Abstract
Background
The spread of Carbapenemase-producing Organisms (CPO) remains a major threat globally. Within clinical settings, the existing method of determining gene load involves traditional culture to determine bacterial load and polymerase-chain-reaction-based Xpert Carba-R Assay to determine carbapenemase gene type. However, there is a need for a fast and accurate method of quantifying CPO colonisation to study the risk of persistent CPO carriage.
Objective
This study evaluated the accuracy of Xpert Carba-R Ct value in estimating carbapenamase producing bacterial loads in stool samples.
Methods
Stool samples were obtained from an ongoing study investigating the household transmission of CPO in Singapore. Stool samples lacking carbapenemase producing organisms were spiked with organism carrying a single carbapenemase gene (blaKPC, blaNDM, blaVIM, blaOXA-48(-like) or blaIMP-1) and serially diluted before being subjected to Xpert Carba-R assay and traditional culture. Standard curves with regression lines showing correlation between Ct values and plate counts were generated. The standard curves were validated with stool samples collected from patients.
Results
The limit of detection of blaNDM, blaKPC, and blaOXA-48 was approximately 103 cfu/mL, while that of blaIMP-1 and blaVIM was approximately 104 cfu/mL. Validation of the blaNDM and blaOXA-48 curves revealed average delta values of 0.56 log(cfu/mL) (95% CI 0.24–0.88) and 0.80 log(cfu/mL) (95% CI 0.53–1.07), respectively.
Conclusions
Our validation data for stool positive for blaNDM and blaOXA-48-type suggests that bacterial loads can be estimated within a reasonable range of error.
Introduction
Conventional culture-based methods of detecting Carbepenemase-producing Organisms (CPO) are often time consuming, with variations in sensitivity and specificity affected by media composition [1, 2]. The development of the on-demand PCR-based Xpert Carba-R Assay has greatly reduced turnaround time for molecular detection of five main carbapenemases, blaKPC (Klebsiella pneumoniae carbapenemase), blaNDM (New Delhi metallo-β-lactamase), blaIMP (Imipenemase), blaVIM (Verona integron-encoded metallo-β-lactamase) and blaOXA-48 (oxacillin-hydrolysing), with higher sensitivity and reliability [1–4]. In 2019, the estimated number of deaths directly attributable to drug-resistant infections was 1.27 million, of which 70% was attributable to fluoroquinolones and β-lactam antibiotics like carbapenems [5]. In a cohort of Carbapeneamse-producing Enterobacterales (CPE) carriers, a recent study has showed a 24.1% incidence of CPE infection with a median time from detection of CPE carriage to infection of 15 days [6]. There is an urgent need for rapidly identifying patients at high risk of CPO infection for infection control and prevention.
A previous study on bronchial specimens investigated the correlation between bacterial count and cycle number (Ct) to differentiate infections from colonisation [7]. A rapid assay to correlate and estimate carbapenemase-producing (CP) bacterial loads would aid future studies on bacterial load dynamics and allow us to determine how well bacterial load potentially predicts the risk of clinical infection. However, such studies are limited for stool samples. In this study, the correlation between Xpert Carba-R Ct values and colony counts of CP bacteria in stool samples was investigated.
Material and methods
Selection of clinical samples
De-identified stool samples were collected as part of an ongoing study investigating the household transmission of CPO in Singapore, reviewed and approved by the Domain Specific Review Board (DSRB) of National Healthcare Group Singapore (DSRB reference 2019/00794). Participants provided written informed consent. Patients from three multidisciplinary public hospitals were recruited and screened for CPOs. The recruitment period lasted from 1st Feburary 2021 to 31st January 2024.
Stool samples from CPO positive patients recruited in the above study were used to perform the validation experiments in this study. CPO positive stool samples were confirmed by a positive Xpert Carba-R run in conjuction with an 18 to 24-hour culture on the ChromID CARBA SMART (bioMérieux, Marcy-l’Étoile, France). Stool samples from CPO negative patients were used to perform the spiking experiments in this study. A CPO negative stool samples is defined as negative for both the Xpert Carba-R run and the ChromID CARBA SMART culture. Fresh stool samples were collected and stored at 4˚C immediately upon receipt, and transferred to -80˚C storage within 48 hours if not used immediately. For this study, only stool samples from participants who consented to storing their samples for future research were used.
