Abstract
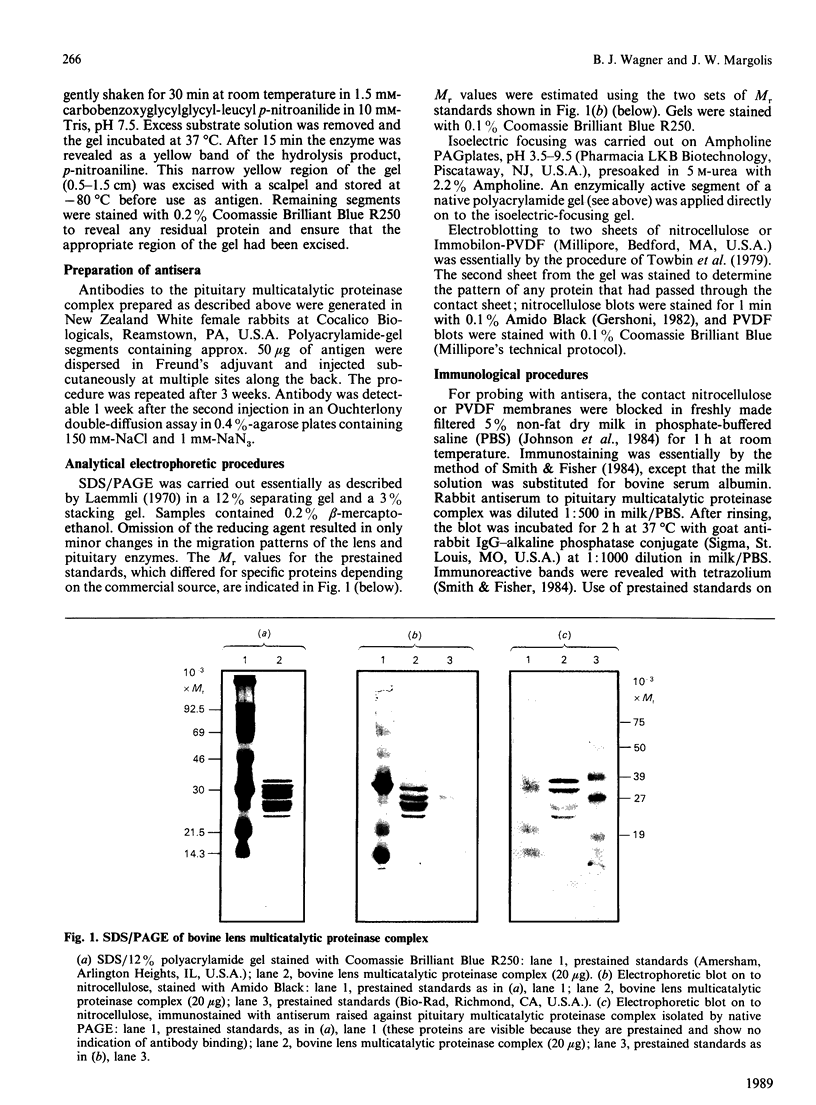
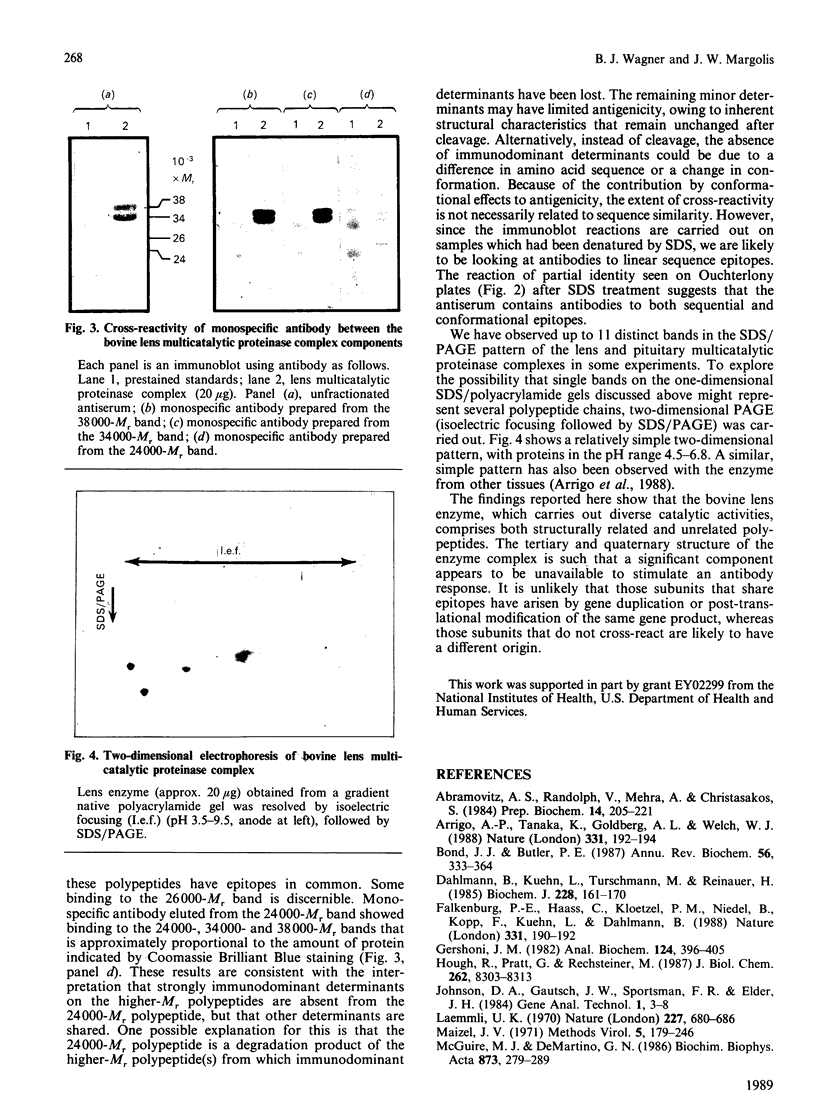
Component polypeptides of both the bovine lens and pituitary multicatalytic proteinase complexes demonstrate different immunoreactivities with a polyclonal antiserum raised against the purified pituitary enzyme. Four (Mr 24000, 26000, 34000 and 38000) of eight bands that have been resolved by SDS/polyacrylamide-gel electrophoresis are stained in immunoblot experiments. Monospecific antibodies obtained from this antiserum by affinity purification from the 38000- and 34000-Mr bands of the lens enzyme bound equally well to either band, but showed little or no binding to the 26000- and 24000-Mr bands upon immunoblotting. Antibody affinity-purified from the 24000-Mr band showed comparable binding to the 24000-, 34000- or 38000-Mr band. One explanation of these results is that the 24000-Mr polypeptide is derived from the higher-Mr polypeptide(s) and has lost some of the common immunodeterminants.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abramovitz A. S., Randolph V., Mehra A., Christakos S. Recovery of native proteins from preparative electrophoresis gel slices by reverse polarity elution. Prep Biochem. 1984 Aug;14(3):205–221. doi: 10.1080/10826068408070629. [DOI] [PubMed] [Google Scholar]
- Arrigo A. P., Tanaka K., Goldberg A. L., Welch W. J. Identity of the 19S 'prosome' particle with the large multifunctional protease complex of mammalian cells (the proteasome). Nature. 1988 Jan 14;331(6152):192–194. doi: 10.1038/331192a0. [DOI] [PubMed] [Google Scholar]
- Bond J. S., Butler P. E. Intracellular proteases. Annu Rev Biochem. 1987;56:333–364. doi: 10.1146/annurev.bi.56.070187.002001. [DOI] [PubMed] [Google Scholar]
- Dahlmann B., Kuehn L., Rutschmann M., Reinauer H. Purification and characterization of a multicatalytic high-molecular-mass proteinase from rat skeletal muscle. Biochem J. 1985 May 15;228(1):161–170. doi: 10.1042/bj2280161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falkenburg P. E., Haass C., Kloetzel P. M., Niedel B., Kopp F., Kuehn L., Dahlmann B. Drosophila small cytoplasmic 19S ribonucleoprotein is homologous to the rat multicatalytic proteinase. Nature. 1988 Jan 14;331(6152):190–192. doi: 10.1038/331190a0. [DOI] [PubMed] [Google Scholar]
- Gershoni J. M., Palade G. E. Electrophoretic transfer of proteins from sodium dodecyl sulfate-polyacrylamide gels to a positively charged membrane filter. Anal Biochem. 1982 Aug;124(2):396–405. doi: 10.1016/0003-2697(82)90056-2. [DOI] [PubMed] [Google Scholar]
