Abstract
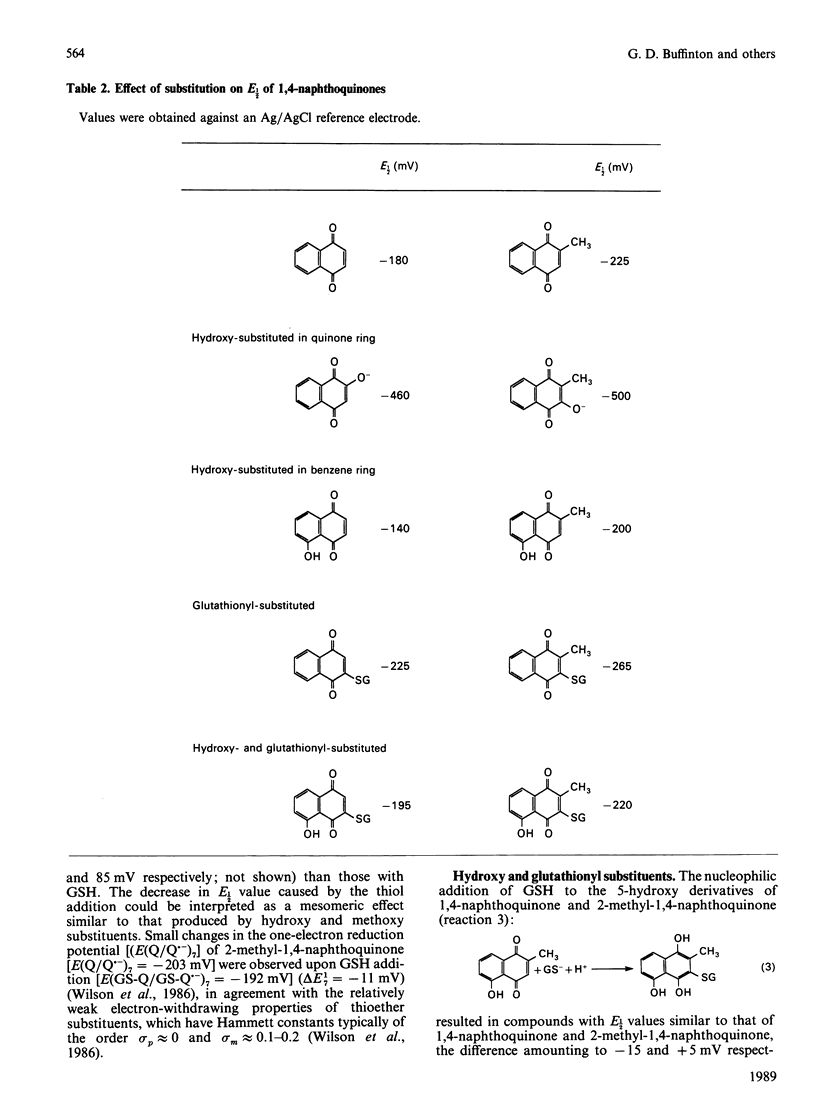
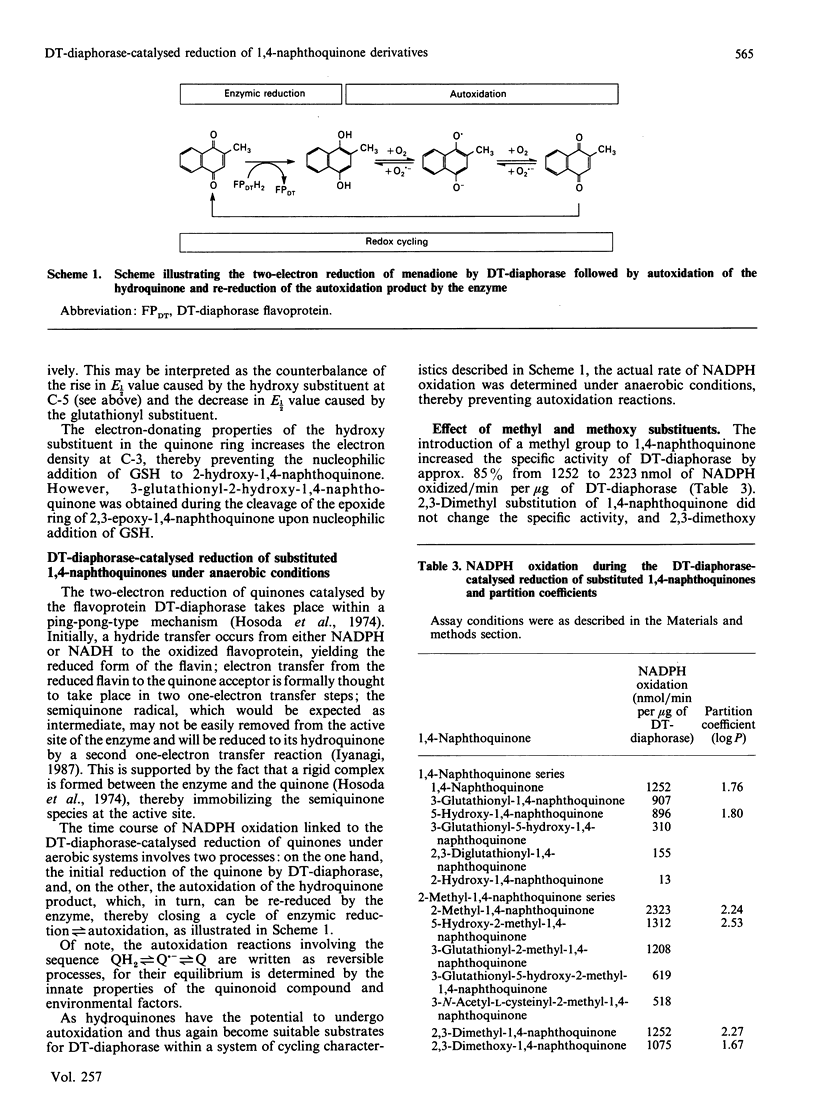
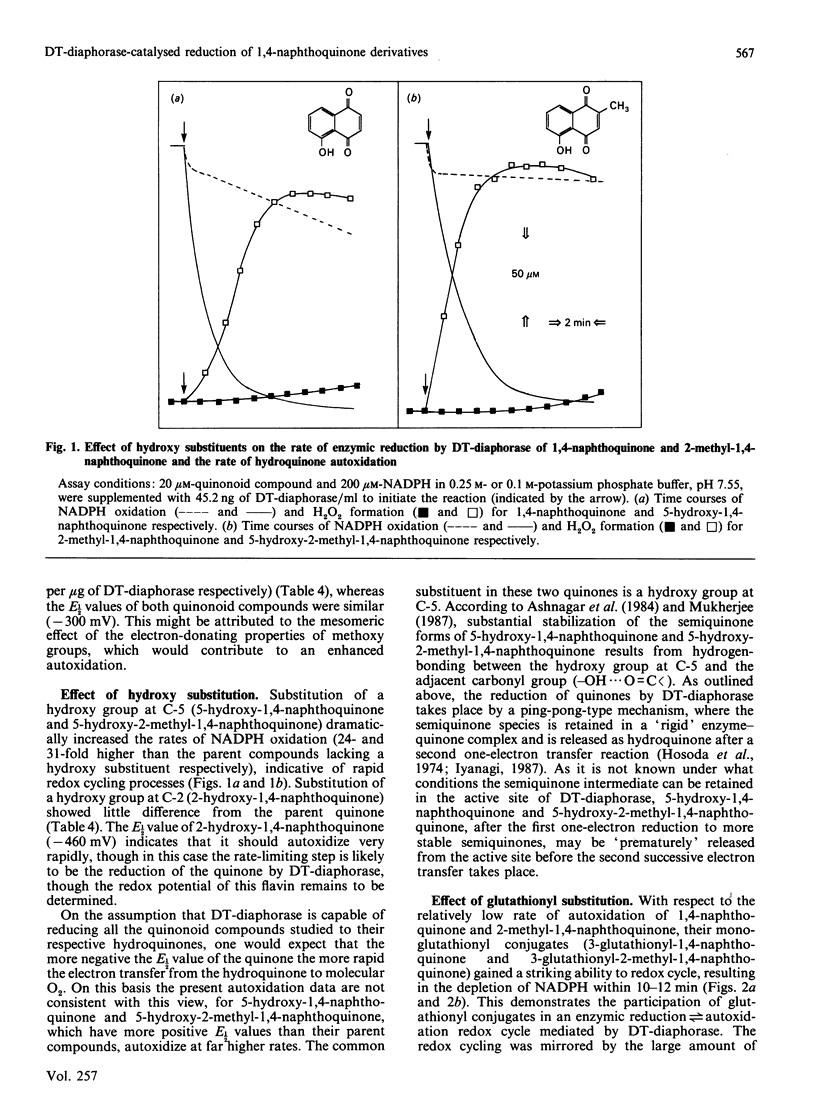
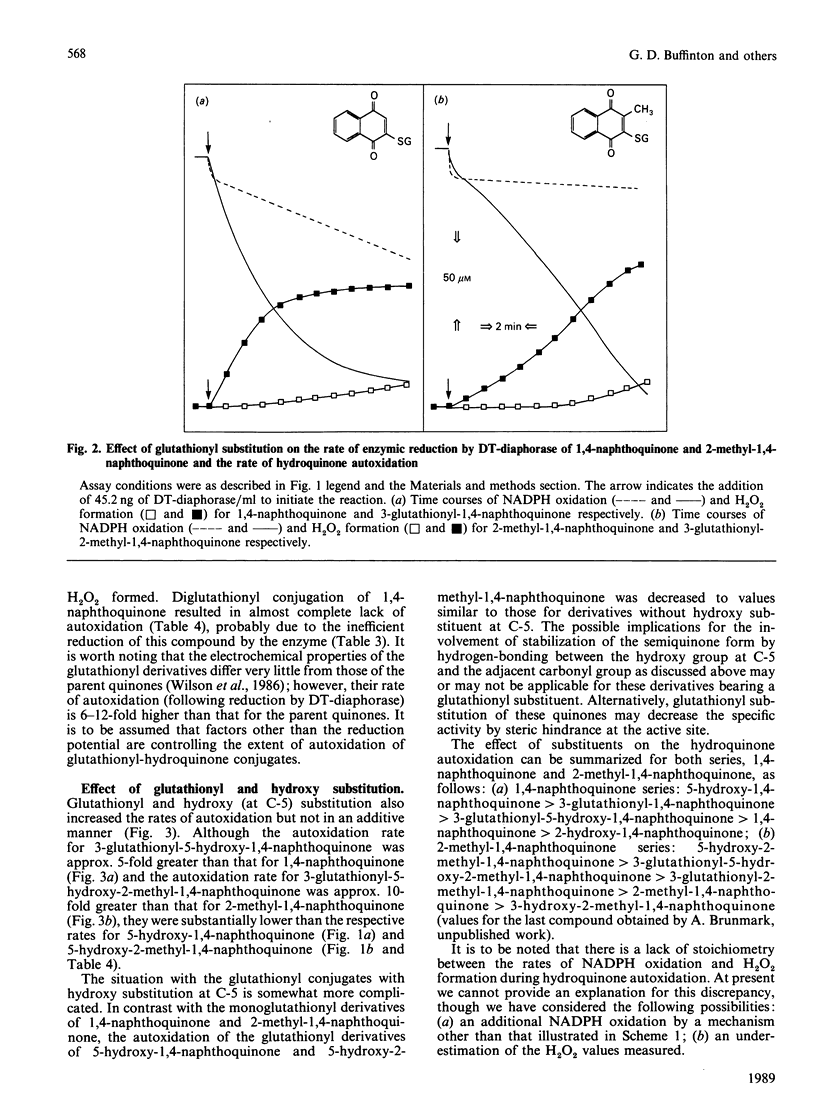
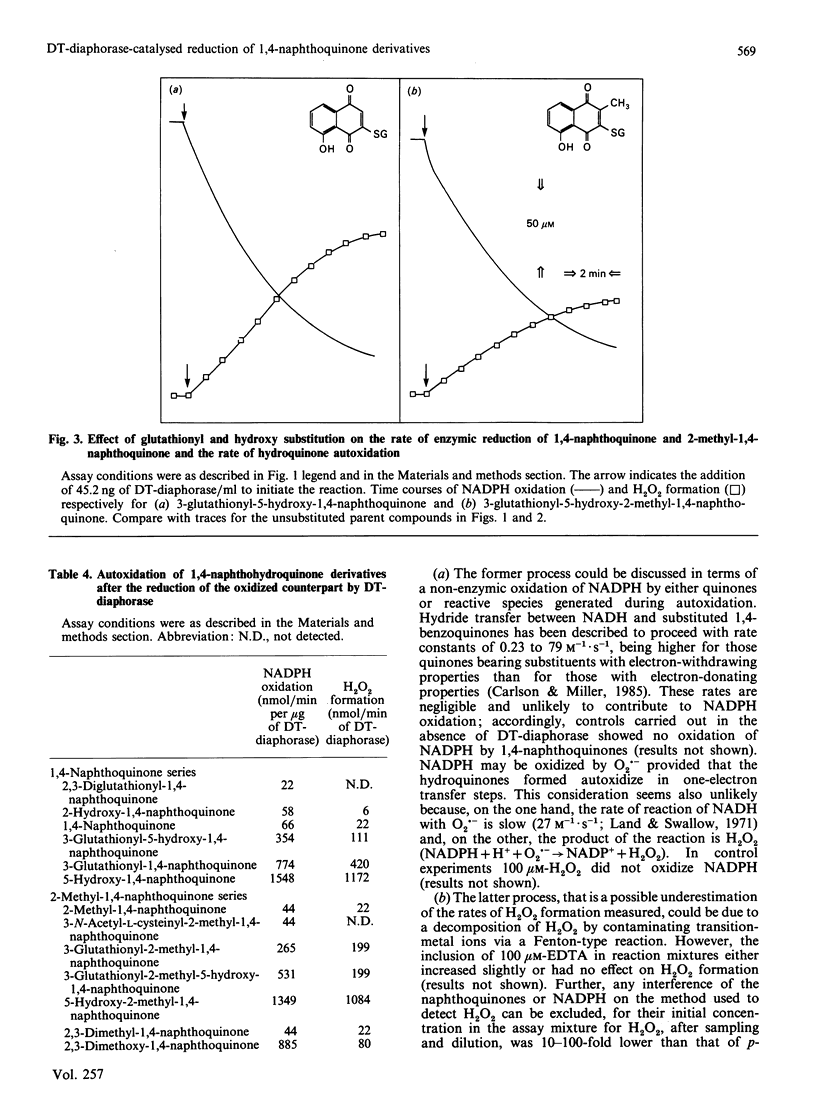
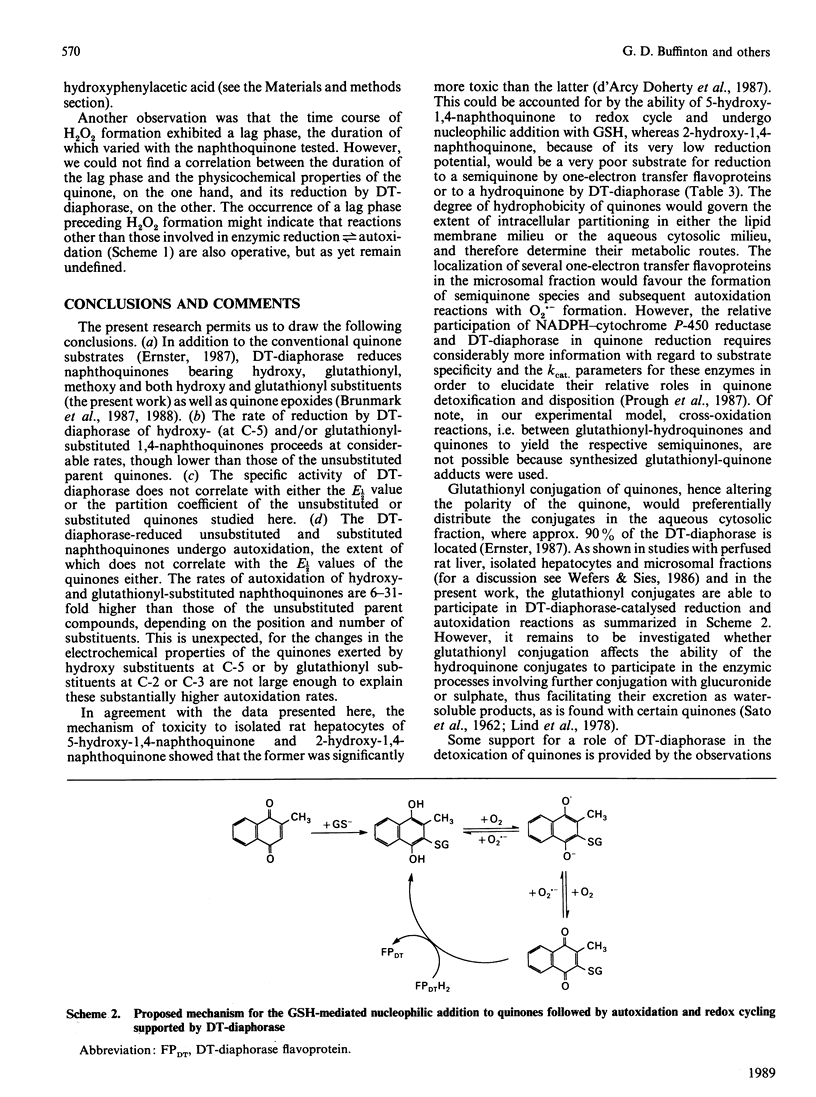
DT-diaphorase catalysed the reduction of 1,4-naphthoquinones with hydroxy, methyl, methoxy and glutathionyl substituents at the expense of reducing equivalents from NADPH. The initial rates of quinone reduction did not correlate with either the half-wave reduction potential (E1/2) value (determined by h.p.l.c. with electrochemical detection against an Ag/AgCl reference electrode) or the partition coefficient of the quinones. After their reduction by DT-diaphorase the 1,4-naphthoquinone derivatives autoxidized at distinct rates, the extent of which was influenced by the nature of the substituents. Thus for the 1,4-naphthoquinone series the following order of rate of autoxidation was found: 5-hydroxy-1,4-naphthoquinone greater than 3-glutathionyl-1,4-naphthoquinone greater than 5-hydroxy-3-glutathionyl-1,4-naphthoquinone greater than 1,4-naphthoquinone greater than 2-hydroxy-1,4-naphthoquinone. For the 2-methyl-1,4-naphthoquinone (menadione) series the following order was observed: 5-hydroxy-2-methyl-1,4-naphthoquinone greater than 3-glutathionyl-5-hydroxy-2-methyl-1,4-naphthoquinone