Abstract
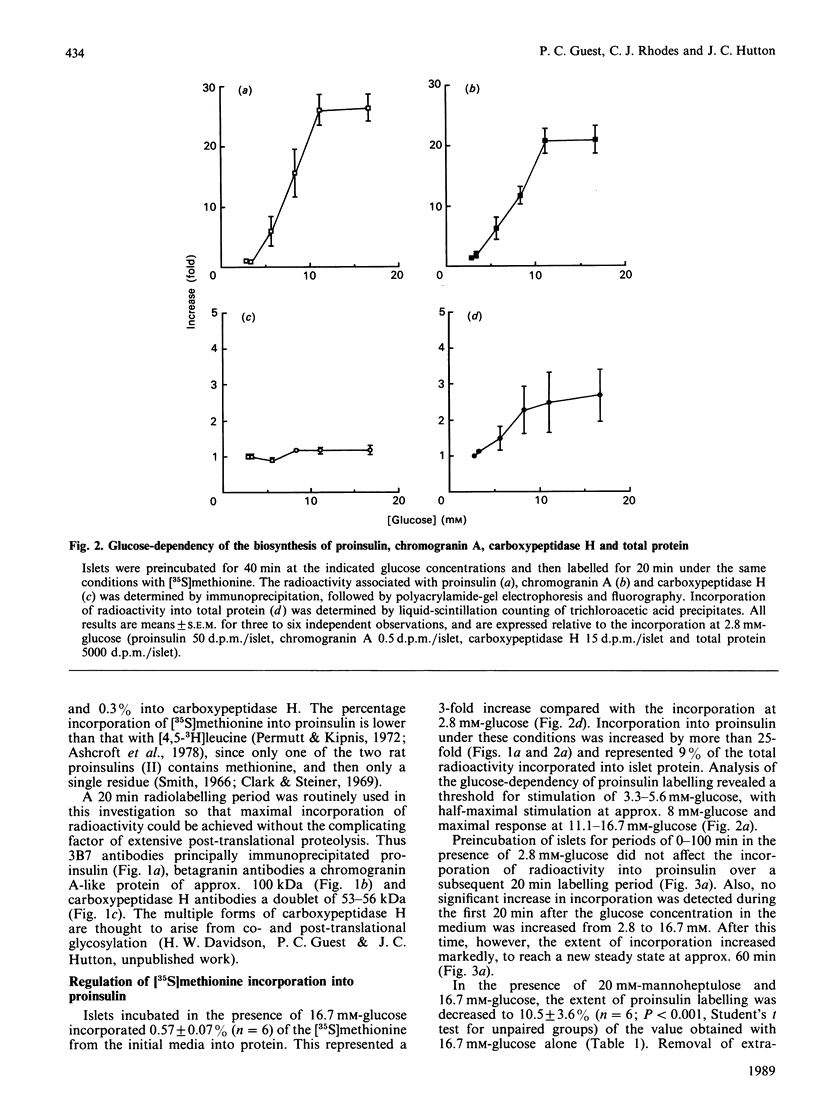
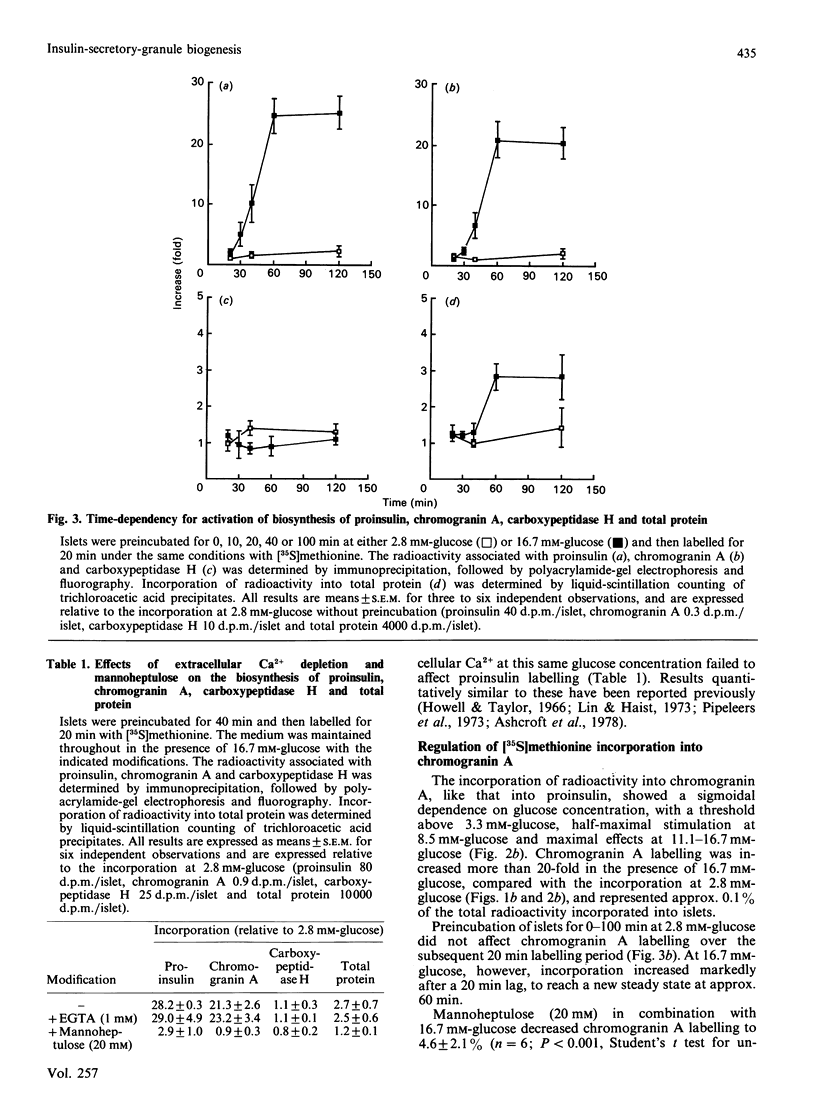
The regulation of the biosynthesis of the insulin-secretory-granule matrix proteins insulin II, chromogranin A and carboxypeptidase H was studied in isolated rat islets of Langerhans. Islets were labelled with [35S]-methionine, and incorporation into total protein was determined by trichloroacetic acid precipitation and that into specific proteins by immunoprecipitation followed by polyacrylamide-gel electrophoresis and fluorography. Islets incubated in the presence of 16.7 mM-glucose incorporated 3 times as much [35S]-methionine into total protein as did islets incubated with 2.8 mM-glucose. The same conditions produced more than a 20-fold increase in incorporation into both proinsulin and chromogranin A, with no observable effect on carboxypeptidase H. The concentration-dependencies of the glucose-stimulated synthesis of chromogranin A and proinsulin were parallel, and in both cases the response to 16.7 mM-glucose was typified by an initial lag of 20 min, followed by a rapid activation to a new steady state over the ensuing 40 min. Synthesis of total protein, although activated to a lesser extent, responded with similar kinetics. Extracellular Ca2+ depletion did not affect the basal or glucose-stimulated biosynthesis of any of the proteins under investigation. Mannoheptulose (20 mM) abolished glucose-stimulated synthesis of insulin, chromogranin A and total protein, but had no effect on the synthesis of carboxypeptidase H. It is concluded that the biosynthesis of insulin and chromogranin A is regulated principally at the translational level by the same intracellular signal generated from the metabolism of glucose. Such regulation is not common to all insulin-secretory-granule proteins, since the synthesis of carboxypeptidase H was unaffected by the same stimulus.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ashcroft S. J., Bunce J., Lowry M., Hansen S. E., Hedeskov C. J. The effect of sugars on (pro)insulin biosynthesis. Biochem J. 1978 Aug 15;174(2):517–526. doi: 10.1042/bj1740517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashcroft S. J., Randle P. J. Enzymes of glucose metabolism in normal mouse pancreatic islets. Biochem J. 1970 Aug;119(1):5–15. doi: 10.1042/bj1190005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Axén R., Porath J., Ernback S. Chemical coupling of peptides and proteins to polysaccharides by means of cyanogen halides. Nature. 1967 Jun 24;214(5095):1302–1304. doi: 10.1038/2141302a0. [DOI] [PubMed] [Google Scholar]
