Abstract
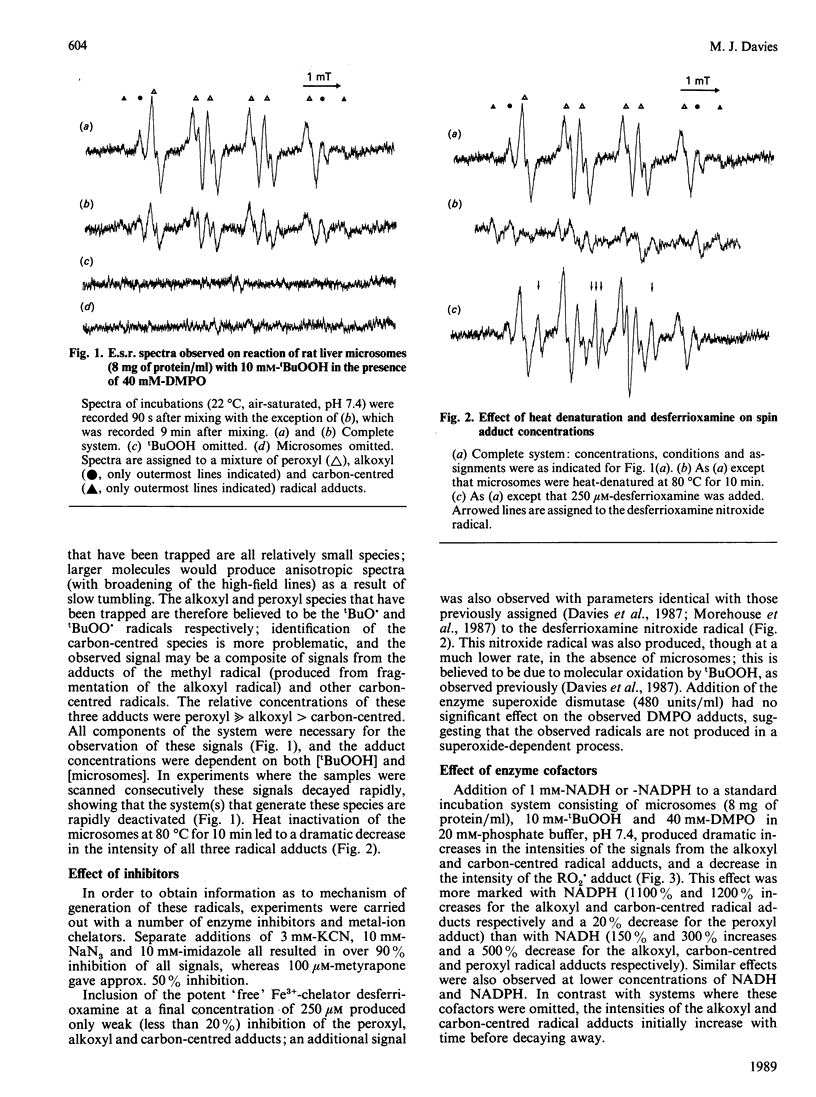
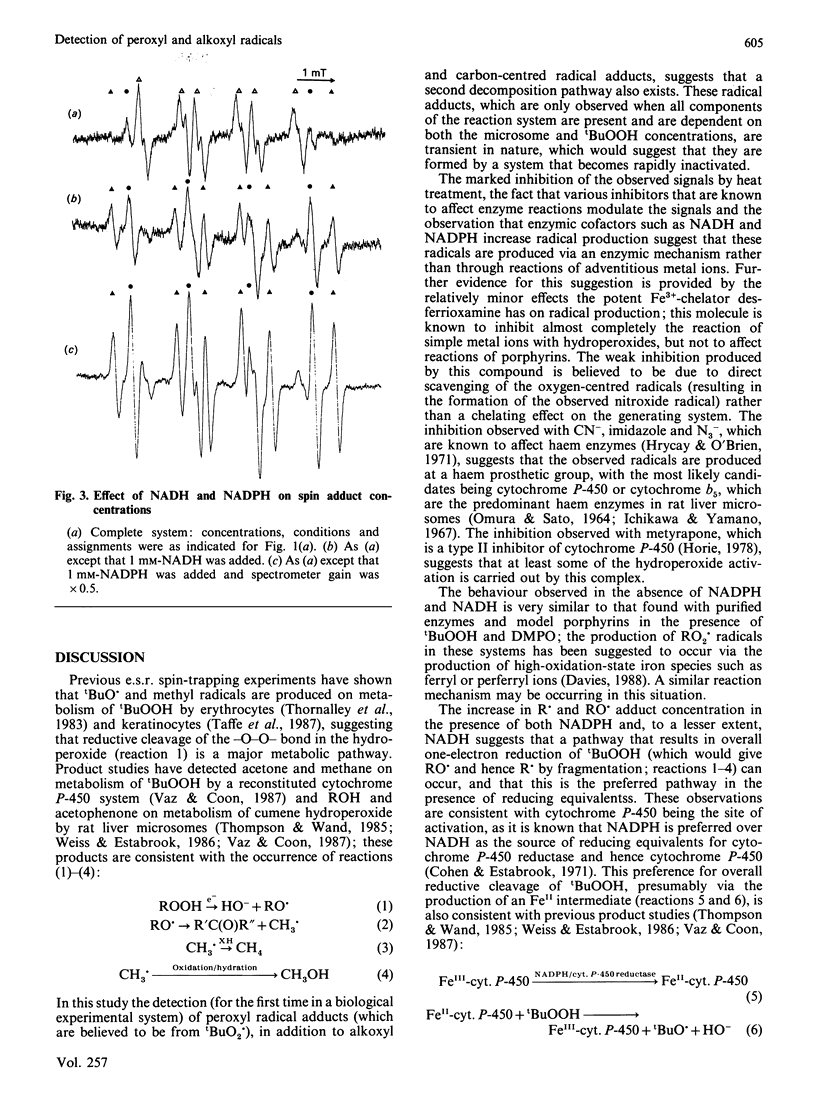
E.s.r. spin trapping using the spin trap 5,5-dimethyl-1-pyrroline N-oxide (DMPO) was used to detect peroxyl, alkoxyl and carbon-centred radicals produced by reaction of t-butyl hydroperoxide (tBuOOH) with rat liver microsomal fraction. The similarity of the hyperfine coupling constants of the peroxyl and alkoxyl radical adducts to those obtained previously with isolated enzymes suggests that these species are the tBuOO. and tBuO. adducts. The effects of metal-ion chelators, heat denaturation, enzyme inhibitors and reducing equivalents demonstrate that these species arise from reaction of tBuOOH with a haem enzyme such as cytochrome P-450 or cytochrome b5. In the absence of NADPH or NADH the previously undetected peroxyl radical adduct is the major species observed. In the presence of these reducing equivalents the alkoxyl and carbon-centred radical adducts predominate, which is in accord with product studies on similar systems. These results demonstrate that both reductive and oxidative decomposition of tBuOOH can occur in rat liver microsomal fraction with the reductive pathway favoured in the presence of NADH or NADPH.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bellomo G., Jewell S. A., Thor H., Orrenius S. Regulation of intracellular calcium compartmentation: studies with isolated hepatocytes and t-butyl hydroperoxide. Proc Natl Acad Sci U S A. 1982 Nov;79(22):6842–6846. doi: 10.1073/pnas.79.22.6842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellomo G., Thor H., Orrenius S. Increase in cytosolic Ca2+ concentration during t-butyl hydroperoxide metabolism by isolated hepatocytes involves NADPH oxidation and mobilization of intracellular Ca2+ stores. FEBS Lett. 1984 Mar 12;168(1):38–42. doi: 10.1016/0014-5793(84)80202-1. [DOI] [PubMed] [Google Scholar]
- Buettner G. R., Oberley L. W. Considerations in the spin trapping of superoxide and hydroxyl radical in aqueous systems using 5,5-dimethyl-1-pyrroline-1-oxide. Biochem Biophys Res Commun. 1978 Jul 14;83(1):69–74. doi: 10.1016/0006-291x(78)90398-4. [DOI] [PubMed] [Google Scholar]