greater than 3-glutathionyl-2-methyl-1,4-naphthoquinone greater than 2-methyl-1,4-naphthoquinone greater than 3-hydroxy-2-methyl-1,4-naphthoquinone. The autoxidized naphthohydroquinone derivatives were re-reduced by DT-diaphorase, thus closing a cycle of enzymic reduction in equilibrium autoxidation. This was expressed as an excess of NADPH oxidized over the initial concentration of quinone present as well as H2O2 formation. These findings demonstrate that glutathionyl conjugates of 1,4-naphthoquinone and 2-methyl-1,4-naphthoquinone and those of their respective 5-hydroxy derivatives are able to act as substrates for DT-diaphorase and that they also autoxidize at rates higher than those for the unsubstituted parent compounds. These results are discussed in terms of the cellular role of DT-diaphorase in the reduction of hydroxy- or glutathionyl-substituted naphthoquinones as well as the further conjugation of these hydroquinones with glucuronide or sulphate within the cellular milieu, thereby facilitating their disposal from the cells.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ashnagar A., Bruce J. M., Dutton P. L., Prince R. C. One- and two-electron reduction of hydroxy-1,4-naphthoquinones and hydroxy-9,10-anthraquinones. The role of internal hydrogen bonding and its bearing on the redox chemistry of the anthracycline antitumour quinones. Biochim Biophys Acta. 1984 Oct 16;801(3):351–359. doi: 10.1016/0304-4165(84)90138-7. [DOI] [PubMed] [Google Scholar]
