Abstract
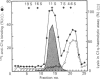
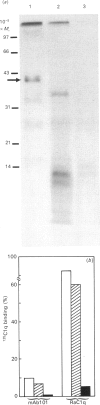
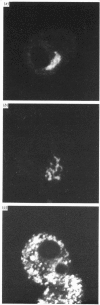
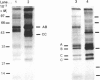
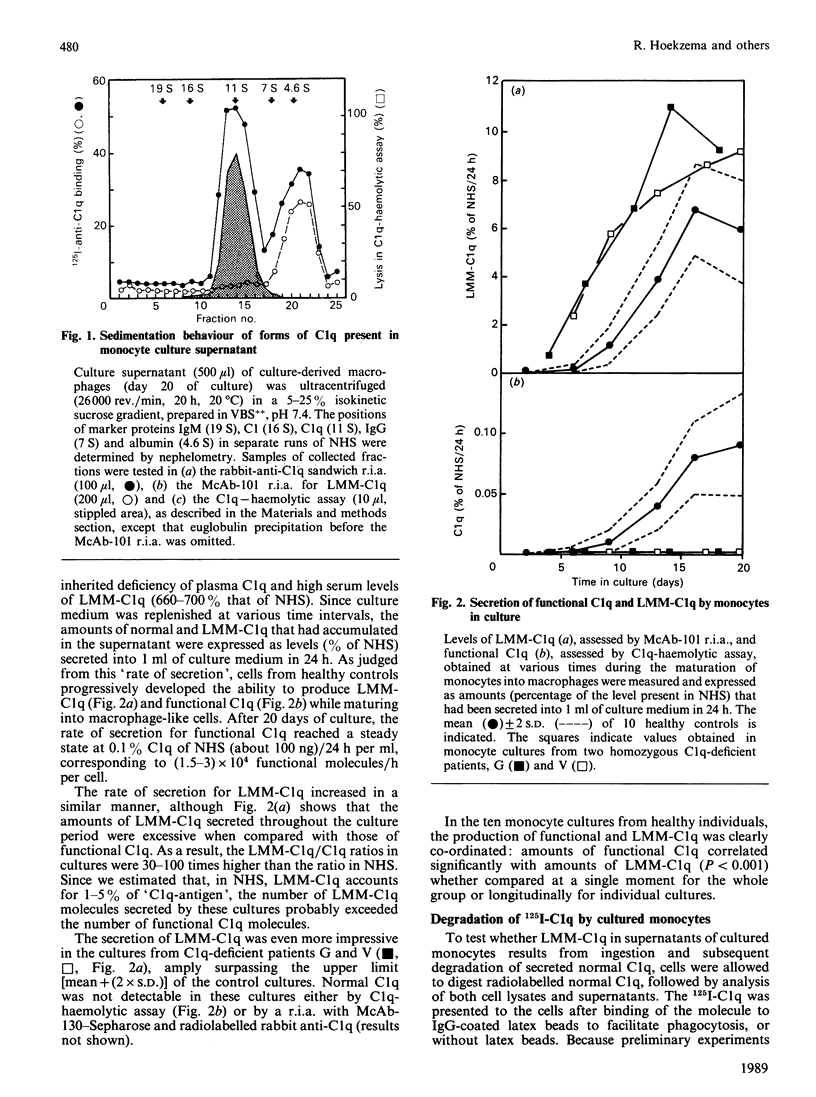
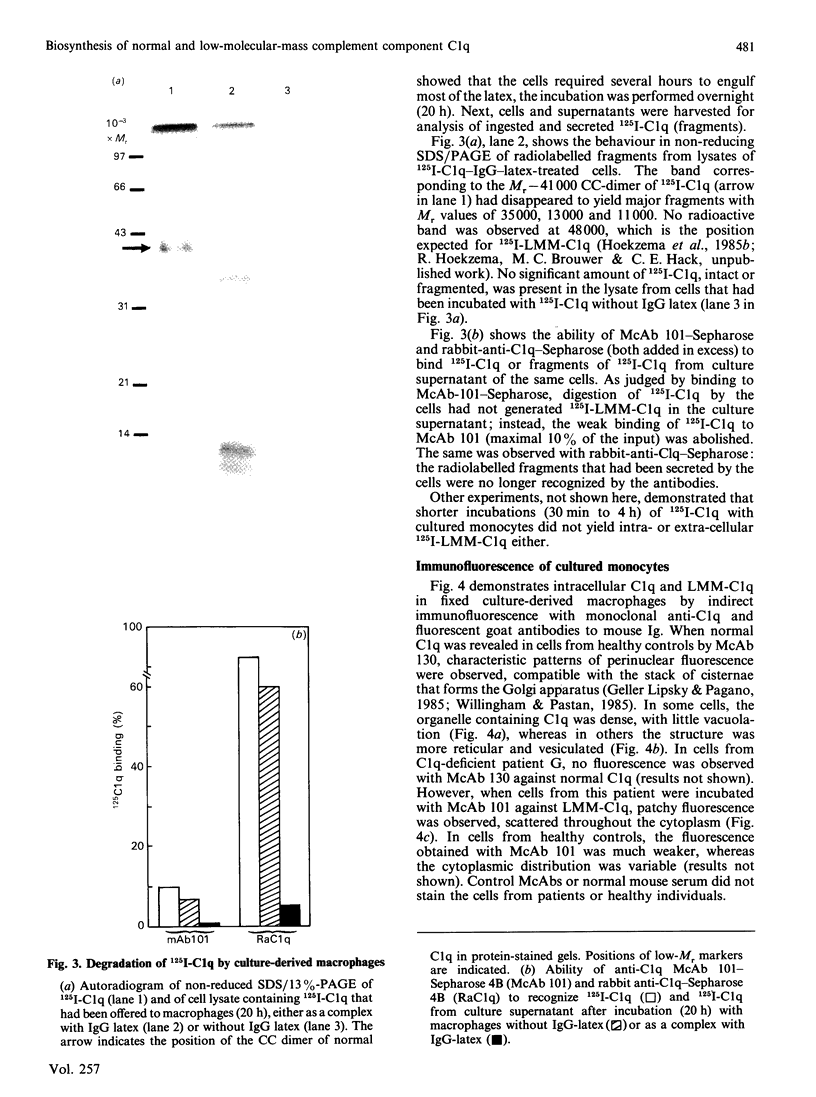
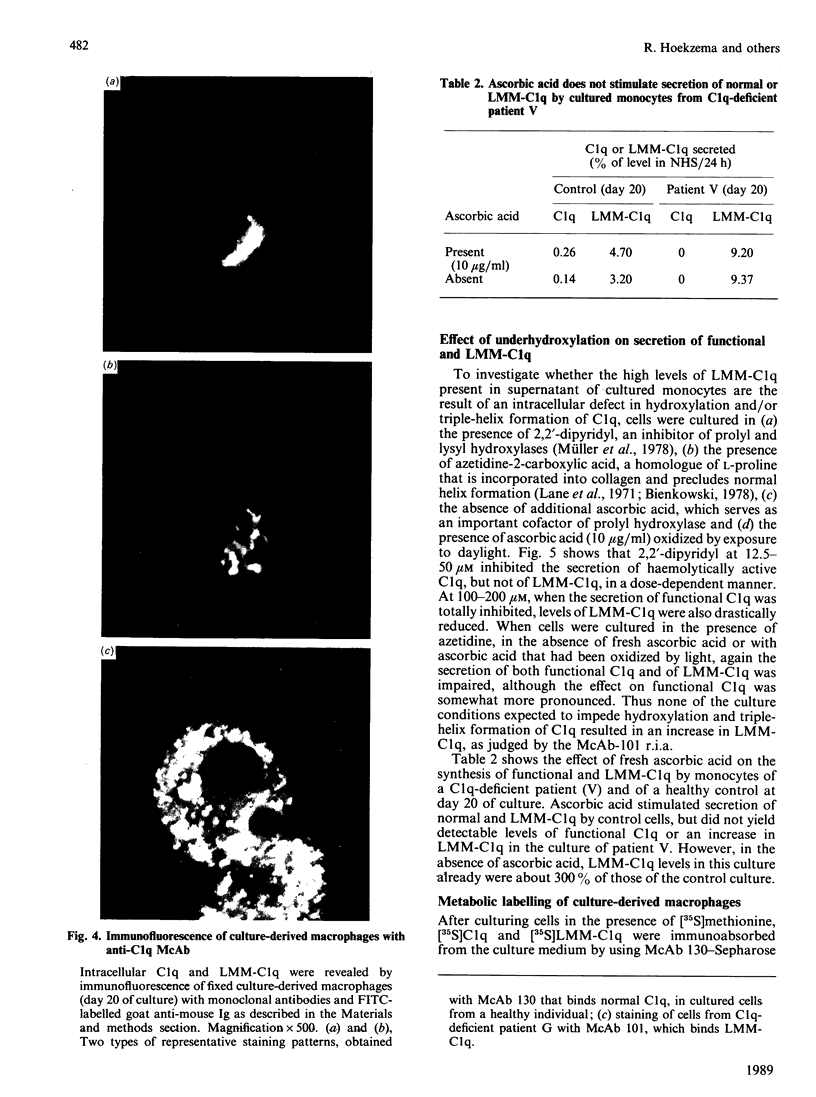
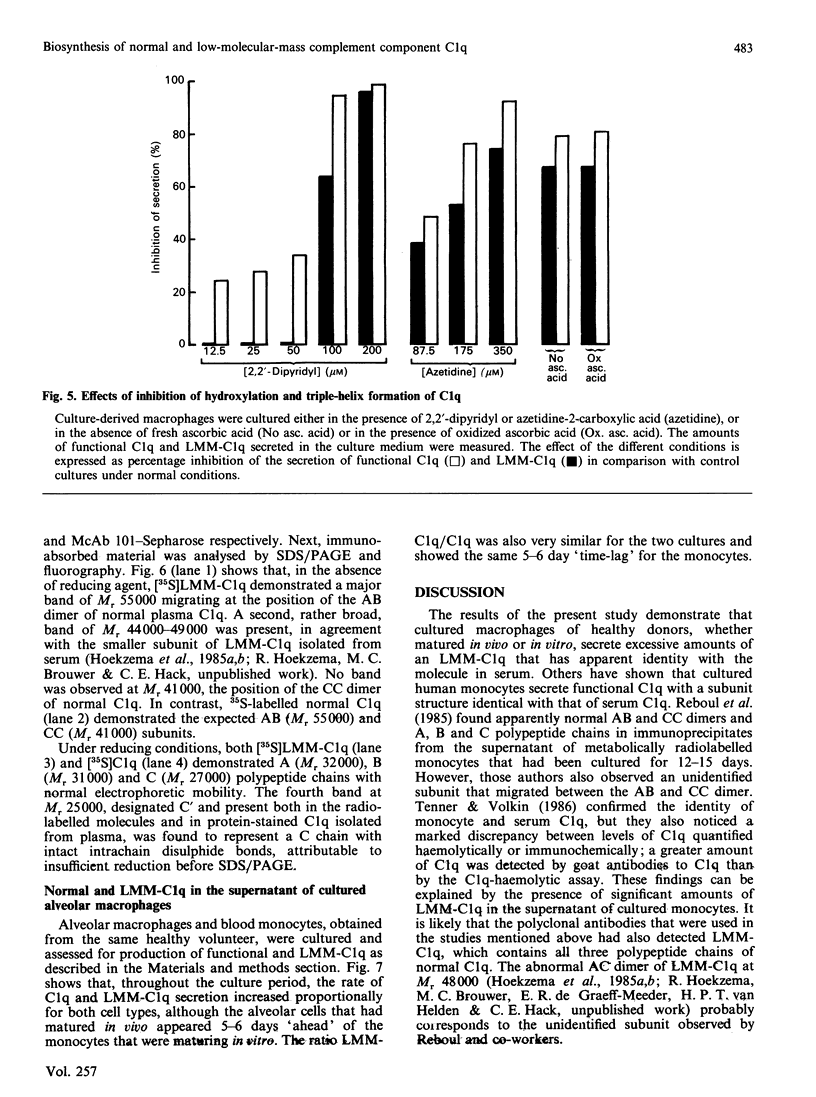
High levels of low-molecular-mass complement component C1q (LMM-C1q), a haemolytically inactive form of C1q, are found in serum of individuals with inherited complete (functional) C1q deficiency and in serum of patients with systemic lupus erythematosus, whereas lower levels are present in normal serum [Hoekzema, Hannema, Swaak, Paardekooper & Hack (1985) J. Immunol. 135, 265-271]. To investigate whether LMM-C1q is a (by-)product of C1q synthesis or the result of degradation of C1q, cultures of blood monocytes and of alveolar macrophages, which secrete functional C1q, were studied. A considerable portion of C1q-like protein secreted by these cells was found to be LMM-C1q. In contrast with the C1q fragments that resulted from degradation of normal C1q during phagocytosis, culture-derived LMM-C1q appeared to be identical with LMM-C1q found in serum, as judged by sedimentation behaviour, subunit structure and recognition by poly- and mono-clonal antibodies raised against C1q. The presence of LMM-C1q in cytoplasmic organelles compatible with the Golgi apparatus and the inability to generate LMM-C1q by impeding hydroxylation and triple-helix formation of C1q further argues against degradation as its source. Monocyte cultures of homozygous probands from two families with complete functional C1q deficiency reflected the abnormalities in serum, i.e. absence of functional C1q, but increased levels of LMM-C1q. By contrast, secretion of C1q and LMM-C1q by cells from healthy individuals was clearly co-ordinate, indicating that LMM-C1q in serum may provide a unique marker of C1q synthesis in vivo.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Al-Adnani M. S., McGee J. O. Clq production and secretion by fibroblasts. Nature. 1976 Sep 9;263(5573):145–146. doi: 10.1038/263145a0. [DOI] [PubMed] [Google Scholar]
