Abstract
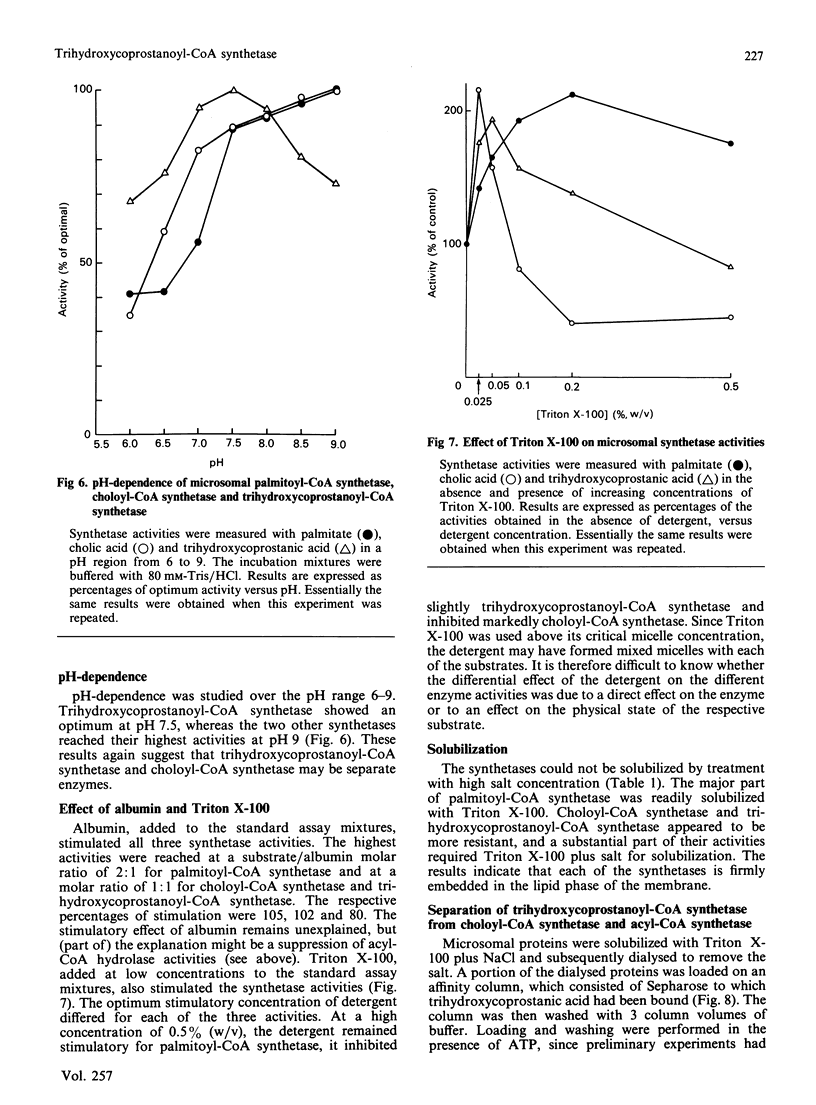
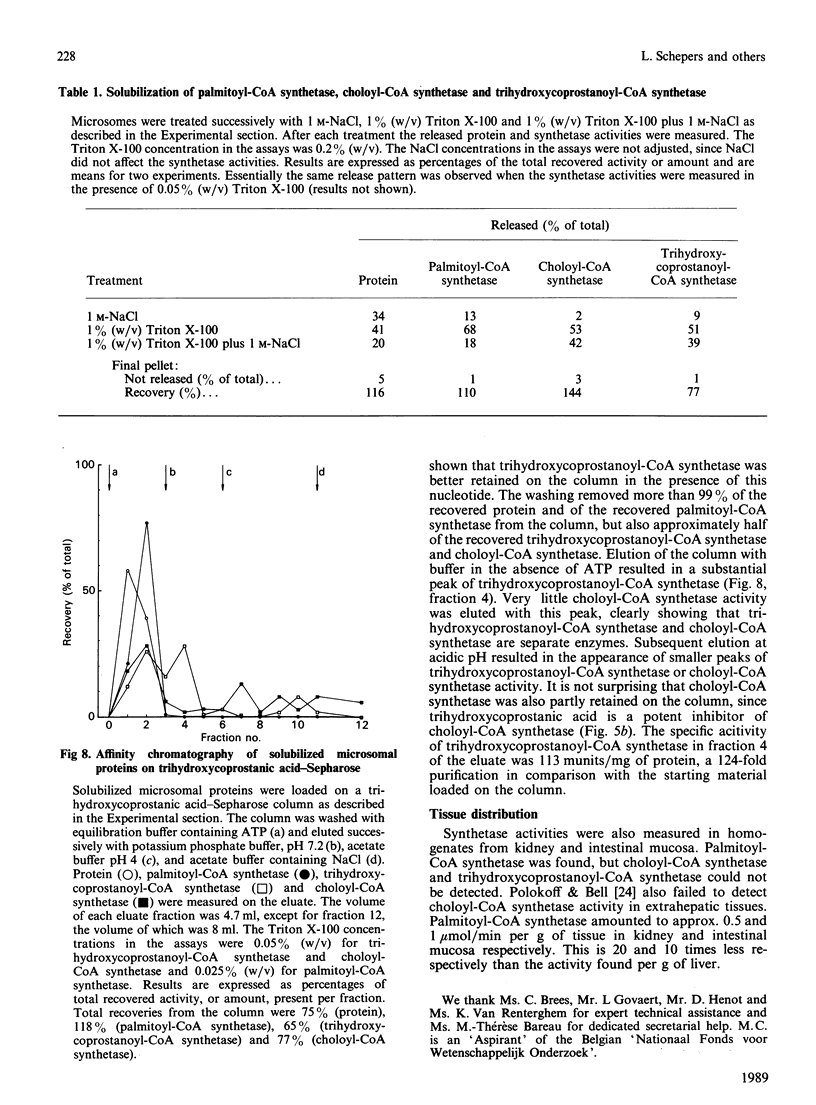
The subcellular distribution and characteristics of trihydroxycoprostanoyl-CoA synthetase were studied in rat liver and were compared with those of palmitoyl-CoA synthetase and choloyl-CoA synthetase. Trihydroxycoprostanoyl-CoA synthetase and choloyl-CoA synthetase were localized almost completely in the endoplasmic reticulum. A quantitatively insignificant part of trihydroxycoprostanoyl-CoA synthetase was perhaps present in mitochondria. Peroxisomes, which convert trihydroxycoprostanoyl-CoA into choloyl-CoA, were devoid of trihydroxycoprostanoyl-CoA synthetase. As already known, palmitoyl-CoA synthetase was distributed among mitochondria, peroxisomes and endoplasmic reticulum. Substrate- and cofactor- (ATP, CoASH) dependence of the three synthesis activities were also studied. Cholic acid and trihydroxycoprostanic acid did not inhibit palmitoyl-CoA synthetase; palmitate inhibited the other synthetases non-competitively. Likewise, cholic acid inhibited trihydroxycoprostanic acid activation non-competitively and vice versa. The pH curves of the synthetases did not coincide. Triton X-100 affected the activity of each of the synthetases differently. Trihydroxycoprostanoyl-CoA synthetase was less sensitive towards inhibition by pyrophosphate than choloyl-CoA synthetase. The synthetases could not be solubilized from microsomal membranes by treatment with 1 M-NaCl, but could be solubilized with Triton X-100 or Triton X-100 plus NaCl. The detergent-solubilized trihydroxycoprostanoyl-CoA synthetase could be separated from the solubilized choloyl-CoA synthetase and palmitoyl-CoA synthetase by affinity chromatograpy on Sepharose to which trihydroxycoprostanic acid was bound. Choloyl-CoA synthetase and trihydroxycoprostanoyl-CoA synthetase could not be detected in homogenates from kidney or intestinal mucosa. The results indicate that long-chain fatty acids, cholic acid and trihydroxycoprostanic acid are activated by three separate enzymes.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bhushan A., Singh R. P., Singh I. Characterization of rat brain microsomal acyl-coenzyme A ligases: different enzymes for the synthesis of palmitoyl-coenzyme A and lignoceroyl-coenzyme A. Arch Biochem Biophys. 1986 Apr;246(1):374–380. doi: 10.1016/0003-9861(86)90482-0. [DOI] [PubMed] [Google Scholar]
- Bronfman M., Inestrosa N. C., Nervi F. O., Leighton F. Acyl-CoA synthetase and the peroxisomal enzymes of beta-oxidation in human liver. Quantitative analysis of their subcellular localization. Biochem J. 1984 Dec 15;224(3):709–720. doi: 10.1042/bj2240709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casteels M., Schepers L., Van Eldere J., Eyssen H. J., Mannaerts G. P. Inhibition of 3 alpha,7 alpha,12 alpha-trihydroxy-5 beta-cholestanoic acid oxidation and of bile acid secretion in rat liver by fatty acids. J Biol Chem. 1988 Apr 5;263(10):4654–4661. [PubMed] [Google Scholar]
