Abstract
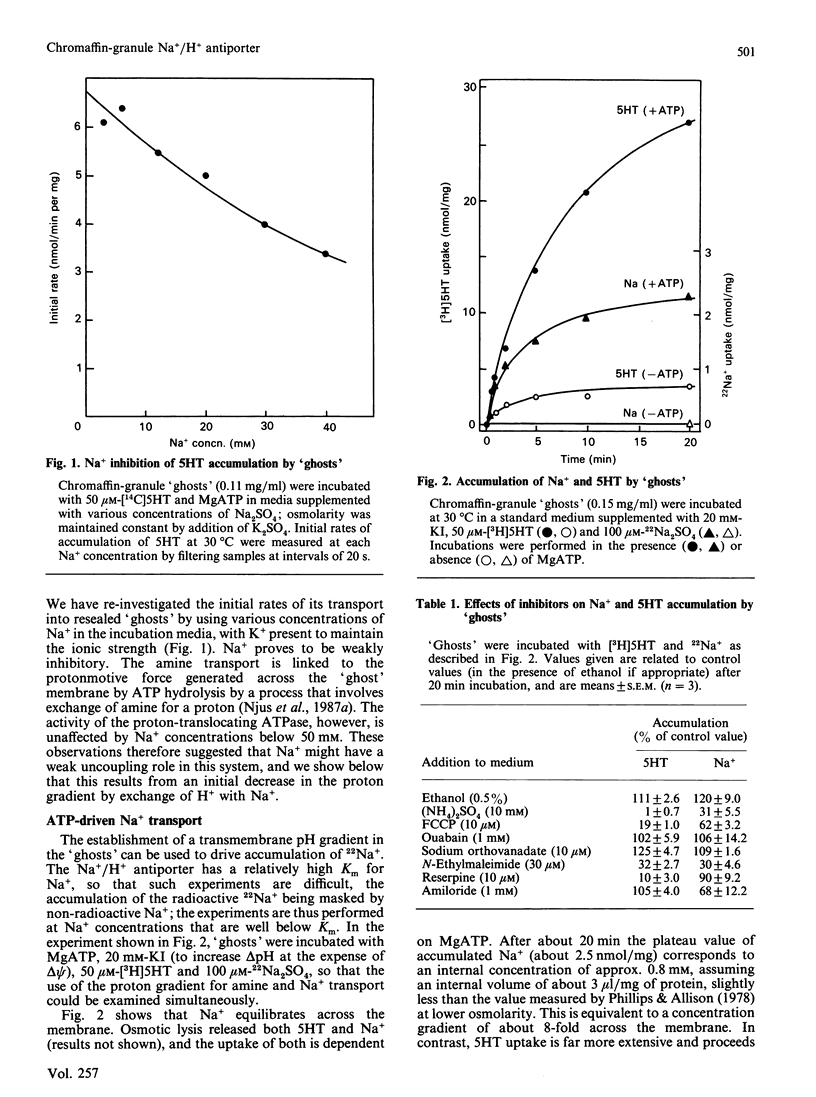
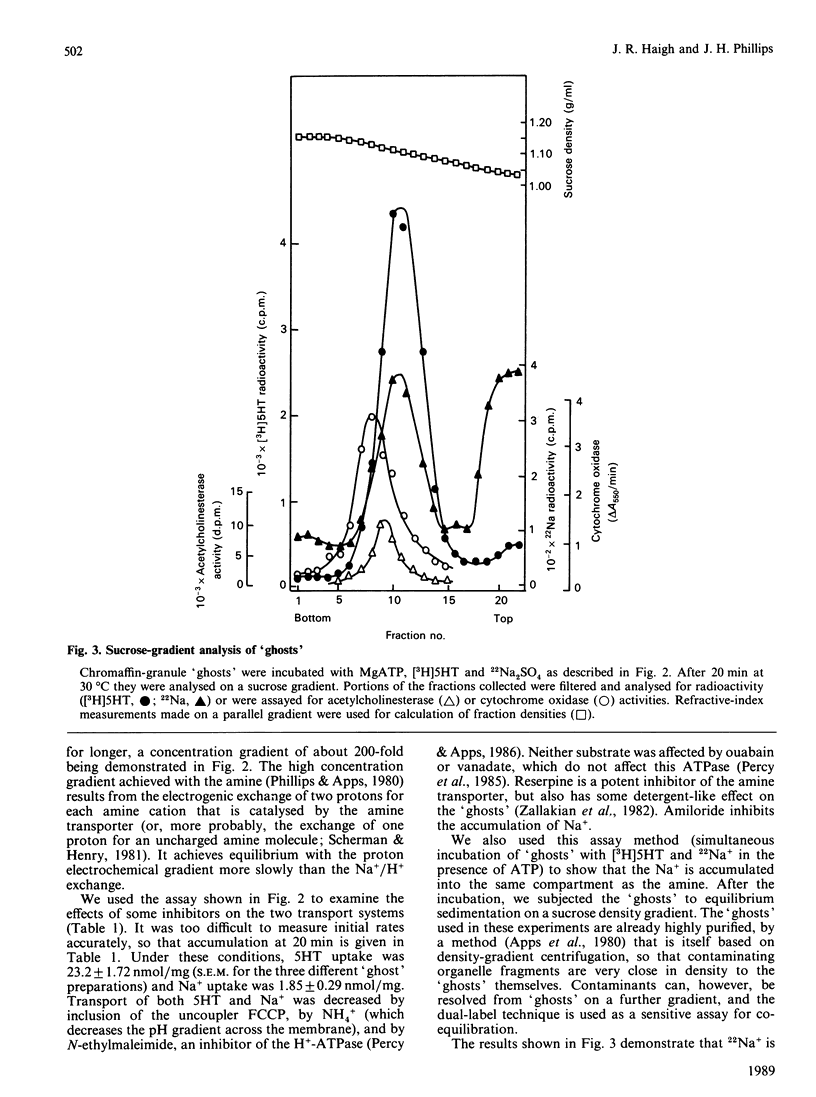
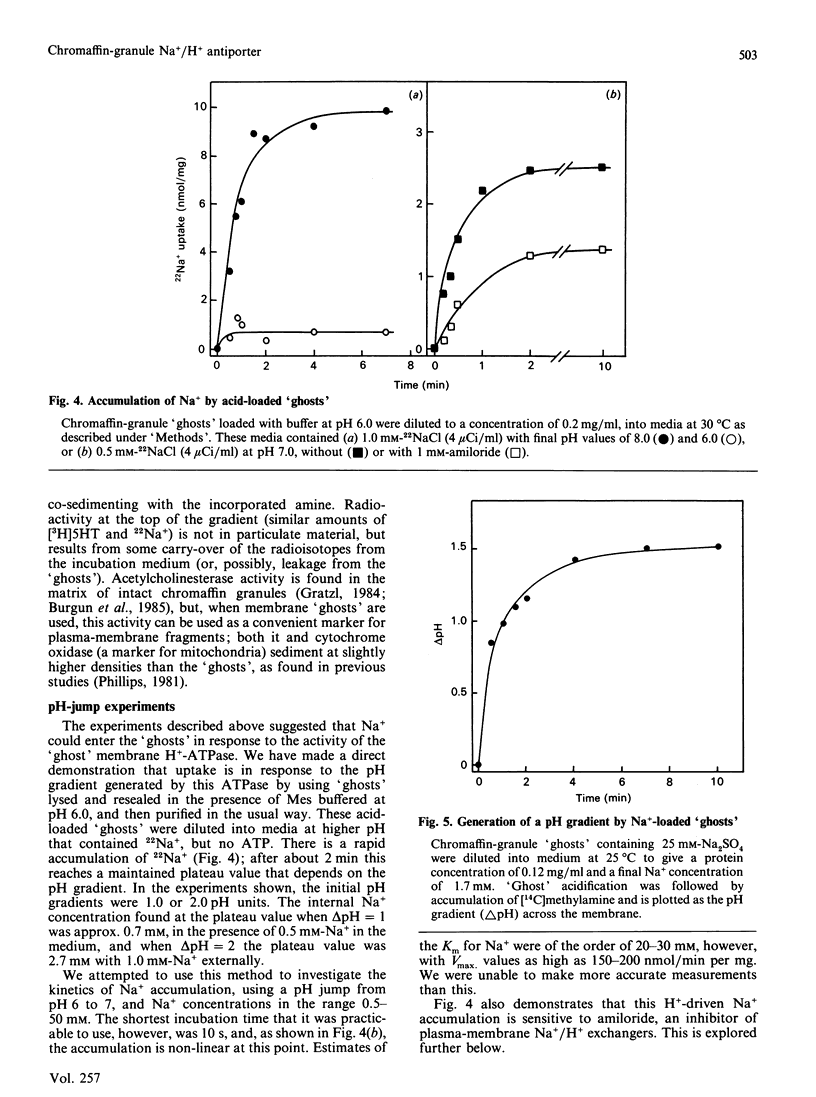
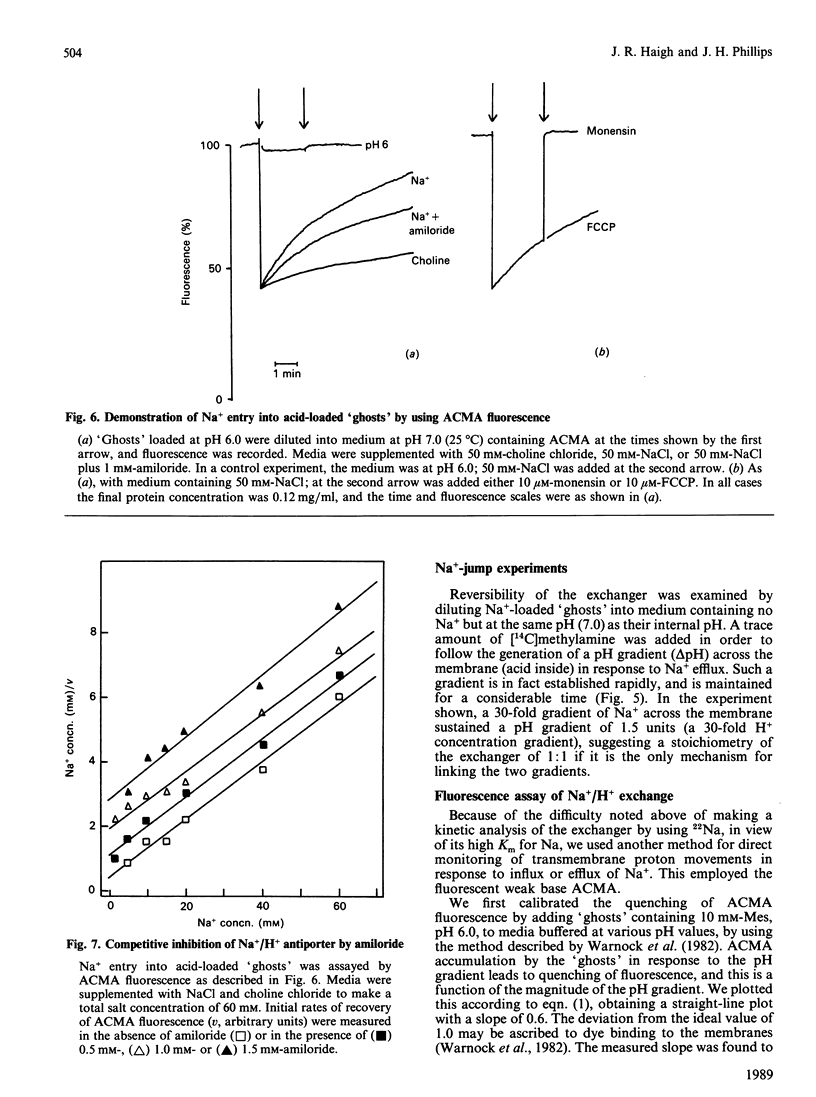
Chromaffin granules, the secretory vesicles of the adrenal medulla, have a Na+/H+ exchange activity in their membranes which brings their proton gradient into equilibrium with a Na+ gradient. This explains why Na+ is mildly inhibitory to amine transport (which is driven by the H+ gradient) The activity can be demonstrated by using accumulation of 22Na+ in response to a pH gradient that is either imposed by diluting membrane 'ghosts' into alkaline media, or generated by ATP hydrolysis. It can also be monitored indirectly by fluorescence measurements in which the pH inside 'ghost' is monitored by quenching of a fluorescent weak base. This method has been used to monitor Na+ entry into acid-loaded 'ghosts' of H+ entry into methylamine accumulation. The exchanger appears to be reversible and non-electrogenic, with a stoichiometry of 1:1. Using an indirect assay we measured an apparent Km for Na+ of 4.7 mM, and a Ki for amiloride, a competitive inhibitor, of 0.26 mM. Direct assays using 22Na+ suggested a higher Km. Ethylisopropylamiloride was not inhibitory.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Apps D. K., Pryde J. G., Sutton R., Phillips J. H. Inhibition of adenosine triphosphatase, 5-hydroxytryptamine transport and proton-translocation activities of resealed chromaffin-granule 'ghosts'. Biochem J. 1980 Aug 15;190(2):273–282. doi: 10.1042/bj1900273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aronson P. S. Kinetic properties of the plasma membrane Na+-H+ exchanger. Annu Rev Physiol. 