- Bensa J. C., Reboul A., Colomb M. G. Biosynthesis in vitro of complement subcomponents C1q, C1s and C1 inhibitor by resting and stimulated human monocytes. Biochem J. 1983 Nov 15;216(2):385–392. doi: 10.1042/bj2160385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bienkowski R. S., Baum B. J., Crystal R. G. Fibroblasts degrade newly synthesised collagen within the cell before secretion. Nature. 1978 Nov 23;276(5686):413–416. doi: 10.1038/276413a0. [DOI] [PubMed] [Google Scholar]
- Bing D. H., Spurlock S. E., Bern M. M. Synthesis of the first component of complement by primary cultures of human tumors of the colon and urogenital tract and comparable normal tissue. Clin Immunol Immunopathol. 1975 Sep;4(3):341–351. doi: 10.1016/0090-1229(75)90003-3. [DOI] [PubMed] [Google Scholar]
- Bobak D. A., Gaither T. A., Frank M. M., Tenner A. J. Modulation of FcR function by complement: subcomponent C1q enhances the phagocytosis of IgG-opsonized targets by human monocytes and culture-derived macrophages. J Immunol. 1987 Feb 15;138(4):1150–1156. [PubMed] [Google Scholar]
- Colten H. R., Gordon J. M., Borsos T., Rapp H. J. Synthesis of the first component of human complement in vitro. J Exp Med. 1968 Oct 1;128(4):595–604. doi: 10.1084/jem.128.4.595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Boer M., Reijneke R., Van de Griend R. J., Loos J. A., Roos D. Large-scale purification and cryopreservation of human monocytes. J Immunol Methods. 1981;43(2):225–239. doi: 10.1016/0022-1759(81)90027-2. [DOI] [PubMed] [Google Scholar]
- Freedman R. Protein chemistry. Folding into the right shape. Nature. 1987 Sep 17;329(6136):196–197. doi: 10.1038/329196a0. [DOI] [PubMed] [Google Scholar]
