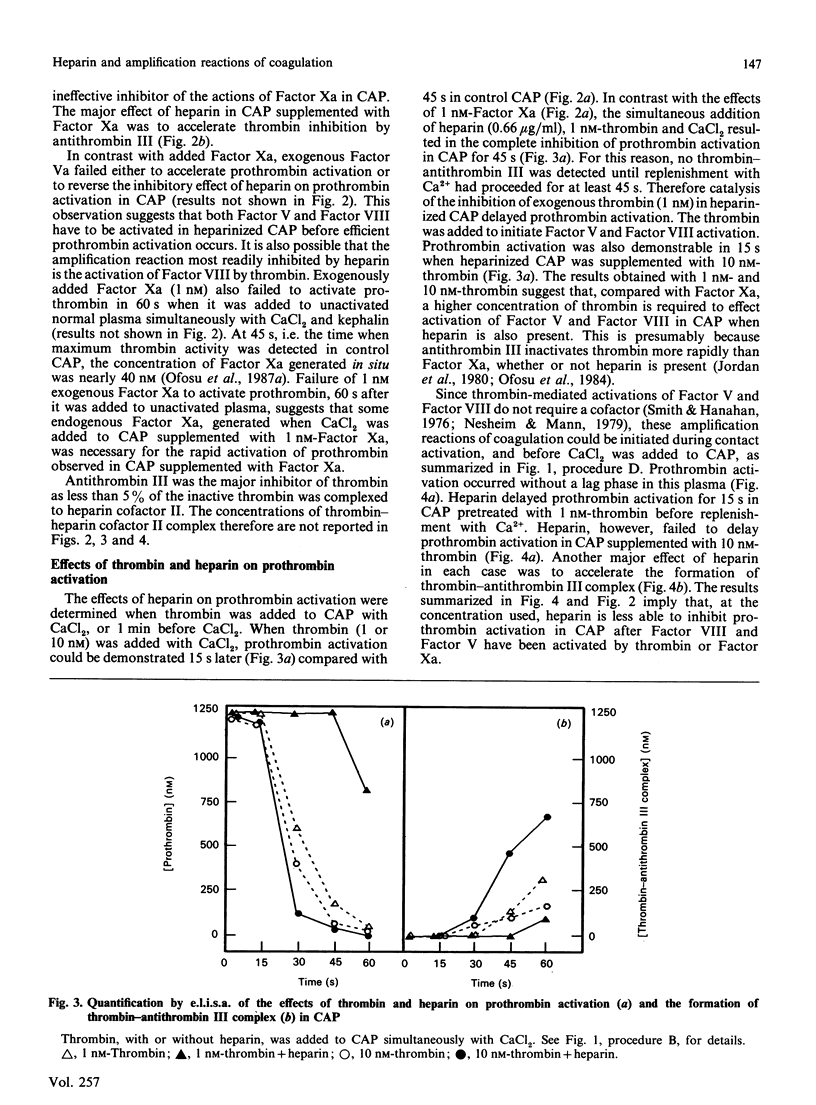
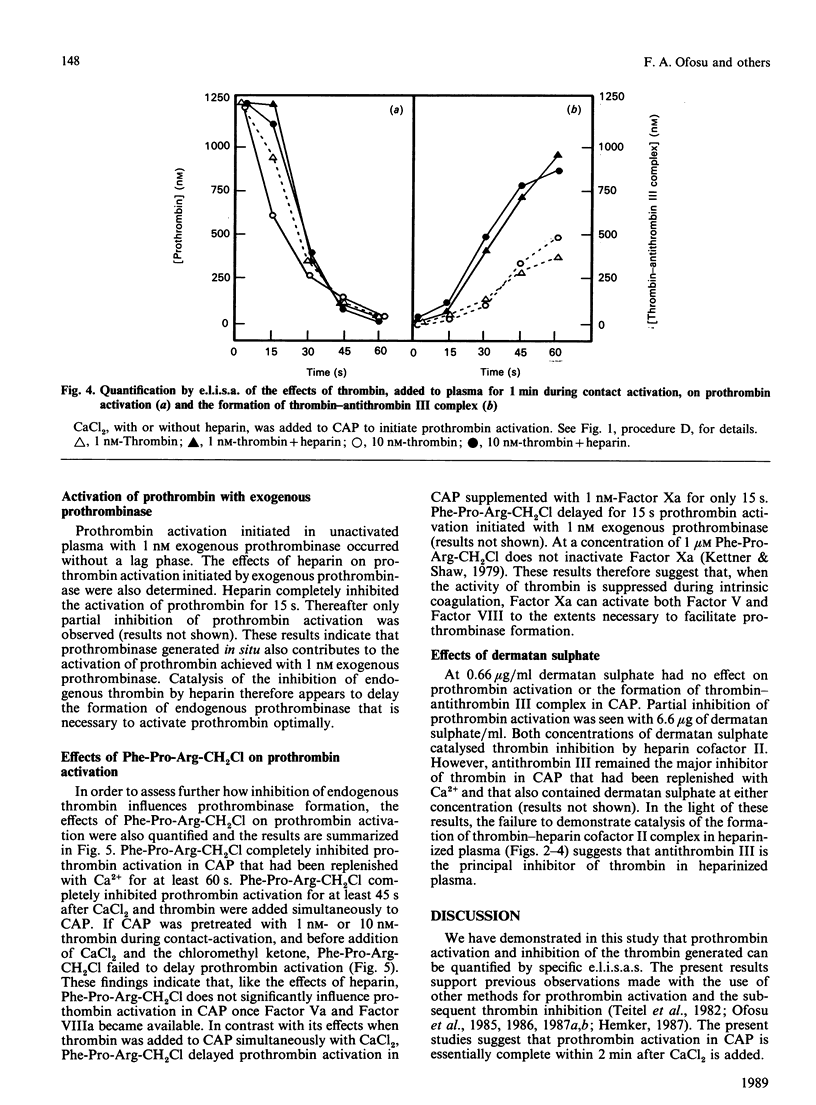
We have proposed previously that the steps in coagulation most sensitive to inhibition by heparin are the thrombin-dependent amplification reactions, and that prothrombinase is formed in heparinized plasma only after Factor Xa activates Factor VIII and Factor V. These propositions were based on the demonstration that both heparin and Phe-Pro-Arg-CH2Cl completely inhibited 125I-prothrombin activation for up to 60 s when contact-activated plasma (CAP) was replenished with Ca2+. Furthermore, the addition of thrombin to CAP before heparin or Phe-Pro-Arg-CH2Cl completely reversed their inhibitory effects. Additional support for the above hypotheses is provided in this study by demonstrating that, when the activity of thrombin is suppressed by heparin (indirectly) or by Phe-Pro-Arg-CH2Cl (directly), exogenous Factor Xa reverses the ability of these two agents to inhibit prothrombin activation. Prothrombin activation was initiated by adding Factor Xa (1 nM) or thrombin (1 or 10 nM) simultaneously with CaCl2 to CAP. In the absence of heparin or Phe-Pro-Arg-CH2Cl, prothrombin activation was seen 15 s later in either case. Heparin failed to delay, and Phe-Pro-Arg-CH2Cl delayed for 15 s, prothrombin activation in CAP supplemented with Factor Xa. In contrast, heparin and Phe-Pro-Arg-CH2Cl completely inhibited prothrombin activation for at least 45 s in CAP supplemented with 1 nM-thrombin. Heparin failed to delay prothrombin activation in CAP supplemented with 10 nM-thrombin, whereas Phe-Pro-Arg-CH2Cl completely inhibited prothrombin activation in this plasma for 45 s. These results suggest that in CAP: (1) Factor Xa can effectively activate Factor VIII and Factor V when the proteolytic activity of thrombin is suppressed; (2) heparin-antithrombin III is less able to inhibit Factor Xa than thrombin; (3) suppression of the thrombin-dependent amplification reactions is the primary anticoagulant effect of heparin.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Esmon C. T. The subunit structure of thrombin-activated factor V. Isolation of activated factor V, separation of subunits, and reconstitution of biological activity. J Biol Chem. 1979 Feb 10;254(3):964–973. [PubMed] [Google Scholar]