- Boveris A. Determination of the production of superoxide radicals and hydrogen peroxide in mitochondria. Methods Enzymol. 1984;105:429–435. doi: 10.1016/s0076-6879(84)05060-6. [DOI] [PubMed] [Google Scholar]
- Brunmark A., Cadenas E. Electronically excited state generation during the reaction of p-benzoquinone with H2O2. Relation to product formation: 2-OH- and 2,3-epoxy-p-benzoquinone. The effect of glutathione. Free Radic Biol Med. 1987;3(3):169–180. doi: 10.1016/0891-5849(87)90002-5. [DOI] [PubMed] [Google Scholar]
- Brunmark A., Cadenas E., Lind C., Segura-Aguilar J., Ernster L. DT-diaphorase-catalyzed two-electron reduction of quinone epoxides. Free Radic Biol Med. 1987;3(3):181–188. doi: 10.1016/0891-5849(87)90003-7. [DOI] [PubMed] [Google Scholar]
- Brunmark A., Cadenas E., Segura-Aguilar J., Lind C., Ernster L. DT-diaphorase-catalyzed two-electron reduction of various p-benzoquinone- and 1,4-naphthoquinone epoxides. Free Radic Biol Med. 1988;5(3):133–143. doi: 10.1016/0891-5849(88)90076-7. [DOI] [PubMed] [Google Scholar]
- Danner D. J., Brignac P. J., Jr, Arceneaux D., Patel V. The oxidation of phenol and its reaction product by horseradish peroxidase and hydrogen peroxide. Arch Biochem Biophys. 1973 Jun;156(2):759–763. doi: 10.1016/0003-9861(73)90329-9. [DOI] [PubMed] [Google Scholar]