1985;47:545–560. doi: 10.1146/annurev.ph.47.030185.002553. [DOI] [PubMed] [Google Scholar]
- Bevington A., Briggs R. W., Radda G. K., Thulborn K. R. Phosphorus-31 nuclear magnetic resonance studies of pig adrenal glands. Neuroscience. 1984 Jan;11(1):281–286. doi: 10.1016/0306-4522(84)90231-8. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Burgun C., Martinez de Muñoz D., Aunis D. Osmotic fragility of chromaffin granules prepared under isoosmotic or hyperosmotic conditions and localization of acetylcholinesterase. Biochim Biophys Acta. 1985 May 8;839(3):219–227. doi: 10.1016/0304-4165(85)90001-7. [DOI] [PubMed] [Google Scholar]
- Carty S. E., Johnson R. G., Vaughan T., Pallant A., Scarpa A. Amine transport into chromaffin ghosts. Kinetic measurements of net uptake of biologically and pharmacologically relevant amines using an on-line amperometric technique. Eur J Biochem. 1985 Mar 15;147(3):447–452. [PubMed] [Google Scholar]
- Gratzl M. Distribution of chromaffin secretory vesicles, acetylcholinesterase, and lysosomal enzymes in sucrose and Percoll gradients. Anal Biochem. 1984 Oct;142(1):148–154. doi: 10.1016/0003-2697(84)90529-3. [DOI] [PubMed] [Google Scholar]
- Holz R. W., Senter R. A., Sharp R. R. Evidence that the H+ electrochemical gradient across membranes of chromaffin granules is not involved in exocytosis. J Biol Chem. 1983 Jun 25;258(12):7506–7513. [PubMed] [Google Scholar]