- Cohen B. S., Estabrook R. W. Microsomal electron transport reactions. II. The use of reduced triphosphopyridine nucleotide and-or reduced diphosphopyridine nucleotide for the oxidative N-demethylation of aminopyrine and other drug substrates. Arch Biochem Biophys. 1971 Mar;143(1):46–53. doi: 10.1016/0003-9861(71)90184-6. [DOI] [PubMed] [Google Scholar]
- Davies M. J. Detection of peroxyl and alkoxyl radicals produced by reaction of hydroperoxides with heme-proteins by electron spin resonance spectroscopy. Biochim Biophys Acta. 1988 Jan 12;964(1):28–35. doi: 10.1016/0304-4165(88)90063-3. [DOI] [PubMed] [Google Scholar]
- Davies M. J., Donkor R., Dunster C. A., Gee C. A., Jonas S., Willson R. L. Desferrioxamine (Desferal) and superoxide free radicals. Formation of an enzyme-damaging nitroxide. Biochem J. 1987 Sep 15;246(3):725–729. doi: 10.1042/bj2460725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies M. J., Slater T. F. Studies on the metal-ion and lipoxygenase-catalysed breakdown of hydroperoxides using electron-spin-resonance spectroscopy. Biochem J. 1987 Jul 1;245(1):167–173. doi: 10.1042/bj2450167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hrycay E. G., O'Brien P. J. Cytochrome P-450 as a microsomal peroxidase utilizing a lipid peroxide substrate. Arch Biochem Biophys. 1971 Nov;147(1):14–27. doi: 10.1016/0003-9861(71)90304-3. [DOI] [PubMed] [Google Scholar]
- Ichikawa Y., Yamano T. Electron spin resonance of microsomal cytochromes. Correlation of the amount of CO-binding species with so-called microsomal e-x in microsomes of of Sudan III-treated animals. Arch Biochem Biophys. 1967 Sep;121(3):742–749. doi: 10.1016/0003-9861(67)90063-x. [DOI] [PubMed] [Google Scholar]
- Kalyanaraman B., Mottley C., Mason R. P. A direct electron spin resonance and spin-trapping investigation of peroxyl free radical formation by hematin/hydroperoxide systems. J Biol Chem. 1983 Mar 25;258(6):3855–3858. [PubMed] [Google Scholar]
- Larroque C., van Lier J. E. Hydroperoxysterols as a probe for the mechanism of cytochrome P-450scc-mediated hydroxylation. Homolytic versus heterolytic oxygen-oxygen bond scission. J Biol Chem. 1986 Jan 25;261(3):1083–1087. [PubMed] [Google Scholar]