- Dodd N. J., Mukherjee T. Free radical formation from anthracycline antitumour agents and model systems--I. Model naphthoquinones and anthraquinones. Biochem Pharmacol. 1984 Feb 1;33(3):379–385. doi: 10.1016/0006-2952(84)90229-6. [DOI] [PubMed] [Google Scholar]
- Doherty M. D., Cohen G. M., Smith M. T. Mechanisms of toxic injury to isolated hepatocytes by 1-naphthol. Biochem Pharmacol. 1984 Feb 15;33(4):543–549. doi: 10.1016/0006-2952(84)90305-8. [DOI] [PubMed] [Google Scholar]
- Dubin P., Wright K. L. Reduction of azo food dyes in cultures of Proteus vulgaris. Xenobiotica. 1975 Sep;5(9):563–571. doi: 10.3109/00498257509056126. [DOI] [PubMed] [Google Scholar]
- Esterbauer H., Pölsler G., Fodor G. Effect of methoxy-p-benzoquinones and methoxy-p-hydroquinones on DNA synthesis in Ehrlich ascites tumor cells. Acta Biochim Biophys Hung. 1987;22(2-3):195–204. [PubMed] [Google Scholar]
- Flaig W., Beutelspacher H., Riemer H., Kälke E. Einfluss von Substituenten auf das Redoxpotential substituierter Benzochinone-(1.4) Justus Liebigs Ann Chem. 1969;719:96–111. doi: 10.1002/jlac.19687190112. [DOI] [PubMed] [Google Scholar]