- Chen R. F. Removal of fatty acids from serum albumin by charcoal treatment. J Biol Chem. 1967 Jan 25;242(2):173–181. [PubMed] [Google Scholar]
- DE DUVE C., PRESSMAN B. C., GIANETTO R., WATTIAUX R., APPELMANS F. Tissue fractionation studies. 6. Intracellular distribution patterns of enzymes in rat-liver tissue. Biochem J. 1955 Aug;60(4):604–617. doi: 10.1042/bj0600604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Declercq P. E., Haagsman H. P., Van Veldhoven P., Debeer L. J., Van Golde L. M., Mannaerts G. P. Rat liver dihydroxyacetone-phosphate acyltransferases and their contribution to glycerolipid synthesis. J Biol Chem. 1984 Jul 25;259(14):9064–9075. [PubMed] [Google Scholar]
- ELLIOTT W. H. Enzymic activation of cholic acid involving coenzyme A. Biochim Biophys Acta. 1955 Jul;17(3):440–441. doi: 10.1016/0006-3002(55)90394-2. [DOI] [PubMed] [Google Scholar]
- Eyssen H., Parmentier G., Compernolle F., Boon J., Eggermont E. Trihydroxycoprostanic acid in the duodenal fluid of two children with intrahepatic bile duct anomalies. Biochim Biophys Acta. 1972 Jun 26;273(1):212–221. doi: 10.1016/0304-4165(72)90209-7. [DOI] [PubMed] [Google Scholar]
- Groot P. H., Scholte H. R., Hülsmann W. C. Fatty acid activation: specificity, localization, and function. Adv Lipid Res. 1976;14:75–126. doi: 10.1016/b978-0-12-024914-5.50009-7. [DOI] [PubMed] [Google Scholar]
- Hashmi M., Stanley W., Singh I. Lignoceroyl-CoASH ligase: enzyme defect in fatty acid beta-oxidation system in X-linked childhood adrenoleukodystrophy. FEBS Lett. 1986 Feb 17;196(2):247–250. doi: 10.1016/0014-5793(86)80256-3. [DOI] [PubMed] [Google Scholar]
- Helenius A., Simons K. Solubilization of membranes by detergents. Biochim Biophys Acta. 1975 Mar 25;415(1):29–79. doi: 10.1016/0304-4157(75)90016-7. [DOI] [PubMed] [Google Scholar]
- Jones C. L., Hajra A. K. Properties of guinea pig liver peroxisomal dihydroxyacetone phosphate acyltransferase. J Biol Chem. 1980 Sep 10;255(17):8289–8295. [PubMed] [Google Scholar]
- Kase B. F., Prydz K., Björkhem I., Pedersen J. I. Conjugation of cholic acid with taurine and glycine by rat liver peroxisomes. Biochem Biophys Res Commun. 1986 Jul 16;138(1):167–173. doi: 10.1016/0006-291x(86)90261-5. [DOI] [PubMed] [Google Scholar]
- Kase F., Björkhem I., Pedersen J. I. Formation of cholic acid from 3 alpha, 7 alpha, 12 alpha-trihydroxy-5 beta-cholestanoic acid by rat liver peroxisomes. J Lipid Res. 1983 Dec;24(12):1560–1567. [PubMed] [Google Scholar]
