Abstract
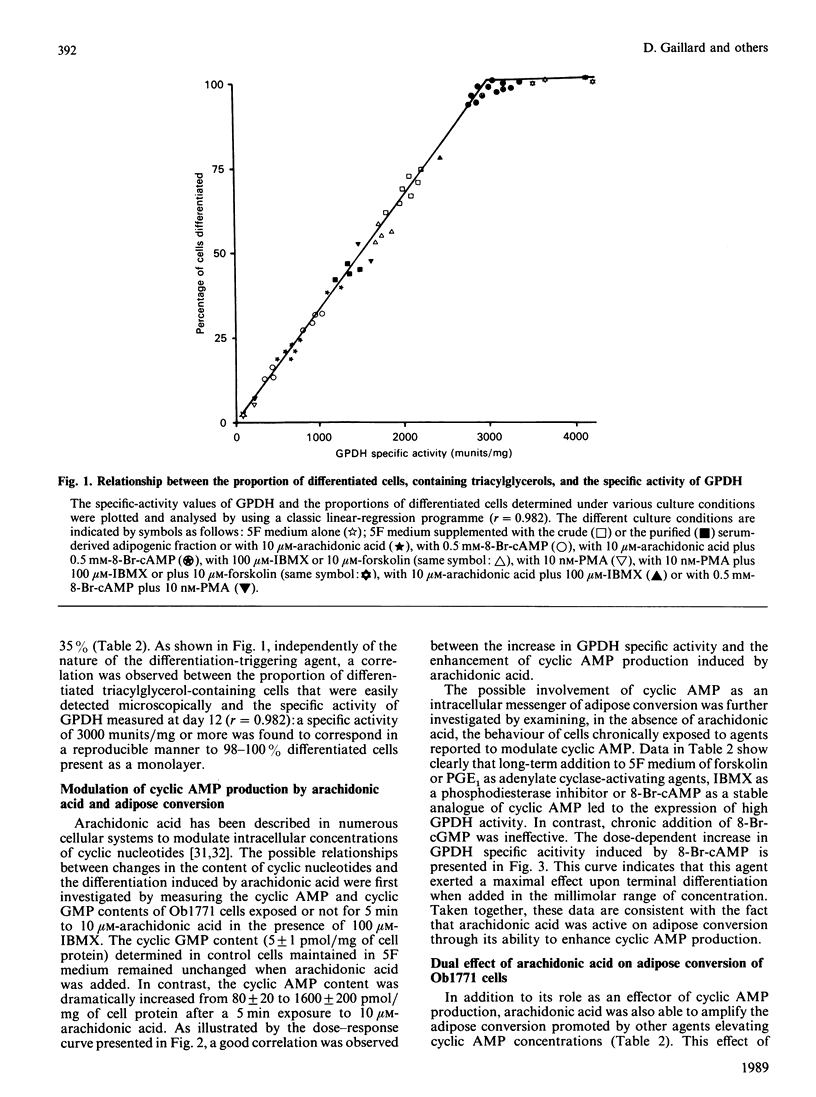
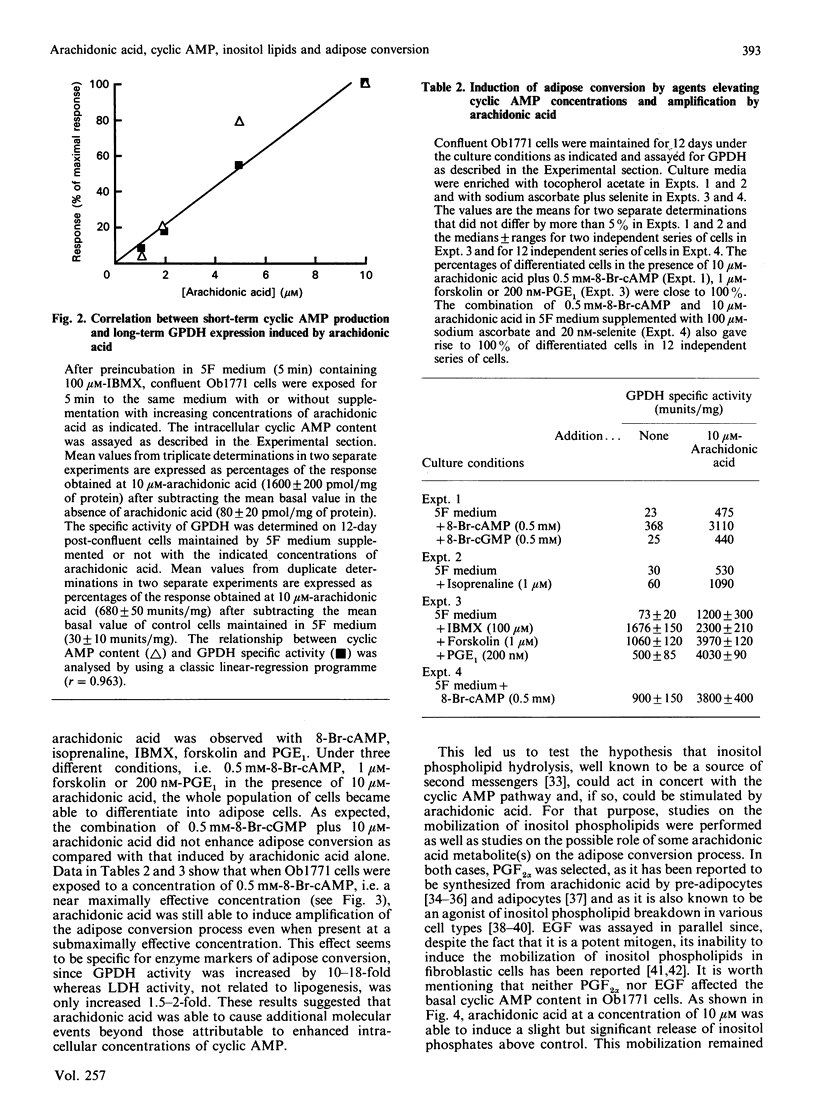
The terminal adipose differentiation of Ob1771 cells, characterized by glycerol-3-phosphate dehydrogenase activity and triacylglycerol accumulation, was studied in serum-free hormone-supplemented medium containing growth hormone, tri-iodothyronine, insulin, transferrin and fetuin. Arachidonic acid was able to substitute for a crude adipogenic fraction isolated from fetal bovine serum but not for growth hormone or tri-iodothyronine. Arachidonic acid was also able to increase in a rapid and dramatic manner cyclic AMP production; moreover it was able to amplify the adipose conversion promoted by other agents elevating cyclic AMP concentrations and to induce inositol phospholipid breakdown. Both phorbol 12-myristate 13-acetate, a protein kinase C activator and ionomycin, a Ca2+-mobilizing agent, showed potent synergy with agents elevating cyclic AMP concentrations for the promotion of adipose conversion, whereas 8-bromo cyclic GMP and 4 alpha-phorbol 12,13-dibutyrate were ineffective. The triggering of both the cyclic AMP and inositol phospholipid pathways was accompanied by a single round of cell division, and within a few days all the cells became differentiated. Similar results were obtained, after exposure to arachidonic acid, with preadipose 3T3-F442A cells and with rat adipose precursor cells in primary culture. The availability of arachidonic acid from intracellular stores and/or of exogenous origin should play a major role for the onset of critical mitoses leading to terminal differentiation in pre-adipose cells.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amri E. Z., Barbaras R., Doglio A., Dani C., Grimaldi P., Ailhaud G. Role of spermidine in the expression of late markers of adipose conversion. Effects of growth hormone. Biochem J. 1986 Oct 15;239(2):363–370. doi: 10.1042/bj2390363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amri E. Z., Dani C., Doglio A., Etienne J., Grimaldi P., Ailhaud G. Adipose cell differentiation: evidence for a two-step process in the polyamine-dependent Ob1754 clonal line. Biochem J. 1986 Aug 15;238(1):115–122. doi: 10.1042/bj2380115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amri E. Z., Dani C., Doglio A., Grimaldi P., Ailhaud G. Coupling of growth arrest and expression of early markers during adipose conversion of preadipocyte cell lines. Biochem Biophys Res Commun. 1986 Jun 13;137(2):903–910. doi: 10.1016/0006-291x(86)91165-4. [DOI] [PubMed] [Google Scholar]