- Grant T. W., Doherty M. D., Odowole D., Sales K. D., Cohen G. M. Semiquinone anion radicals formed by the reaction of quinones with glutathione or amino acids. FEBS Lett. 1986 Jun 9;201(2):296–300. doi: 10.1016/0014-5793(86)80627-5. [DOI] [PubMed] [Google Scholar]
- Hinsberg W. D., 3rd, Milby K. H., Zare R. N. Determination of insulin in serum by enzyme immunoassay with fluorimetric detection. Anal Chem. 1981 Aug;53(9):1509–1512. doi: 10.1021/ac00232a048. [DOI] [PubMed] [Google Scholar]
- Hodnett E. M., Wongwiechintana C., Dunn W. J., 3rd, Marrs P. Substituted 1,4-naphthoquinones vs. the ascitic sarcoma 180 of mice. J Med Chem. 1983 Apr;26(4):570–574. doi: 10.1021/jm00358a021. [DOI] [PubMed] [Google Scholar]
- Hosoda S., Nakamura W., Hayashi K. Properties and reaction mechanism of DT diaphorase from rat liver. J Biol Chem. 1974 Oct 25;249(20):6416–6423. [PubMed] [Google Scholar]
- Höjeberg B., Blomberg K., Stenberg S., Lind C. Biospecific adsorption of hepatic DT-diaphorase on immobilized dicoumarol. I. Purification of cytosolic DT-diaphorase from control and 3-methylcholanthrene-treated rats. Arch Biochem Biophys. 1981 Mar;207(1):205–216. doi: 10.1016/0003-9861(81)90026-6. [DOI] [PubMed] [Google Scholar]
- Iyanagi T., Yamazaki I. One-electron-transfer reactions in biochemical systems. V. Difference in the mechanism of quinone reduction by the NADH dehydrogenase and the NAD(P)H dehydrogenase (DT-diaphorase). Biochim Biophys Acta. 1970 Sep 1;216(2):282–294. doi: 10.1016/0005-2728(70)90220-3. [DOI] [PubMed] [Google Scholar]
