Abstract
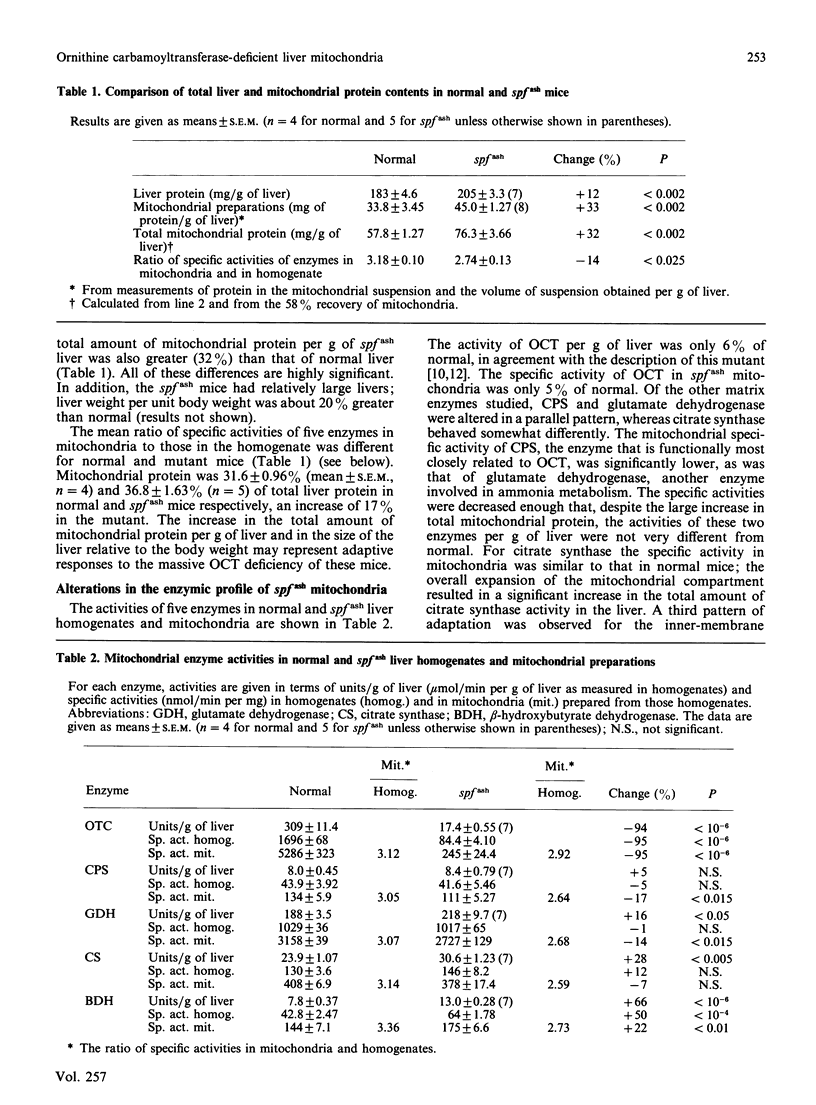
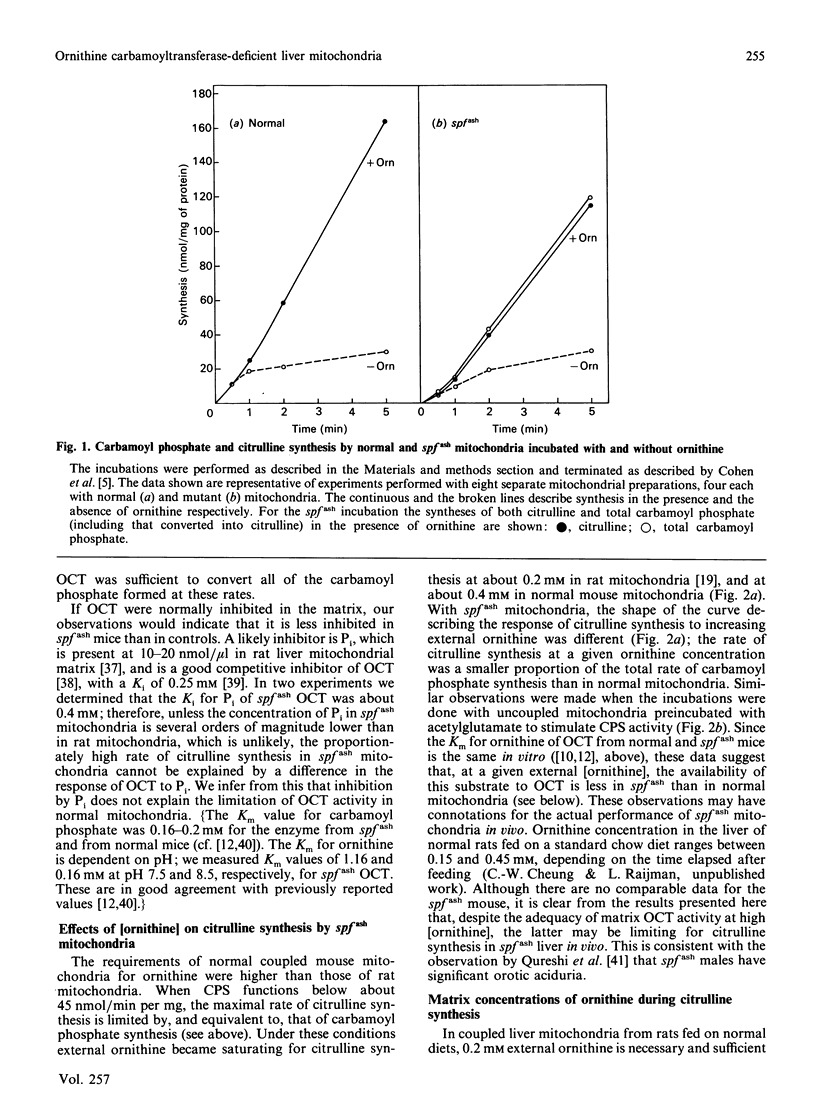
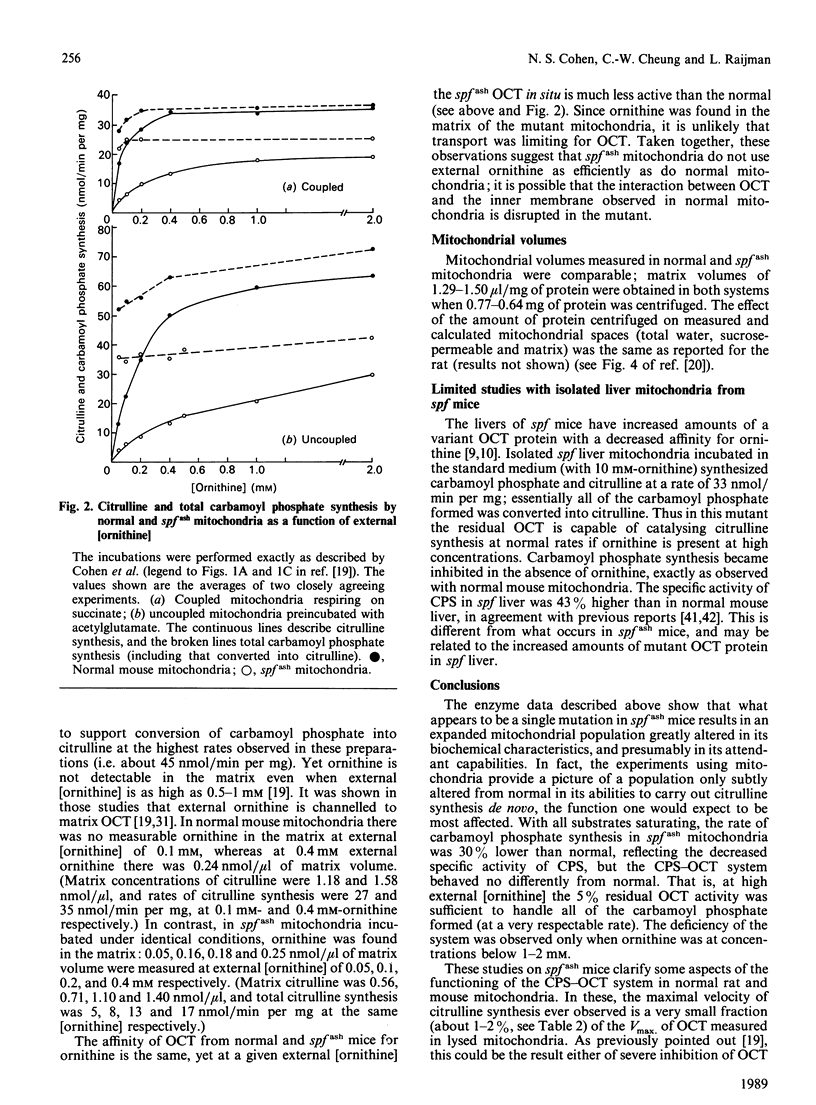
Male mice carrying the spfash mutation have 5-10% of the normal activity of ornithine carbamoyltransferase, yet are only slightly hyperammonaemic and develop quite well. A study of liver mitochondria from normal and spfash males showed that they differ in important ways. (1) The spfash liver contains about 33% more mitochondrial protein per g than does normal liver. (2) The specific activities of carbamoyl-phosphate synthetase (ammonia) and glutamate dehydrogenase are about 15% lower than normal in mitochondria from spfash mice, whereas those of beta-hydroxybutyrate dehydrogenase and cytochrome oxidase are 22% higher and 30% lower respectively. (3) In the presence of 10 mM-ornithine and the substrates for carbamoyl phosphate synthesis, coupled and uncoupled mitochondria from spfash mice synthesize citrulline at unexpectedly high rates, about 25 and 44 nmol/min per mg respectively. Though these are somewhat lower than the corresponding rates obtained with normal mitochondria, the difference does not arise from the deficiency in ornithine carbamoyltransferase, but from the lower carbamoyl-phosphate synthetase activity of the mutant mitochondria. (4) At lower external [ornithine] (less than 2 mM), a smaller fraction of the carbamoyl phosphate synthesized is converted into citrulline in spfash than in normal mitochondria. These studies show that what appears to be a single mutation brings about major adaptations in the mitochondrial component of liver. In addition, they clarify the role of ornithine transport and of protein-protein interactions in citrulline synthesis in normal mitochondria.