- Brunstedt J., Chan S. J. Direct effect of glucose on the preproinsulin mRNA level in isolated pancreatic islets. Biochem Biophys Res Commun. 1982 Jun 30;106(4):1383–1389. doi: 10.1016/0006-291x(82)91267-0. [DOI] [PubMed] [Google Scholar]
- Chick W. L., Warren S., Chute R. N., Like A. A., Lauris V., Kitchen K. C. A transplantable insulinoma in the rat. Proc Natl Acad Sci U S A. 1977 Feb;74(2):628–632. doi: 10.1073/pnas.74.2.628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark J. L., Steiner D. F. Insulin biosynthesis in the rat: demonstration of two proinsulins. Proc Natl Acad Sci U S A. 1969 Jan;62(1):278–285. doi: 10.1073/pnas.62.1.278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson H. W., Hutton J. C. The insulin-secretory-granule carboxypeptidase H. Purification and demonstration of involvement in proinsulin processing. Biochem J. 1987 Jul 15;245(2):575–582. doi: 10.1042/bj2450575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson H. W., Rhodes C. J., Hutton J. C. Intraorganellar calcium and pH control proinsulin cleavage in the pancreatic beta cell via two distinct site-specific endopeptidases. Nature. 1988 May 5;333(6168):93–96. doi: 10.1038/333093a0. [DOI] [PubMed] [Google Scholar]
- Docherty K., Hutton J. C. Carboxypeptidase activity in the insulin secretory granule. FEBS Lett. 1983 Oct 3;162(1):137–141. doi: 10.1016/0014-5793(83)81065-5. [DOI] [PubMed] [Google Scholar]
- Eiden L. E. Is chromogranin a prohormone? Nature. 1987 Jan 22;325(6102):301–301. doi: 10.1038/325301a0. [DOI] [PubMed] [Google Scholar]
- Fricker L. D., Snyder S. H. Purification and characterization of enkephalin convertase, an enkephalin-synthesizing carboxypeptidase. J Biol Chem. 1983 Sep 25;258(18):10950–10955. [PubMed] [Google Scholar]
- Grimaldi K. A., Siddle K., Hutton J. C. Biosynthesis of insulin secretory granule membrane proteins. Control by glucose. Biochem J. 1987 Jul 15;245(2):567–573. doi: 10.1042/bj2450567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howell S. L., Montague W. Adenylate cyclase activity in isolated rat islets of Langerhans. Effects of agents which alter rates of insulin secretion. Biochim Biophys Acta. 1973 Aug 17;320(1):44–52. doi: 10.1016/0304-4165(73)90163-3. [DOI] [PubMed] [Google Scholar]
- Huttner W. B., Benedum U. M. Chromogranin A and pancreastatin. Nature. 1987 Jan 22;325(6102):305–305. doi: 10.1038/325305b0. [DOI] [PubMed] [Google Scholar]