- Land E. J., Swallow A. J. One-electron reactions in biochemical systems as studied by pulse radiolysis. IV. Oxidation of dihydronicotinamide-adenine dinucleotide. Biochim Biophys Acta. 1971 Apr 6;234(1):34–42. doi: 10.1016/0005-2728(71)90126-5. [DOI] [PubMed] [Google Scholar]
- Lind C., Vadi H., Ernster L. Metabolism of benzo(a)pyrene-3,6-quinone and 3-hydroxybenzo(a)pyrene in liver microsomes from 3-methylcholanthrene-treated rats. A possible role of DT-diaphorase in the formation of glucuronyl conjugates. Arch Biochem Biophys. 1978 Sep;190(1):97–108. doi: 10.1016/0003-9861(78)90256-4. [DOI] [PubMed] [Google Scholar]
- Matsuura K., Bowyer J. R., Ohnishi T., Dutton P. L. Inhibition of electron transfer by 3-alkyl-2-hydroxy-1,4-naphthoquinones in the ubiquinol-cytochrome c oxidoreductases of Rhodopseudomonas sphaeroides and mammalian mitochondria. Interaction with a ubiquinone-binding site and the Rieske iron-sulfur cluster. J Biol Chem. 1983 Feb 10;258(3):1571–1579. [PubMed] [Google Scholar]
- Moore G. A., Rossi L., Nicotera P., Orrenius S., O'Brien P. J. Quinone toxicity in hepatocytes: studies on mitochondrial Ca2+ release induced by benzoquinone derivatives. Arch Biochem Biophys. 1987 Dec;259(2):283–295. doi: 10.1016/0003-9861(87)90495-4. [DOI] [PubMed] [Google Scholar]