- Hough R., Pratt G., Rechsteiner M. Purification of two high molecular weight proteases from rabbit reticulocyte lysate. J Biol Chem. 1987 Jun 15;262(17):8303–8313. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- McGuire M. J., Croall D. E., DeMartino G. N. ATP-stimulated proteolysis in soluble extracts of BHK 21/C13 cells. Evidence for multiple pathways and a role for an enzyme related to the high-molecular-weight protease, macropain. Arch Biochem Biophys. 1988 Apr;262(1):273–285. doi: 10.1016/0003-9861(88)90189-0. [DOI] [PubMed] [Google Scholar]
- McGuire M. J., DeMartino G. N. Purification and characterization of a high molecular weight proteinase (macropain) from human erythrocytes. Biochim Biophys Acta. 1986 Sep 26;873(2):279–289. doi: 10.1016/0167-4838(86)90055-5. [DOI] [PubMed] [Google Scholar]
- Olmsted J. B. Affinity purification of antibodies from diazotized paper blots of heterogeneous protein samples. J Biol Chem. 1981 Dec 10;256(23):11955–11957. [PubMed] [Google Scholar]
- Ray K., Harris H. Lens neutral endopeptidase occurs in other bovine and human tissues. Biochem J. 1987 Dec 15;248(3):643–648. doi: 10.1042/bj2480643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray K., Harris H. Purification of neutral lens endopeptidase: close similarity to a neutral proteinase in pituitary. Proc Natl Acad Sci U S A. 1985 Nov;82(22):7545–7549. doi: 10.1073/pnas.82.22.7545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivett A. J. Purification of a liver alkaline protease which degrades oxidatively modified glutamine synthetase. Characterization as a high molecular weight cysteine proteinase. J Biol Chem. 1985 Oct 15;260(23):12600–12606. [PubMed] [Google Scholar]
- Smith D. E., Fisher P. A. Identification, developmental regulation, and response to heat shock of two antigenically related forms of a major nuclear envelope protein in Drosophila embryos: application of an improved method for affinity purification of antibodies using polypeptides immobilized on nitrocellulose blots. J Cell Biol. 1984 Jul;99(1 Pt 1):20–28. doi: 10.1083/jcb.99.1.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WALEY S. G., VAN HEYNINGEN R. Neutral proteinases in the lens. Biochem J. 1962 May;83:274–283. doi: 10.1042/bj0830274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner B. J., Fu S. C., Margolis J. W., Fleshman K. R. A synthetic endopeptidase substrate hydrolyzed by the bovine lens neutral proteinase preparation. Exp Eye Res. 1984 May;38(5):477–483. doi: 10.1016/0014-4835(84)90125-8. [DOI] [PubMed] [Google Scholar]