- Hutton J. C., Davidson H. W., Grimaldi K. A., Peshavaria M. Biosynthesis of betagranin in pancreatic beta-cells. Identification of a chromogranin A-like precursor and its parallel processing with proinsulin. Biochem J. 1987 Jun 1;244(2):449–456. doi: 10.1042/bj2440449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutton J. C., Davidson H. W., Peshavaria M. The mechanism of chromogranin A processing. 1987 Feb 26-Mar 4Nature. 325(6107):766–766. doi: 10.1038/325766b0. [DOI] [PubMed] [Google Scholar]
- Hutton J. C., Peshavaria M., Johnston C. F., Ravazzola M., Orci L. Immunolocalization of betagranin: a chromogranin A-related protein of the pancreatic B-cell. Endocrinology. 1988 Mar;122(3):1014–1020. doi: 10.1210/endo-122-3-1014. [DOI] [PubMed] [Google Scholar]
- Hutton J. C. Secretory granules. Experientia. 1984 Oct 15;40(10):1091–1098. doi: 10.1007/BF01971456. [DOI] [PubMed] [Google Scholar]
- Iacangelo A., Affolter H. U., Eiden L. E., Herbert E., Grimes M. Bovine chromogranin A sequence and distribution of its messenger RNA in endocrine tissues. Nature. 1986 Sep 4;323(6083):82–86. doi: 10.1038/323082a0. [DOI] [PubMed] [Google Scholar]
- Itoh N., Sei T., Nose K., Okamoto H. Glucose stimulation of the proinsulin synthesis in isolated pancreatic islets without increasing amount of proinsulin mRNA. FEBS Lett. 1978 Sep 15;93(2):343–347. doi: 10.1016/0014-5793(78)81136-3. [DOI] [PubMed] [Google Scholar]
- Jarrett R. J., Keen H., Track N. Glucose and RNA synthesis in mammalian islets of Langerhans. Nature. 1967 Feb 11;213(5076):634–635. doi: 10.1038/213634a0. [DOI] [PubMed] [Google Scholar]
- Lacy P. E., Kostianovsky M. Method for the isolation of intact islets of Langerhans from the rat pancreas. Diabetes. 1967 Jan;16(1):35–39. doi: 10.2337/diab.16.1.35. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lin B. J., Haist R. E. Effects of some modifiers of insulin secretion on insulin biosynthesis. Endocrinology. 1973 Mar;92(3):735–742. doi: 10.1210/endo-92-3-735. [DOI] [PubMed] [Google Scholar]
- Morris G. E., Korner A. RNA synthesis and the stimulation of insulin biosynthesis by glucose. FEBS Lett. 1970 Oct 5;10(3):165–168. doi: 10.1016/0014-5793(70)80444-6. [DOI] [PubMed] [Google Scholar]
- Nielsen D. A., Welsh M., Casadaban M. J., Steiner D. F. Control of insulin gene expression in pancreatic beta-cells and in an insulin-producing cell line, RIN-5F cells. I. Effects of glucose and cyclic AMP on the transcription of insulin mRNA. J Biol Chem. 1985 Nov 5;260(25):13585–13589. [PubMed] [Google Scholar]
- Nolan J. A., Trojanowski J. Q., Hogue-Angeletti R. Neurons and neuroendocrine cells contain chromogranin: detection of the molecule in normal bovine tissues by immunochemical and immunohistochemical methods. J Histochem Cytochem. 1985 Aug;33(8):791–798. doi: 10.1177/33.8.3894497. [DOI] [PubMed] [Google Scholar]