- Fenton J. W., 2nd, Fasco M. J., Stackrow A. B. Human thrombins. Production, evaluation, and properties of alpha-thrombin. J Biol Chem. 1977 Jun 10;252(11):3587–3598. [PubMed] [Google Scholar]
- Foster W. B., Nesheim M. E., Mann K. G. The factor Xa-catalyzed activation of factor V. J Biol Chem. 1983 Nov 25;258(22):13970–13977. [PubMed] [Google Scholar]
- Hoogendoorn H., Cerskus A., Ofosu F., Blajchman M., Hirsh J. Preparation and partial characterization of human plasma depleted of antithrombin-III by heparin-sepharose affinity chromatography. Thromb Res. 1980 Oct 1;20(1):77–83. doi: 10.1016/0049-3848(80)90058-4. [DOI] [PubMed] [Google Scholar]
- Jordan R. E., Oosta G. M., Gardner W. T., Rosenberg R. D. The kinetics of hemostatic enzyme-antithrombin interactions in the presence of low molecular weight heparin. J Biol Chem. 1980 Nov 10;255(21):10081–10090. [PubMed] [Google Scholar]
- Kettner C., Shaw E. D-Phe-Pro-ArgCH2C1-A selective affinity label for thrombin. Thromb Res. 1979;14(6):969–973. doi: 10.1016/0049-3848(79)90014-8. [DOI] [PubMed] [Google Scholar]
- Modi G. J., Blajchman M. A., Ofosu F. A. The isolation of prothrombin, Factor IX and Factor X from human Factor IX concentrates. Thromb Res. 1984 Dec 15;36(6):537–547. doi: 10.1016/0049-3848(84)90193-2. [DOI] [PubMed] [Google Scholar]
- Nesheim M. E., Mann K. G. Thrombin-catalyzed activation of single chain bovine factor V. J Biol Chem. 1979 Feb 25;254(4):1326–1334. [PubMed] [Google Scholar]
- Ofosu F. A., Blajchman M. A., Modi G. J., Smith L. M., Buchanan M. R., Hirsh J. The importance of thrombin inhibition for the expression of the anticoagulant activities of heparin, dermatan sulphate, low molecular weight heparin and pentosan polysulphate. Br J Haematol. 1985 Aug;60(4):695–704. doi: 10.1111/j.1365-2141.1985.tb07474.x. [DOI] [PubMed] [Google Scholar]
- Ofosu F. A., Blajchman M. A., Modi G., Cerskus A. L., Hirsh J. Activation of factor X and prothrombin in antithrombin-III depleted plasma: the effects of heparin. 1981 Aug 15-Sep 1Thromb Res. 23(4-5):331–345. doi: 10.1016/0049-3848(81)90194-8. [DOI] [PubMed] [Google Scholar]
- Ofosu F. A., Cerskus A. L., Hirsh J., Smith L. M., Modi G. J., Blajchman M. A. The inhibition of the anticoagulant activity of heparin by platelets, brain phospholipids, and tissue factor. Br J Haematol. 1984 Jun;57(2):229–238. [PubMed] [Google Scholar]