- Johnson R. G., Beers M. F., Scarpa A. H+ ATPase of chromaffin granules. Kinetics, regulation, and stoichiometry. J Biol Chem. 1982 Sep 25;257(18):10701–10707. [PubMed] [Google Scholar]
- Johnson R. G., Scarpa A. Internal pH of isolated chromaffin vesicles. J Biol Chem. 1976 Apr 10;251(7):2189–2191. [PubMed] [Google Scholar]
- Kopell W. N., Westhead E. W. Osmotic pressures of solutions of ATP and catecholamines relating to storage in chromaffin granules. J Biol Chem. 1982 May 25;257(10):5707–5710. [PubMed] [Google Scholar]
- Krieger-Brauer H., Gratzl M. Uptake of Ca2+ by isolated secretory vesicles from adrenal medulla. Biochim Biophys Acta. 1982 Sep 24;691(1):61–70. doi: 10.1016/0005-2736(82)90214-0. [DOI] [PubMed] [Google Scholar]
- Lazdunski M., Frelin C., Vigne P. The sodium/hydrogen exchange system in cardiac cells: its biochemical and pharmacological properties and its role in regulating internal concentrations of sodium and internal pH. J Mol Cell Cardiol. 1985 Nov;17(11):1029–1042. doi: 10.1016/s0022-2828(85)80119-x. [DOI] [PubMed] [Google Scholar]
- Mellman I., Fuchs R., Helenius A. Acidification of the endocytic and exocytic pathways. Annu Rev Biochem. 1986;55:663–700. doi: 10.1146/annurev.bi.55.070186.003311. [DOI] [PubMed] [Google Scholar]
- Njus D., Kelley P. M., Harnadek G. J. Bioenergetics of secretory vesicles. Biochim Biophys Acta. 1986;853(3-4):237–265. doi: 10.1016/0304-4173(87)90003-6. [DOI] [PubMed] [Google Scholar]