- Geesin J., Murad S., Pinnell S. R. Ascorbic acid stimulates collagen production without altering intracellular degradation in cultured human skin fibroblasts. Biochim Biophys Acta. 1986 Apr 29;886(2):272–274. doi: 10.1016/0167-4889(86)90145-x. [DOI] [PubMed] [Google Scholar]
- Hack C. E., Eerenberg-Belmer A. J., Hannema A. J., Out T. A., Aalberse R. C. Polyethylene glycol enhances the binding of C1q to circulating immune complexes. J Immunol Methods. 1981;44(2):211–221. doi: 10.1016/0022-1759(81)90349-5. [DOI] [PubMed] [Google Scholar]
- Hannema A. J., Kluin-Nelemans J. C., Hack C. E., Eerenberg-Belmer A. J., Mallée C., van Helden H. P. SLE like syndrome and functional deficiency of C1q in members of a large family. Clin Exp Immunol. 1984 Jan;55(1):106–114. [PMC free article] [PubMed] [Google Scholar]
- Hoekzema R., Hannema A. J., Swaak T. J., Paardekooper J., Hack C. E. Low molecular weight C1q in systemic lupus erythematosus. J Immunol. 1985 Jul;135(1):265–271. [PubMed] [Google Scholar]
- Hoekzema R., Martens M., Brouwer M. C., Hack C. E. The distortive mechanism for the activation of complement component C1 supported by studies with a monoclonal antibody against the "arms" of C1q. Mol Immunol. 1988 May;25(5):485–494. doi: 10.1016/0161-5890(88)90169-1. [DOI] [PubMed] [Google Scholar]
- Johnston C. S., Kolb W. P., Haskell B. E. The effect of vitamin C nutriture on complement component C1q concentrations in guinea pig plasma. J Nutr. 1987 Apr;117(4):764–768. doi: 10.1093/jn/117.4.764. [DOI] [PubMed] [Google Scholar]
- Kohler P. F. Maturation of the human complement system. I. Onset time and sites of fetal C1q, C4, C3, and C5 synthesis. J Clin Invest. 1973 Mar;52(3):671–677. doi: 10.1172/JCI107228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai A Fat R. F., van Furth R. In vitro synthesis of some complement components (C1q, C3 and C4) by lymphoid tissues and circulating leucocytes in man. Immunology. 1975 Feb;28(2):359–368. [PMC free article] [PubMed] [Google Scholar]