- Axelrod L., Levine L. Prostacyclin production by isolated adipocytes. Diabetes. 1981 Feb;30(2):163–167. doi: 10.2337/diab.30.2.163. [DOI] [PubMed] [Google Scholar]
- Azarnia R., Russell T. R. Cyclic AMP effects on cell-to-cell junctional membrane permeability during adipocyte differentiation of 3T3-L1 fibroblasts. J Cell Biol. 1985 Jan;100(1):265–269. doi: 10.1083/jcb.100.1.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge M. J., Irvine R. F. Inositol trisphosphate, a novel second messenger in cellular signal transduction. Nature. 1984 Nov 22;312(5992):315–321. doi: 10.1038/312315a0. [DOI] [PubMed] [Google Scholar]
- Bevan S., Wood J. N. Arachidonic-acid metabolites as second messengers. Nature. 1987 Jul 2;328(6125):20–20. doi: 10.1038/328020a0. [DOI] [PubMed] [Google Scholar]
- Björntorp P., Karlsson M., Pertoft H., Pettersson P., Sjöström L., Smith U. Isolation and characterization of cells from rat adipose tissue developing into adipocytes. J Lipid Res. 1978 Mar;19(3):316–324. [PubMed] [Google Scholar]
- Björntorp P., Karlsson M., Pettersson P., Sypniewska G. Differentiation and function of rat adipocyte precursor cells in primary culture. J Lipid Res. 1980 Aug;21(6):714–723. [PubMed] [Google Scholar]
- Catalioto R. M., Négrel R., Gaillard D., Ailhaud G. Growth-promoting activity in serum-free medium of kallikreinlike arginylesteropeptidases from rat submaxillary gland. J Cell Physiol. 1987 Mar;130(3):352–360. doi: 10.1002/jcp.1041300307. [DOI] [PubMed] [Google Scholar]
- Chapman A. B., Knight D. M., Ringold G. M. Glucocorticoid regulation of adipocyte differentiation: hormonal triggering of the developmental program and induction of a differentiation-dependent gene. J Cell Biol. 1985 Oct;101(4):1227–1235. doi: 10.1083/jcb.101.4.1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook J. R., Kozak L. P. Sn-glycerol-3-phosphate dehydrogenase gene expression during mouse adipocyte development in vivo. Dev Biol. 1982 Aug;92(2):440–448. doi: 10.1016/0012-1606(82)90189-0. [DOI] [PubMed] [Google Scholar]
- Darmon M., Serrero G., Rizzino A., Sato G. Isolation of myoblastic, fibro-adipogenic, and fibroblastic clonal cell lines from a common precursor and study of their requirements for growth and differentiation. Exp Cell Res. 1981 Apr;132(2):313–327. doi: 10.1016/0014-4827(81)90107-5. [DOI] [PubMed] [Google Scholar]