- Ofosu F. A., Modi G. J., Blajchman M. A., Buchanan M. R., Johnson E. A. Increased sulphation improves the anticoagulant activities of heparan sulphate and dermatan sulphate. Biochem J. 1987 Dec 15;248(3):889–896. doi: 10.1042/bj2480889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ofosu F. A., Modi G. J., Hirsh J., Buchanan M. R., Blajchman M. A. Mechanisms for inhibition of the generation of thrombin activity by sulfated polysaccharides. Ann N Y Acad Sci. 1986;485:41–55. doi: 10.1111/j.1749-6632.1986.tb34566.x. [DOI] [PubMed] [Google Scholar]
- Ofosu F. A., Sie P., Modi G. J., Fernandez F., Buchanan M. R., Blajchman M. A., Boneu B., Hirsh J. The inhibition of thrombin-dependent positive-feedback reactions is critical to the expression of the anticoagulant effect of heparin. Biochem J. 1987 Apr 15;243(2):579–588. doi: 10.1042/bj2430579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosing J., Tans G., Govers-Riemslag J. W., Zwaal R. F., Hemker H. C. The role of phospholipids and factor Va in the prothrombinase complex. J Biol Chem. 1980 Jan 10;255(1):274–283. [PubMed] [Google Scholar]
- Smith C. M., Hanahan D. J. The activation of factor V by factor Xa or alpha-chymotrypsin and comparison with thrombin and RVV-V action. An improved factor V isolation procedure. Biochemistry. 1976 May 4;15(9):1830–1838. doi: 10.1021/bi00654a007. [DOI] [PubMed] [Google Scholar]
- Teitel J. M., Bauer K. A., Lau H. K., Rosenberg R. D. Studies of the prothrombin activation pathway utilizing radioimmunoassays for the F2/F1 + 2 fragment and thrombin--antithrombin complex. Blood. 1982 May;59(5):1086–1097. [PubMed] [Google Scholar]
- Vehar G. A., Davie E. W. Preparation and properties of bovine factor VIII (antihemophilic factor). Biochemistry. 1980 Feb 5;19(3):401–410. doi: 10.1021/bi00544a001. [DOI] [PubMed] [Google Scholar]
- Yamagishi R., Niwa M., Kondo S., Sakuragawa N., Koide T. Purification and biological property of heparin cofactor II: activation of heparin cofactor II and antithrombin III by dextran sulfate and various glycosaminoglycans. Thromb Res. 1984 Dec 15;36(6):633–642. doi: 10.1016/0049-3848(84)90202-0. [DOI] [PubMed] [Google Scholar]
- van Dieijen G., Tans G., Rosing J., Hemker H. C. The role of phospholipid and factor VIIIa in the activation of bovine factor X. J Biol Chem. 1981 Apr 10;256(7):3433–3442. [PubMed] [Google Scholar]