- Krisans S. K., Mortensen R. M., Lazarow P. B. Acyl-CoA synthetase in rat liver peroxisomes. Computer-assisted analysis of cell fractionation experiments. J Biol Chem. 1980 Oct 25;255(20):9599–9607. [PubMed] [Google Scholar]
- Mannaerts G. P., Van Veldhoven P., Van Broekhoven A., Vandebroek G., Debeer L. J. Evidence that peroxisomal acyl-CoA synthetase is located at the cytoplasmic side of the peroxisomal membrane. Biochem J. 1982 Apr 15;204(1):17–23. doi: 10.1042/bj2040017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcel Y. L., Suzue G. Kinetic studies on the specificity of long chain acyl coenzyme A synthetase from rat liver microsomes. J Biol Chem. 1972 Jul 25;247(14):4433–4436. [PubMed] [Google Scholar]
- Miller D. A., DeLuca H. F. Activation of retinoic acid by coenzyme A for the formation of ethyl retinoate. Proc Natl Acad Sci U S A. 1985 Oct;82(19):6419–6422. doi: 10.1073/pnas.82.19.6419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyazawa S., Hashimoto T., Yokota S. Identity of long-chain acyl-coenzyme A synthetase of microsomes, mitochondria, and peroxisomes in rat liver. J Biochem. 1985 Sep;98(3):723–733. doi: 10.1093/oxfordjournals.jbchem.a135330. [DOI] [PubMed] [Google Scholar]
- Nagamatsu K., Soeda S., Mori M., Kishimoto Y. Lignoceroyl-coenzyme A synthetase from developing rat brain: partial purification, characterization and comparison with palmitoyl-coenzyme A synthetase activity and liver enzyme. Biochim Biophys Acta. 1985 Aug 22;836(1):80–88. doi: 10.1016/0005-2760(85)90223-1. [DOI] [PubMed] [Google Scholar]
- Peterson G. L. A simplification of the protein assay method of Lowry et al. which is more generally applicable. Anal Biochem. 1977 Dec;83(2):346–356. doi: 10.1016/0003-2697(77)90043-4. [DOI] [PubMed] [Google Scholar]
- Polokoff M. A., Bell R. M. Characterization of liver cholic acid coenzyme A ligase activity. Evidence that separate microsomal enzymes are responsible for cholic acid and fatty acid activation. J Biol Chem. 1977 Feb 25;252(4):1167–1171. [PubMed] [Google Scholar]
- Roughan P. G., Slack C. R. Long-chain acyl-coenzyme A synthetase activity of spinach chloroplasts is concentrated in the envelope. Biochem J. 1977 Feb 15;162(2):457–459. doi: 10.1042/bj1620457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SIPERSTEIN M. D., MURRAY A. W. Enzymatic synthesis of cholyl coA and taurocholic acid. Science. 1956 Mar 2;123(3192):377–378. doi: 10.1126/science.123.3192.377. [DOI] [PubMed] [Google Scholar]
- Salen G., Shefer S. Bile acid synthesis. Annu Rev Physiol. 1983;45:679–685. doi: 10.1146/annurev.ph.45.030183.003335. [DOI] [PubMed] [Google Scholar]