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akerboom T. P., Bookelman H., Zuurendonk P. F., van der Meer R., Tager J. M. Intramitochondrial and extramitochondrial concentrations of adenine nucleotides and inorganic phosphate in isolated hepatocytes from fasted rats. Eur J Biochem. 1978 Mar 15;84(2):413–420. doi: 10.1111/j.1432-1033.1978.tb12182.x. [DOI] [PubMed] [Google Scholar]
- Attardi G., Chomyn A., Montoya J., Ojala D. Identification and mapping of human mitochondrial genes. Cytogenet Cell Genet. 1982;32(1-4):85–98. doi: 10.1159/000131689. [DOI] [PubMed] [Google Scholar]
- Bachmann C., Colombo J. P. Increase of tryptophan and 5-hydroxyindole acetic acid in the brain of ornithine carbamoyltransferase deficient sparse-fur mice. Pediatr Res. 1984 Apr;18(4):372–375. doi: 10.1203/00006450-198404000-00014. [DOI] [PubMed] [Google Scholar]
- Bock H. O., Fleischer S. Purification of D-beta-hydroxybutyrate apodehydrogenase, a lecithin-requiring enzyme. Methods Enzymol. 1974;32:374–391. [PubMed] [Google Scholar]
- Briand P., Francois B., Rabier D., Cathelineau L. Ornithine transcarbamylase deficiencies in human males. Kinetic and immunochemical classification. Biochim Biophys Acta. 1982 May 21;704(1):100–106. doi: 10.1016/0167-4838(82)90136-4. [DOI] [PubMed] [Google Scholar]
- CERIOTTI G., SPANDRIO L. A spectrophotometric method for determination of urea. Clin Chim Acta. 1963 Mar;8:295–299. doi: 10.1016/0009-8981(63)90171-2. [DOI] [PubMed] [Google Scholar]
- Cheung C. W., Raijman L. The regulation of carbamyl phosphate synthetase (ammonia) in rat liver mitochondria. Effects of acetylglutamate concentration and ATP translocation. J Biol Chem. 1980 Jun 10;255(11):5051–5057. [PubMed] [Google Scholar]
- Clarke S. A major polypeptide component of rat liver mitochondria: carbamyl phosphate synthetase. J Biol Chem. 1976 Feb 25;251(4):950–961. [PubMed] [Google Scholar]