- Davis J. S., Weakland L. L., Weiland D. A., Farese R. V., West L. A. Prostaglandin F2 alpha stimulates phosphatidylinositol 4,5-bisphosphate hydrolysis and mobilizes intracellular Ca2+ in bovine luteal cells. Proc Natl Acad Sci U S A. 1987 Jun;84(11):3728–3732. doi: 10.1073/pnas.84.11.3728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deslex S., Negrel R., Ailhaud G. Development of a chemically defined serum-free medium for differentiation of rat adipose precursor cells. Exp Cell Res. 1987 Jan;168(1):15–30. doi: 10.1016/0014-4827(87)90412-5. [DOI] [PubMed] [Google Scholar]
- Djian P., Grimaldi P., Négrel R., Ailhaud G. Adipose conversion of OB17 preadipocytes. Relationships between cell division and fat cell cluster formation. Exp Cell Res. 1982 Dec;142(2):273–281. doi: 10.1016/0014-4827(82)90368-8. [DOI] [PubMed] [Google Scholar]
- Doglio A., Dani C., Grimaldi P., Ailhaud G. Growth hormone regulation of the expression of differentiation-dependent genes in preadipocyte Ob1771 cells. Biochem J. 1986 Aug 15;238(1):123–129. doi: 10.1042/bj2380123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaillard D., Ailhaud G., Négrel R. Fetuin modulates growth and differentiation of Ob17 preadipose cells in serum-free hormone-supplemented medium. Biochim Biophys Acta. 1985 Jul 30;846(1):185–191. doi: 10.1016/0167-4889(85)90125-9. [DOI] [PubMed] [Google Scholar]
- Gaillard D., Négrel R., Serrero-Davé G., Cermolacce C., Ailhaud G. Growth of preadipocyte cell lines and cell strains from rodents in serum-free hormone-supplemented medium. In Vitro. 1984 Feb;20(2):79–88. doi: 10.1007/BF02626647. [DOI] [PubMed] [Google Scholar]
- Gaillard D., Poli P., Négrel R. Characterization of ouabain-resistant mutants of the preadipocyte Ob17 clonal line. Adipose conversion in vitro and in vivo. Exp Cell Res. 1985 Feb;156(2):513–527. doi: 10.1016/0014-4827(85)90558-0. [DOI] [PubMed] [Google Scholar]
- Gharbi-Chihi J., Grimaldi P., Torresani J., Ailhaud G. Triiodothyronine and adipose conversion of OB17 preadipocytes : binding to high affinity sites and effects on fatty acid synthetizing and esterifying enzymes. J Recept Res. 1981;2(2):153–173. doi: 10.3109/10799898109039259. [DOI] [PubMed] [Google Scholar]
- Green H., Kehinde O. Formation of normally differentiated subcutaneous fat pads by an established preadipose cell line. J Cell Physiol. 1979 Oct;101(1):169–171. doi: 10.1002/jcp.1041010119. [DOI] [PubMed] [Google Scholar]
- Green H., Kehinde O. Spontaneous heritable changes leading to increased adipose conversion in 3T3 cells. Cell. 1976 Jan;7(1):105–113. doi: 10.1016/0092-8674(76)90260-9. [DOI] [PubMed] [Google Scholar]