- NICKERSON W. J., FALCONE G., STRAUSS G. STUDIES ON QUINONE-THIOETHERS. I. MECHANISM OF FORMATION AND PROPERTIES OF THIODIONE. Biochemistry. 1963 May-Jun;2:537–543. doi: 10.1021/bi00903a025. [DOI] [PubMed] [Google Scholar]
- Powis G., Appel P. L. Relationship of the single-electron reduction potential of quinones to their reduction by flavoproteins. Biochem Pharmacol. 1980 Oct 1;29(19):2567–2572. doi: 10.1016/0006-2952(80)90068-4. [DOI] [PubMed] [Google Scholar]
- Pritsos C. A., Jensen D. E., Pisani D., Pardini R. S. Involvement of superoxide in the interaction of 2,3-dichloro-1,4-naphthoquinone with mitochondrial membranes. Arch Biochem Biophys. 1982 Aug;217(1):98–109. doi: 10.1016/0003-9861(82)90483-0. [DOI] [PubMed] [Google Scholar]
- Ross D., Thor H., Orrenius S., Moldeus P. Interaction of menadione (2-methyl-1,4-naphthoquinone) with glutathione. Chem Biol Interact. 1985 Oct;55(1-2):177–184. doi: 10.1016/s0009-2797(85)80126-5. [DOI] [PubMed] [Google Scholar]
- Takahashi N., Schreiber J., Fischer V., Mason R. P. Formation of glutathione-conjugated semiquinones by the reaction of quinones with glutathione: an ESR study. Arch Biochem Biophys. 1987 Jan;252(1):41–48. doi: 10.1016/0003-9861(87)90006-3. [DOI] [PubMed] [Google Scholar]