- O'Connor D. T. Chromogranin: widespread immunoreactivity in polypeptide hormone producing tissues and in serum. Regul Pept. 1983 Jul;6(3):263–280. doi: 10.1016/0167-0115(83)90145-3. [DOI] [PubMed] [Google Scholar]
- Permutt M. A., Kipnis D. M. Insulin biosynthesis. I. On the mechanism of glucose stimulation. J Biol Chem. 1972 Feb 25;247(4):1194–1199. [PubMed] [Google Scholar]
- Perrie W. T., Perry S. V. An electrophoretic study of the low-molecular-weight components of myosin. Biochem J. 1970 Aug;119(1):31–38. doi: 10.1042/bj1190031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pipeleers D. G., Marichal M., Malaisse W. J. The stimulus-secretion coupling of glucose-induced insulin release. XIV. Glucose regulation of insular biosynthetic activity. Endocrinology. 1973 Nov;93(5):1001–1011. doi: 10.1210/endo-93-5-1001. [DOI] [PubMed] [Google Scholar]
- Smith L. F. Species variation in the amino acid sequence of insulin. Am J Med. 1966 May;40(5):662–666. doi: 10.1016/0002-9343(66)90145-8. [DOI] [PubMed] [Google Scholar]
- Sopwith A. M., Hales C. N., Hutton J. C. Pancreatic B-cells secrete a range of novel peptides besides insulin. Biochim Biophys Acta. 1984 Apr 16;803(4):342–345. doi: 10.1016/0167-4889(84)90127-7. [DOI] [PubMed] [Google Scholar]
- Strieder N., Ziegler E., Winkler H., Smith A. D. Some properties of soluble proteins from chromaffin granules of different species. Biochem Pharmacol. 1968 Aug;17(8):1553–1556. doi: 10.1016/0006-2952(68)90214-1. [DOI] [PubMed] [Google Scholar]
- Tatemoto K., Efendić S., Mutt V., Makk G., Feistner G. J., Barchas J. D. Pancreastatin, a novel pancreatic peptide that inhibits insulin secretion. Nature. 1986 Dec 4;324(6096):476–478. doi: 10.1038/324476a0. [DOI] [PubMed] [Google Scholar]
- Welsh M., Nielsen D. A., MacKrell A. J., Steiner D. F. Control of insulin gene expression in pancreatic beta-cells and in an insulin-producing cell line, RIN-5F cells. II. Regulation of insulin mRNA stability. J Biol Chem. 1985 Nov 5;260(25):13590–13594. [PubMed] [Google Scholar]
- Welsh M., Scherberg N., Gilmore R., Steiner D. F. Translational control of insulin biosynthesis. Evidence for regulation of elongation, initiation and signal-recognition-particle-mediated translational arrest by glucose. Biochem J. 1986 Apr 15;235(2):459–467. doi: 10.1042/bj2350459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson B. S., Lloyd R. V. Detection of chromogranin in neuroendocrine cells with a monoclonal antibody. Am J Pathol. 1984 Jun;115(3):458–468. [PMC free article] [PubMed] [Google Scholar]