- Cohen N. S., Cheung C. W. Differential effects of N-acetylglutamate on citrulline synthesis by coupled and uncoupled mitochondria. Arch Biochem Biophys. 1984 Oct;234(1):31–44. doi: 10.1016/0003-9861(84)90321-7. [DOI] [PubMed] [Google Scholar]
- Cohen N. S., Cheung C. W., Kyan F. S., Jones E. E., Raijman L. Mitochondrial carbamyl phosphate and citrulline synthesis at high matrix acetylglutamate. J Biol Chem. 1982 Jun 25;257(12):6898–6907. [PubMed] [Google Scholar]
- Cohen N. S., Cheung C. W., Raijman L. Channeling of extramitochondrial ornithine to matrix ornithine transcarbamylase. J Biol Chem. 1987 Jan 5;262(1):203–208. [PubMed] [Google Scholar]
- Cohen N. S., Cheung C. W., Raijman L. Measurements of mitochondrial volumes are affected by the amount of mitochondria used in the determinations. Biochem J. 1987 Jul 15;245(2):375–379. doi: 10.1042/bj2450375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen N. S., Cheung C. W., Raijman L. The effects of ornithine on mitochondrial carbamyl phosphate synthesis. J Biol Chem. 1980 Nov 10;255(21):10248–10255. [PubMed] [Google Scholar]
- Cohen N. S., Kyan F. S., Kyan S. S., Cheung C. W., Raijman L. The apparent Km of ammonia for carbamoyl phosphate synthetase (ammonia) in situ. Biochem J. 1985 Jul 1;229(1):205–211. doi: 10.1042/bj2290205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeMars R., LeVan S. L., Trend B. L., Russell L. B. Abnormal ornithine carbamoyltransferase in mice having the sparse-fur mutation. Proc Natl Acad Sci U S A. 1976 May;73(5):1693–1697. doi: 10.1073/pnas.73.5.1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doolittle D. P., Hulbert L. L., Cordy C. A new allele of the sparse fur gene in the mouse. J Hered. 1974 May-Jun;65(3):194–195. doi: 10.1093/oxfordjournals.jhered.a108500. [DOI] [PubMed] [Google Scholar]