- Schepers L., Casteels M., Vamecq J., Parmentier G., Van Veldhoven P. P., Mannaerts G. P. Beta-oxidation of the carboxyl side chain of prostaglandin E2 in rat liver peroxisomes and mitochondria. J Biol Chem. 1988 Feb 25;263(6):2724–2731. [PubMed] [Google Scholar]
- Simion F. A., Fleischer B., Fleischer S. Subcellular distribution of cholic acid:coenzyme a ligase and deoxycholic acid:Coenzyme a ligase activities in rat liver. Biochemistry. 1983 Oct 11;22(21):5029–5034. doi: 10.1021/bi00290a023. [DOI] [PubMed] [Google Scholar]
- Singh H., Derwas N., Poulos A. Very long chain fatty acid beta-oxidation by rat liver mitochondria and peroxisomes. Arch Biochem Biophys. 1987 Dec;259(2):382–390. doi: 10.1016/0003-9861(87)90504-2. [DOI] [PubMed] [Google Scholar]
- Tanaka T., Hosaka K., Hoshimaru M., Numa S. Purification and properties of long-chain acyl-coenzyme-A synthetase from rat liver. Eur J Biochem. 1979 Jul;98(1):165–172. doi: 10.1111/j.1432-1033.1979.tb13173.x. [DOI] [PubMed] [Google Scholar]
- Tserng K. Y., Klein P. D. Formylated bile acids: improved synthesis, properties, and partial deformylation. Steroids. 1977 May;29(5):635–648. doi: 10.1016/0039-128x(77)90015-0. [DOI] [PubMed] [Google Scholar]
- Vamecq J., de Hoffmann E., Van Hoof F. The microsomal dicarboxylyl-CoA synthetase. Biochem J. 1985 Sep 15;230(3):683–693. doi: 10.1042/bj2300683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vessey D. A., Zakim D. Characterization of microsomal choloyl-coenzyme A synthetase. Biochem J. 1977 May 1;163(2):357–362. doi: 10.1042/bj1630357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wanders R. J., van Roermund C. W., van Wijland M. J., Schutgens R. B., Schram A. W., van den Bosch H., Tager J. M. Studies on the peroxisomal oxidation of palmitate and lignocerate in rat liver. Biochim Biophys Acta. 1987 May 13;919(1):21–25. doi: 10.1016/0005-2760(87)90213-x. [DOI] [PubMed] [Google Scholar]
- Wanders R. J., van Roermund C. W., van Wijland M. J., Schutgens R. B., van den Bosch H., Schram A. W., Tager J. M. Direct demonstration that the deficient oxidation of very long chain fatty acids in X-linked adrenoleukodystrophy is due to an impaired ability of peroxisomes to activate very long chain fatty acids. Biochem Biophys Res Commun. 1988 Jun 16;153(2):618–624. doi: 10.1016/s0006-291x(88)81140-9. [DOI] [PubMed] [Google Scholar]
- Williamson J. R., Browning E. T., Thurman R. G., Scholz R. Inhibition of glucagon effects in perfused rat liver by (+)decanoylcarnitine. J Biol Chem. 1969 Sep 25;244(18):5055–5064. [PubMed] [Google Scholar]
- Wilson D. B., Prescott S. M., Majerus P. W. Discovery of an arachidonoyl coenzyme A synthetase in human platelets. J Biol Chem. 1982 Apr 10;257(7):3510–3515. [PubMed] [Google Scholar]