- Lötscher H. R., Winterhalter K. H., Carafoli E., Richter C. Hydroperoxide-induced loss of pyridine nucleotides and release of calcium from rat liver mitochondria. J Biol Chem. 1980 Oct 10;255(19):9325–9330. [PubMed] [Google Scholar]
- Lötscher H. R., Winterhalter K. H., Carafoli E., Richter C. Hydroperoxides can modulate the redox state of pyridine nucleotides and the calcium balance in rat liver mitochondria. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4340–4344. doi: 10.1073/pnas.76.9.4340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy M. B., White R. E. Competing modes of peroxyacid flux through cytochrome P-450. J Biol Chem. 1983 Oct 10;258(19):11610–11616. [PubMed] [Google Scholar]
- Morehouse K. M., Flitter W. D., Mason R. P. The enzymatic oxidation of Desferal to a nitroxide free radical. FEBS Lett. 1987 Oct 5;222(2):246–250. doi: 10.1016/0014-5793(87)80379-4. [DOI] [PubMed] [Google Scholar]
- OMURA T., SATO R. THE CARBON MONOXIDE-BINDING PIGMENT OF LIVER MICROSOMES. I. EVIDENCE FOR ITS HEMOPROTEIN NATURE. J Biol Chem. 1964 Jul;239:2370–2378. [PubMed] [Google Scholar]
- Rosen G. M., Rauckman E. J. Spin trapping of the primary radical involved in the activation of the carcinogen N-hydroxy-2-acetylaminofluorene by cumene hydroperoxide-hematin. Mol Pharmacol. 1980 Mar;17(2):233–238. [PubMed] [Google Scholar]
- Rush G. F., Gorski J. R., Ripple M. G., Sowinski J., Bugelski P., Hewitt W. R. Organic hydroperoxide-induced lipid peroxidation and cell death in isolated hepatocytes. Toxicol Appl Pharmacol. 1985 May;78(3):473–483. doi: 10.1016/0041-008x(85)90255-8. [DOI] [PubMed] [Google Scholar]
- Rush G. F., Yodis L. A., Alberts D. Protection of rat hepatocytes from tert-butyl hydroperoxide-induced injury by catechol. Toxicol Appl Pharmacol. 1986 Jul;84(3):607–616. doi: 10.1016/0041-008x(86)90267-x. [DOI] [PubMed] [Google Scholar]
- Sies H., Gerstenecker C., Menzel H., Flohé L. Oxidation in the NADP system and release of GSSG from hemoglobin-free perfused rat liver during peroxidatic oxidation of glutathione by hydroperoxides. FEBS Lett. 1972 Oct 15;27(1):171–175. doi: 10.1016/0014-5793(72)80434-4. [DOI] [PubMed] [Google Scholar]