- Wagner B. J., Margolis J. W., Abramovitz A. S., Fu S. C. Differential inhibition of two proteolytic activities in bovine lens neutral-proteinase preparations. Biochem J. 1985 Jun 1;228(2):517–519. doi: 10.1042/bj2280517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner B. J., Margolis J. W., Abramovitz A. S. The bovine lens neutral proteinase comprises a family of cysteine-dependent proteolytic activities. Curr Eye Res. 1986 Nov;5(11):863–868. doi: 10.3109/02713688609029238. [DOI] [PubMed] [Google Scholar]
- Wagner B. J., Margolis J. W., Fu S. C., Abramovitz A. S. Lens neutral proteinase preparations hydrolyze glutamoyl bonds. Exp Eye Res. 1985 Jun;40(6):879–882. doi: 10.1016/0014-4835(85)90132-0. [DOI] [PubMed] [Google Scholar]
- Wagner B. J., Margolis J. W., Garland D., Roseman J. E. Bovine lens neutral proteinase preferentially hydrolyses oxidatively modified glutamine synthetase. Exp Eye Res. 1986 Dec;43(6):1141–1143. doi: 10.1016/0014-4835(86)90091-6. [DOI] [PubMed] [Google Scholar]
- Wilk S., Orlowski M. Cation-sensitive neutral endopeptidase: isolation and specificity of the bovine pituitary enzyme. J Neurochem. 1980 Nov;35(5):1172–1182. doi: 10.1111/j.1471-4159.1980.tb07873.x. [DOI] [PubMed] [Google Scholar]
- Wilk S., Orlowski M. Evidence that pituitary cation-sensitive neutral endopeptidase is a multicatalytic protease complex. J Neurochem. 1983 Mar;40(3):842–849. doi: 10.1111/j.1471-4159.1983.tb08056.x. [DOI] [PubMed] [Google Scholar]
- Yamamoto T., Nojima M., Ishiura S., Sugita H. Purification of the two forms of the high-molecular-weight neutral proteinase ingensin from rat liver. Biochim Biophys Acta. 1986 Jul 16;882(3):297–304. doi: 10.1016/0304-4165(86)90251-5. [DOI] [PubMed] [Google Scholar]
- Zolfaghari R., Baker C. R., Amirgholami A., Canizaro P. C., Behal F. J. A multicatalytic high-molecular-weight neutral endopeptidase from human kidney. Arch Biochem Biophys. 1987 Oct;258(1):42–50. doi: 10.1016/0003-9861(87)90320-1. [DOI] [PubMed] [Google Scholar]
- Zolfaghari R., Baker C. R., Jr, Canizaro P. C., Amirgholami A., Behal F. J. A high-molecular-mass neutral endopeptidase-24.5 from human lung. Biochem J. 1987 Jan 1;241(1):129–135. doi: 10.1042/bj2410129. [DOI] [PMC free article] [PubMed] [Google Scholar]