- Lane J. M., Dehm P., Prockop D. J. Effect of the proline analogue azetidine-2-carboxylic acid on collagen synthesis in vivo. I. Arrest of collagen accumulation in growing chick embryos. Biochim Biophys Acta. 1971 Jun 29;236(3):517–527. doi: 10.1016/0005-2795(71)90235-2. [DOI] [PubMed] [Google Scholar]
- Lipsky N. G., Pagano R. E. A vital stain for the Golgi apparatus. Science. 1985 May 10;228(4700):745–747. doi: 10.1126/science.2581316. [DOI] [PubMed] [Google Scholar]
- Loos M., Heinz H. P. Component deficiencies. 1. The first component: C1q, C1r, C1s. Prog Allergy. 1986;39:212–231. [PubMed] [Google Scholar]
- Loos M. The functions of endogenous C1q, a subcomponent of the first component of complement, as a receptor on the membrane of macrophages. Mol Immunol. 1982 Oct;19(10):1229–1238. doi: 10.1016/0161-5890(82)90288-7. [DOI] [PubMed] [Google Scholar]
- Martin H., Heinz H. P., Reske K., Loos M. Macrophage C1q: characterization of a membrane form of C1q and of multimers of C1q subunits. J Immunol. 1987 Jun 1;138(11):3863–3867. [PubMed] [Google Scholar]
- Morris K. M., Colten H. R., Bing D. H. The first component of complement. A quantitative comparison of its biosynthesis in culture by human epithelial and mesenchymal cells. J Exp Med. 1978 Oct 1;148(4):1007–1019. doi: 10.1084/jem.148.4.1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murad S., Grove D., Lindberg K. A., Reynolds G., Sivarajah A., Pinnell S. R. Regulation of collagen synthesis by ascorbic acid. Proc Natl Acad Sci U S A. 1981 May;78(5):2879–2882. doi: 10.1073/pnas.78.5.2879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murad S., Tajima S., Johnson G. R., Sivarajah S., Pinnell S. R. Collagen synthesis in cultured human skin fibroblasts: effect of ascorbic acid and its analogs. J Invest Dermatol. 1983 Aug;81(2):158–162. doi: 10.1111/1523-1747.ep12543573. [DOI] [PubMed] [Google Scholar]
- Müller W., Hanauske-Abel H., Loos M. Biosynthesis of the first component of complement by human and guinea pig peritoneal macrophages: evidence for an independent production of the C1 subunits. J Immunol. 1978 Oct;121(4):1578–1584. [PubMed] [Google Scholar]