- Grimaldi P., Djian P., Negrel R., Ailhaud G. Differentiation of Ob17 preadipocytes to adipocytes: requirement of adipose conversion factor(s) for fat cell cluster formation. EMBO J. 1982;1(6):687–692. doi: 10.1002/j.1460-2075.1982.tb01231.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimaldi P., Négrel R., Ailhaud G. Induction of the triglyceride pathway enzymes and of lipolytic enzymes during differentiation in a 'preadipocyte' cell line. Eur J Biochem. 1978 Mar 15;84(2):369–376. doi: 10.1111/j.1432-1033.1978.tb12177.x. [DOI] [PubMed] [Google Scholar]
- Hauner H., Schmid P., Pfeiffer E. F. Glucocorticoids and insulin promote the differentiation of human adipocyte precursor cells into fat cells. J Clin Endocrinol Metab. 1987 Apr;64(4):832–835. doi: 10.1210/jcem-64-4-832. [DOI] [PubMed] [Google Scholar]
- Hesketh T. R., Moore J. P., Morris J. D., Taylor M. V., Rogers J., Smith G. A., Metcalfe J. C. A common sequence of calcium and pH signals in the mitogenic stimulation of eukaryotic cells. Nature. 1985 Feb 7;313(6002):481–484. doi: 10.1038/313481a0. [DOI] [PubMed] [Google Scholar]
- Hyman B. T., Stoll L. L., Spector A. A. Prostaglandin production by 3T3-L1 cells in culture. Biochim Biophys Acta. 1982 Nov 12;713(2):375–385. doi: 10.1016/0005-2760(82)90256-9. [DOI] [PubMed] [Google Scholar]
- Kodama H. A., Amagai Y., Koyama H., Kasai S. Hormonal responsiveness of a preadipose cell line derived from newborn mouse calvaria. J Cell Physiol. 1982 Jul;112(1):83–88. doi: 10.1002/jcp.1041120113. [DOI] [PubMed] [Google Scholar]
- L'Allemain G., Pouysségur EGF and insulin action in fibroblasts. Evidence that phosphoinositide hydrolysis is not an essential mitogenic signalling pathway. FEBS Lett. 1986 Mar 3;197(1-2):344–348. doi: 10.1016/0014-5793(86)80354-4. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Löffler G., Feick P., Hauner H., Herberg L. An adipogenic serum factor in genetically obese rodents. FEBS Lett. 1983 Mar 7;153(1):179–182. doi: 10.1016/0014-5793(83)80143-4. [DOI] [PubMed] [Google Scholar]
- Macphee C. H., Drummond A. H., Otto A. M., Jimenez de Asua L. Prostaglandin F2 alpha stimulates phosphatidylinositol turnover and increases the cellular content of 1,2-diacylglycerol in confluent resting Swiss 3T3 cells. J Cell Physiol. 1984 Apr;119(1):35–40. doi: 10.1002/jcp.1041190107. [DOI] [PubMed] [Google Scholar]
- Mitchell M. D., Cleland W. H., Smith M. E., Simpson E. R., Mendelson C. R. Inhibition of prostaglandin biosynthesis in human adipose tissue by glucocorticosteroids. J Clin Endocrinol Metab. 1983 Oct;57(4):771–776. doi: 10.1210/jcem-57-4-771. [DOI] [PubMed] [Google Scholar]