- Njus D., Kelley P. M., Harnadek G. J., Pacquing Y. V. Mechanism of ascorbic acid regeneration mediated by cytochrome b561. Ann N Y Acad Sci. 1987;493:108–119. doi: 10.1111/j.1749-6632.1987.tb27188.x. [DOI] [PubMed] [Google Scholar]
- Ornberg R. L., Kuijpers G. A., Leapman R. D. Electron probe microanalysis of the subcellular compartments of bovine adrenal chromaffin cells. Comparison of chromaffin granules in situ and in vitro. J Biol Chem. 1988 Jan 25;263(3):1488–1493. [PubMed] [Google Scholar]
- Percy J. M., Apps D. K. Proton-translocating adenosine triphosphatase of chromaffin-granule membranes. The active site is in the largest (70 kDa) subunit. Biochem J. 1986 Oct 1;239(1):77–81. doi: 10.1042/bj2390077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Percy J. M., Pryde J. G., Apps D. K. Isolation of ATPase I, the proton pump of chromaffin-granule membranes. Biochem J. 1985 Nov 1;231(3):557–564. doi: 10.1042/bj2310557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips J. H., Allison V. P. Proton translocation of the bovine chromaffin-granule membrane. Biochem J. 1978 Mar 15;170(3):661–672. doi: 10.1042/bj1700661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips J. H., Apps D. K. Stoichiometry of catecholamine/proton exchange across the chromaffin-granule membrane. Biochem J. 1980 Oct 15;192(1):273–278. doi: 10.1042/bj1920273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips J. H. Steady-state kinetics of catecholamine transport by chromaffin-granule "ghosts". Biochem J. 1974 Nov;144(2):319–325. doi: 