- Gamble J. G., Lehninger A. L. Transport of ornithine and citrulline across the mitochondrial membrane. J Biol Chem. 1973 Jan 25;248(2):610–618. [PubMed] [Google Scholar]
- Girgis N., McGravey V., Shah B. L., Herrin J., Shih V. E. Lethal ornithine transcarbamylase deficiency in a female neonate. J Inherit Metab Dis. 1987;10(3):274–275. doi: 10.1007/BF01800079. [DOI] [PubMed] [Google Scholar]
- Hay R., Böhni P., Gasser S. How mitochondria import proteins. Biochim Biophys Acta. 1984 Jan 27;779(1):65–87. doi: 10.1016/0304-4157(84)90004-2. [DOI] [PubMed] [Google Scholar]
- JONES M. E., ANDERSON A. D., ANDERSON C., HODES S. Citrulline synthesis in rat tissues. Arch Biochem Biophys. 1961 Dec;95:499–507. doi: 10.1016/0003-9861(61)90182-5. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lusty C. J., Jilka R. L., Nietsch E. H. Ornithine transcarbamylase of rat liver. Kinetic, physical, and chemical properties. J Biol Chem. 1979 Oct 25;254(20):10030–10036. [PubMed] [Google Scholar]
- Marshall M., Cohen P. P. Ornithine transcarbamylase from Streptococcus faecalis and bovine liver. I. Isolation and subunit structure. J Biol Chem. 1972 Mar 25;247(6):1641–1653. [PubMed] [Google Scholar]
- Marshall M., Cohen P. P. Ornithine transcarbamylase from Streptococcus faecalis and bovine liver. II. Multiple binding sites for carbamyl-P and L-norvaline, correlation with steady state kinetics. J Biol Chem. 1972 Mar 25;247(6):1654–1668. [PubMed] [Google Scholar]
- Meijer A. J., Van Woerkom G. M., Wanders R. J., Lof C. Transport of N-acetylglutamate in rat-liver mitochondria. Eur J Biochem. 1982 May 17;124(2):325–330. doi: 10.1111/j.1432-1033.1982.tb06595.x. [DOI] [PubMed] [Google Scholar]
- Meijer A. J., van Woerkom G. M. Control of the rate of citrulline synthesis by short-term changes in N-acetylglutamate levels in isolated rat-liver mitochondria. FEBS Lett. 1978 Feb 1;86(1):117–121. doi: 10.1016/0014-5793(78)80111-2. [DOI] [PubMed] [Google Scholar]