- Thor H., Smith M. T., Hartzell P., Bellomo G., Jewell S. A., Orrenius S. The metabolism of menadione (2-methyl-1,4-naphthoquinone) by isolated hepatocytes. A study of the implications of oxidative stress in intact cells. J Biol Chem. 1982 Oct 25;257(20):12419–12425. [PubMed] [Google Scholar]
- Wefers H., Sies H. Generation of photoemissive species during quinone redox cycling. Biochem Pharmacol. 1986 Jan 1;35(1):22–24. doi: 10.1016/0006-2952(86)90548-4. [DOI] [PubMed] [Google Scholar]
- Wefers H., Sies H. Hepatic low-level chemiluminescence during redox cycling of menadione and the menadione-glutathione conjugate: relation to glutathione and NAD(P)H:quinone reductase (DT-diaphorase) activity. Arch Biochem Biophys. 1983 Jul 15;224(2):568–578. doi: 10.1016/0003-9861(83)90244-8. [DOI] [PubMed] [Google Scholar]
- Wilson I., Wardman P., Lin T. S., Sartorelli A. C. One-electron reduction of 2- and 6-methyl-1,4-naphthoquinone bioreductive alkylating agents. J Med Chem. 1986 Aug;29(8):1381–1384. doi: 10.1021/jm00158a010. [DOI] [PubMed] [Google Scholar]
- d'Arcy Doherty M., Rodgers A., Cohen G. M. Mechanisms of toxicity of 2- and 5-hydroxy-1,4-naphthoquinone; absence of a role for redox cycling in the toxicity of 2-hydroxy-1,4-naphthoquinone to isolated hepatocytes. J Appl Toxicol. 1987 Apr;7(2):123–129. doi: 10.1002/jat.2550070209. [DOI] [PubMed] [Google Scholar]