- Morikawa M., Nixon T., Green H. Growth hormone and the adipose conversion of 3T3 cells. Cell. 1982 Jul;29(3):783–789. doi: 10.1016/0092-8674(82)90440-8. [DOI] [PubMed] [Google Scholar]
- Murphy M. G., Négrel R., Ailhaud G. Lipoprotein lipase and monoacylglycerol lipase activities during maturation of ob17 preadipocytes. Biochim Biophys Acta. 1981 May 22;664(2):240–248. doi: 10.1016/0005-2760(81)90046-1. [DOI] [PubMed] [Google Scholar]
- Negrel R., Grimaldi P., Ailhaud G. Differentiation of ob 17 preadipocytes to adipocytes. Effects of prostaglandin F2alpha and relationship to prostaglandin synthesis. Biochim Biophys Acta. 1981 Oct 23;666(1):15–24. doi: 10.1016/0005-2760(81)90086-2. [DOI] [PubMed] [Google Scholar]
- Nishizuka Y. Perspectives on the role of protein kinase C in stimulus-response coupling. J Natl Cancer Inst. 1986 Mar;76(3):363–370. [PubMed] [Google Scholar]
- Nixon T., Green H. Contribution of growth hormone to the adipogenic activity of serum. Endocrinology. 1984 Feb;114(2):527–532. doi: 10.1210/endo-114-2-527. [DOI] [PubMed] [Google Scholar]
- Négrel R., Ailhaud G. Metabolism of arachidonic acid and prostaglandin synthesis in the preadipocyte clonal line ob17. Biochem Biophys Res Commun. 1981 Feb 12;98(3):768–777. doi: 10.1016/0006-291x(81)91178-5. [DOI] [PubMed] [Google Scholar]
- Négrel R., Grimaldi P., Ailhaud G. Establishment of preadipocyte clonal line from epididymal fat pad of ob/ob mouse that responds to insulin and to lipolytic hormones. Proc Natl Acad Sci U S A. 1978 Dec;75(12):6054–6058. doi: 10.1073/pnas.75.12.6054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Négrel R., Grimaldi P., Forest C., Ailhaud G. Establishment and characterization of fibroblast-like cell lines derived from adipocytes with the capacity to redifferentiate into adipocyte-like cells. Methods Enzymol. 1985;109:377–385. doi: 10.1016/0076-6879(85)09103-0. [DOI] [PubMed] [Google Scholar]
- Orlicky D. J., Silio M., Williams C., Gordon J., Gerschenson L. E. Regulation of inositol phosphate levels by prostaglandins in cultured endometrial cells. J Cell Physiol. 1986 Jul;128(1):105–112. doi: 10.1002/jcp.1041280116. [DOI] [PubMed] [Google Scholar]
- Parham E. S., King S. L., Bedell M. L., Martersteck S. Weight control content of women's magazines: bias and accuracy. Int J Obes. 1986;10(1):19–27. [PubMed] [Google Scholar]
- Pilgrim C. DNA synthesis and differentiation in developing white adipose tissue. Dev Biol. 1971 Sep;26(1):69–76. doi: 10.1016/0012-1606(71)90108-4. [DOI] [PubMed] [Google Scholar]