10.1042/bj1440319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips J. H. Transport of Ca2+ and Na+ across the chromaffin-granule membrane. Biochem J. 1981 Oct 15;200(1):99–107. doi: 10.1042/bj2000099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips J. H. Transport of catecholamines by resealed chromaffin-grnaule "ghosts". Biochem J. 1974 Nov;144(2):311–318. doi: 10.1042/bj1440311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherman D., Henry J. P. pH-dependence of the ATP-driven uptake of noradrenaline by bovine chromaffin-granule ghosts. Eur J Biochem. 1981 Jun 1;116(3):535–539. doi: 10.1111/j.1432-1033.1981.tb05369.x. [DOI] [PubMed] [Google Scholar]
- Seiler S. M., Cragoe E. J., Jr, Jones L. R. Demonstration of a Na+/H+ exchange activity in purified canine cardiac sarcolemmal vesicles. J Biol Chem. 1985 Apr 25;260(8):4869–4876. [PubMed] [Google Scholar]
- Warnock D. G., Reenstra W. W., Yee V. J. Na+/H+ antiporter of brush border vesicles: studies with acridine orange uptake. Am J Physiol. 1982 Jun;242(6):F733–F739. doi: 10.1152/ajprenal.1982.242.6.F733. [DOI] [PubMed] [Google Scholar]
- Weber A., Winkler H. Specificity and mechanism of nucleotide uptake by adrenal chromaffin granules. Neuroscience. 1981;6(11):2269–2276. doi: 10.1016/0306-4522(81)90016-6. [DOI] [PubMed] [Google Scholar]
- Zallakian M., Knoth J., Metropoulos G. E., Njus D. Multiple effects of reserpine on chromaffin-granule in membranes. Biochemistry. 1982 Mar 2;21(5):1051–1055. doi: 10.1021/bi00534a035. [DOI] [PubMed] [Google Scholar]