- Rabs U., Martin H., Hitschold T., Golan M. D., Heinz H. P., Loos M. Isolation and characterization of macrophage-derived C1q and its similarities to serum C1q. Eur J Immunol. 1986 Sep;16(9):1183–1186. doi: 10.1002/eji.1830160926. [DOI] [PubMed] [Google Scholar]
- Reboul A., Prandini M. H., Bensa J. C., Colomb M. G. Characterization of C1q, C1s and C-1 Inh synthesized by stimulated human monocytes in vitro. FEBS Lett. 1985 Oct 7;190(1):65–68. doi: 10.1016/0014-5793(85)80428-2. [DOI] [PubMed] [Google Scholar]
- Reid K. B., Solomon E. Biosynthesis of the first component of complement by human fibroblasts. Biochem J. 1977 Dec 1;167(3):647–660. doi: 10.1042/bj1670647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schur P. H. Complement and lupus erythematosus. Arthritis Rheum. 1982 Jul;25(7):793–798. doi: 10.1002/art.1780250715. [DOI] [PubMed] [Google Scholar]
- Skok J., Solomon E., Reid K. B., Thompson R. A. Distinct genes for fibroblast and serum C1q. Nature. 1981 Aug 6;292(5823):549–551. doi: 10.1038/292549a0. [DOI] [PubMed] [Google Scholar]
- Sobel A. T., Bokisch V. A., Müller-Eberhard H. J. C1q deviation test for the detection of immune complexes, aggregates of IgG, and bacterial products in human serum. J Exp Med. 1975 Jul 1;142(1):139–150. doi: 10.1084/jem.142.1.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stecher V. J., Morse J. H., Thorbecke G. J. Sites of production of primate serum proteins associated with complement system. Proc Soc Exp Biol Med. 1967 Feb;124(2):433–438. doi: 10.3181/00379727-124-31758. [DOI] [PubMed] [Google Scholar]
- Tajima S., Pinnell S. R. Regulation of collagen synthesis by ascorbic acid. Ascorbic acid increases type I procollagen mRNA. Biochem Biophys Res Commun. 1982 May 31;106(2):632–637. doi: 10.1016/0006-291x(82)91157-3. [DOI] [PubMed] [Google Scholar]