- Powell W. S. Rapid extraction of arachidonic acid metabolites from biological samples using octadecylsilyl silica. Methods Enzymol. 1982;86:467–477. doi: 10.1016/0076-6879(82)86218-6. [DOI] [PubMed] [Google Scholar]
- Rubin C. S., Hirsch A., Fung C., Rosen O. M. Development of hormone receptors and hormonal responsiveness in vitro. Insulin receptors and insulin sensitivity in the preadipocyte and adipocyte forms of 3T3-L1 cells. J Biol Chem. 1978 Oct 25;253(20):7570–7578. [PubMed] [Google Scholar]
- Russell T. R., Ho R. Conversion of 3T3 fibroblasts into adipose cells: triggering of differentiation by prostaglandin F2alpha and 1-methyl-3-isobutyl xanthine. Proc Natl Acad Sci U S A. 1976 Dec;73(12):4516–4520. doi: 10.1073/pnas.73.12.4516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuelsson B., Goldyne M., Granström E., Hamberg M., Hammarström S., Malmsten C. Prostaglandins and thromboxanes. Annu Rev Biochem. 1978;47:997–1029. doi: 10.1146/annurev.bi.47.070178.005025. [DOI] [PubMed] [Google Scholar]
- Schiwek D. R., Löffler G. Glucocorticoid hormones contribute to the adipogenic activity of human serum. Endocrinology. 1987 Feb;120(2):469–474. doi: 10.1210/endo-120-2-469. [DOI] [PubMed] [Google Scholar]
- Smets L. A., Van Rooy H. Mitogenic and antimitogenic effects of cholera toxin-mediated cyclic AMP levels in 3T3 cells. J Cell Physiol. 1987 Nov;133(2):395–399. doi: 10.1002/jcp.1041330227. [DOI] [PubMed] [Google Scholar]
- Spiegelman B. M., Green H. Cyclic AMP-mediated control of lipogenic enzyme synthesis during adipose differentiation of 3T3 cells. Cell. 1981 May;24(2):503–510. doi: 10.1016/0092-8674(81)90341-x. [DOI] [PubMed] [Google Scholar]
- Vannier C., Gaillard D., Grimaldi P., Amri E. Z., Djian P., Cermolacce C., Forest C., Etienne J., Negrel R., Ailhaud G. Adipose conversion of ob17 cells and hormone-related events. Int J Obes. 1985;9 (Suppl 1):41–53. [PubMed] [Google Scholar]
- Vannier C., Jansen H., Négrel R., Ailhaud G. Study of lipoprotein lipase content in Ob17 preadipocytes during adipose conversion. Immunofluorescent localization of the enzyme. J Biol Chem. 1982 Oct 25;257(20):12387–12393. [PubMed] [Google Scholar]
- Wiederer O., Löffler G. Hormonal regulation of the differentiation of rat adipocyte precursor cells in primary culture. J Lipid Res. 1987 Jun;28(6):649–658. [PubMed] [Google Scholar]
- Wise L. S., Green H. Participation of one isozyme of cytosolic glycerophosphate dehydrogenase in the adipose conversion of 3T3 cells. J Biol Chem. 1979 Jan 25;254(2):273–275. [PubMed] [Google Scholar]