- Mori M., Miura S., Morita T., Takiguchi M., Tatibana M. Synthesis, intracellular transport and processing of mitochondrial urea cycle enzymes. Adv Enzyme Regul. 1983;21:121–132. doi: 10.1016/0065-2571(83)90011-0. [DOI] [PubMed] [Google Scholar]
- Powers-Lee S. G., Mastico R. A., Bendayan M. The interaction of rat liver carbamoyl phosphate synthetase and ornithine transcarbamoylase with inner mitochondrial membranes. J Biol Chem. 1987 Nov 15;262(32):15683–15688. [PubMed] [Google Scholar]
- Raijman L., Bartulis T. Effect of ATP translocation on citrulline and oxaloacetate synthesis by isolated rat liver mitochondria. Arch Biochem Biophys. 1979 Jun;195(1):188–197. doi: 10.1016/0003-9861(79)90340-0. [DOI] [PubMed] [Google Scholar]
- Raijman L. Citrulline synthesis in rat tissues and liver content of carbamoyl phosphate and ornithine. Biochem J. 1974 Feb;138(2):225–232. doi: 10.1042/bj1380225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raijman L., Jones M. E. Purification, composition, and some properties of rat liver carbamyl phosphate synthetase (ammonia). Arch Biochem Biophys. 1976 Jul;175(1):270–278. doi: 10.1016/0003-9861(76)90508-7. [DOI] [PubMed] [Google Scholar]
- Rosenberg L. E., Kalousek F., Orsulak M. D. Biogenesis of ornithine transcarbamylase in spfash mutant mice: two cytoplasmic precursors, one mitochondrial enzyme. Science. 1983 Oct 28;222(4622):426–428. doi: 10.1126/science.6623083. [DOI] [PubMed] [Google Scholar]
- Yokota S., Mori M. Immunoelectron microscopical localization of ornithine transcarbamylase in hepatic parenchymal cells of the rat. Histochem J. 1986 Aug;18(8):451–457. doi: 10.1007/BF01675338. [DOI] [PubMed] [Google Scholar]