- Tenner A. J., Cooper N. R. Analysis of receptor-mediated C1q binding to human peripheral blood mononuclear cells. J Immunol. 1980 Oct;125(4):1658–1664. [PubMed] [Google Scholar]
- Tenner A. J., Cooper N. R. Identification of types of cells in human peripheral blood that bind C1q. J Immunol. 1981 Mar;126(3):1174–1179. [PubMed] [Google Scholar]
- Tenner A. J., Cooper N. R. Stimulation of a human polymorphonuclear leukocyte oxidative response by the C1q subunit of the first complement component. J Immunol. 1982 Jun;128(6):2547–2552. [PubMed] [Google Scholar]
- Tenner A. J., Lesavre P. H., Cooper N. R. Purification and radiolabeling of human C1q. J Immunol. 1981 Aug;127(2):648–653. [PubMed] [Google Scholar]
- Tenner A. J., Volkin D. B. Complement subcomponent C1q secreted by cultured human monocytes has subunit structure identical with that of serum C1q. Biochem J. 1986 Jan 15;233(2):451–458. doi: 10.1042/bj2330451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veerhuis R., Klar-Mohamad N., van Es L. A., Daha M. R. Enhanced binding and degradation of the C1q subcomponent of complement by thioglycollate-stimulated guinea pig peritoneal macrophages. Scand J Immunol. 1986 May;23(5):581–587. doi: 10.1111/j.1365-3083.1986.tb01991.x. [DOI] [PubMed] [Google Scholar]
- Veerhuis R., Van Es L. A., Daha M. R. Effects of soluble aggregates of IgG on the binding, uptake and degradation of the C1q subcomponent of complement by adherent guinea pig peritoneal macrophages. Eur J Immunol. 1985 Sep;15(9):881–887. doi: 10.1002/eji.1830150904. [DOI] [PubMed] [Google Scholar]
- Zubler R. H., Lange G., Lambert P. H., Miescher P. A. Detection of immune complexes in unheated sera by modified 125I-Clq binding test. Effect of heating on the binding of Clq by immune complexes and application of the test to systemic lupus erythematosus. J Immunol. 1976 Jan;116(1):232–235. [PubMed] [Google Scholar]
- de Boer M., Roos D. Metabolic comparison between basophils and other leukocytes from human blood. J Immunol. 1986 May 1;136(9):3447–3454. [PubMed] [Google Scholar]