- Sies H., Grosskopf Oxidation of cytochrome b5 by hydroperoxides in rat liver. Eur J Biochem. 1975 Sep 15;57(2):513–520. doi: 10.1111/j.1432-1033.1975.tb02326.x. [DOI] [PubMed] [Google Scholar]
- Slater T. F., Sawyer B. C. The stimulatory effects of carbon tetrachloride and other halogenoalkanes on peroxidative reactions in rat liver fractions in vitro. General features of the systems used. Biochem J. 1971 Aug;123(5):805–814. doi: 10.1042/bj1230805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taffe B. G., Takahashi N., Kensler T. W., Mason R. P. Generation of free radicals from organic hydroperoxide tumor promoters in isolated mouse keratinocytes. Formation of alkyl and alkoxyl radicals from tert-butyl hydroperoxide and cumene hydroperoxide. J Biol Chem. 1987 Sep 5;262(25):12143–12149. [PubMed] [Google Scholar]
- Thompson J. A., Wand M. D. Interaction of cytochrome P-450 with a hydroperoxide derived from butylated hydroxytoluene. Mechanism of isomerization. J Biol Chem. 1985 Sep 5;260(19):10637–10644. [PubMed] [Google Scholar]
- Thor H., Hartzell P., Orrenius S. Potentiation of oxidative cell injury in hepatocytes which have accumulated Ca2+. J Biol Chem. 1984 May 25;259(10):6612–6615. [PubMed] [Google Scholar]
- Thornalley P. J., Trotta R. J., Stern A. Free radical involvement in the oxidative phenomena induced by tert-butyl hydroperoxide in erythrocytes. Biochim Biophys Acta. 1983 Aug 23;759(1-2):16–22. doi: 10.1016/0304-4165(83)90183-6. [DOI] [PubMed] [Google Scholar]
- Vaz A. D., Coon M. J. Hydrocarbon formation in the reductive cleavage of hydroperoxides by cytochrome P-450. Proc Natl Acad Sci U S A. 1987 Mar;84(5):1172–1176. doi: 10.1073/pnas.84.5.1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss R. H., Estabrook R. W. The mechanism of cumene hydroperoxide-dependent lipid peroxidation: the function of cytochrome P-450. Arch Biochem Biophys. 1986 Nov 15;251(1):348–360. doi: 10.1016/0003-9861(86)90082-2. [DOI] [PubMed] [Google Scholar]