Sample preparation for Xpert Carba-R assay
To prepare stool suspensions, an Eswab (Copan Diagnostics, Murrieta, CA) was dipped into the stool sample and resuspended in Amies medium by vortexing for 10 seconds. We created a series of turbidity standards to standardise the amount of stool for each Xpert Carba-R assay run (S1 Appendix). Each Amies-stool suspension was prepared to match the most turbid standard. From the Amies-stool suspension, 300 μL was added into the Xpert sample reagent and vortexed [1]. From this mixture, 1.7 mL was transferred into an Xpert Carba-R cartridge and loaded on the GeneXpert platform according to the manufacturer’s instructions (Xpert Carba-R package insert; 301–2438, Rev. F).
Generation of standard curve
Each CPO-negative stool sample was singly-spiked with a carbapenemase-producing organism carrying a single Xpert Carba-R target gene in its resistome. The isolates used were a strain of NDM-1-producing Enterobacter cloacae, IMP-1-producing Pseudomonas aeruginosa, KPC-2-producing Escherichia coli, VIM-2-producing P. aeruginosa, and OXA-48-producing E. coli. These isolates were previously obtained from purity plates of clinical or surveillance samples and stored in CryoCare vials (Key Scientific Products, Stamford, Texas) at -80˚C. For each gene, the experiment was repeated independently on three stool samples. The organisms were prepared in 0.9% sterile saline to a 0.5 McFarland standard (approximately 1 x 108 cfu/mL) using a Densichek instrument (bioMérieux). Samples were then subjected to 10-fold serial dilutions to estimated concentrations of 101, 102, 103, 104, 105, 106, and 107 cfu/mL in the Amies-stool suspension. Following which, the Xpert Carba-R assay was performed as described in previous section.
To determine bacterial counts, 100 μL of each sample was also inoculated on ChromID CARBA (bioMérieux) or ChromID OXA-48 (bioMérieux) plates in triplicates. Samples with estimated concentrations greater than 103 cfu/mL were subjected to 10-fold serial dilutions to achieve 103 cfu/mL suspensions, while the remaining samples were plated neat. Colonies were counted after incubation at 37˚C for 18 hours. As a negative control, an aliquot of the Amies-stool sample was loaded on the GeneXpert, as well as cultured on both ChromID CARBA and ChromID OXA-48 plates without prior spiking.
Validation of standard curve
Stool samples from the ongoing study investigating the household transmission of CPO in Singapore that tested positive for carbapenemase gene by GeneXpert were used for validation. Based on the Ct value obtained, an estimated bacterial concentration was determined using the standard curves. The processed stool samples were diluted accordingly and plated on ChromID CARBA or ChromID OXA-48 plates in triplicates. The plate counts were recorded and compared with estimated values. We define delta values as the absolute difference between Carba-R estimated log(cfu/mL) and colony count log(cfu/mL). For each sample, delta values were recorded for evaluation. For samples that yielded multiple morphologies on ChromID CARBA or ChromID OXA-48 plates, GeneXpert was performed on each distinct morphology to check for CP status. Only those that were confirmed to be carbapenemase-producers were included in the counts.
Statistical analysis
Regression lines showing the correlation between Ct values and final plate counts were plotted using Graphpad Prism. Statistical calculations to determine standard deviation and 95% confidence intervals of delta values were performed using Microsoft Excel.
Results
Evaluation of limit of detection for Xpert Carba-R assay
Runs with a Ct value but deemed analyte negative by the GeneXpert platform were recorded as negative. The highest Ct values measured for the five CPO isolates were, 36.9, 35.3, 37.7, 37.2 and 37.3 for the detection of blaNDM, blaIMP-1, blaKPC, blaVIM and blaOXA-48-type, respectively, corresponding to plate counts of 1.88, 3.64, 1.87, 3.01 and 1.12 log(cfu/mL). blaNDM, blaKPC and blaOXA-48-type were consistently detected when spiked at an estimated concentration of 103 to 107 cfu/mL compared to blaIMP-1 and blaVIM, which were only consistently detected at estimated concentrations of 104 to 107 cfu/mL (S1 Table). The limit of detection (LOD), defined as the lowest concentration at which all repeat runs are positive [1], was higher for blaIMP-1 and blaVIM (104 cfu/mL) compared to blaNDM, blaKPC and blaOXA-48-type (103 cfu/mL). All genes detected by the Xpert Carba-R assay correspond with the carbapenemase gene carried by the spiked organism; no false positives were detected in all samples.
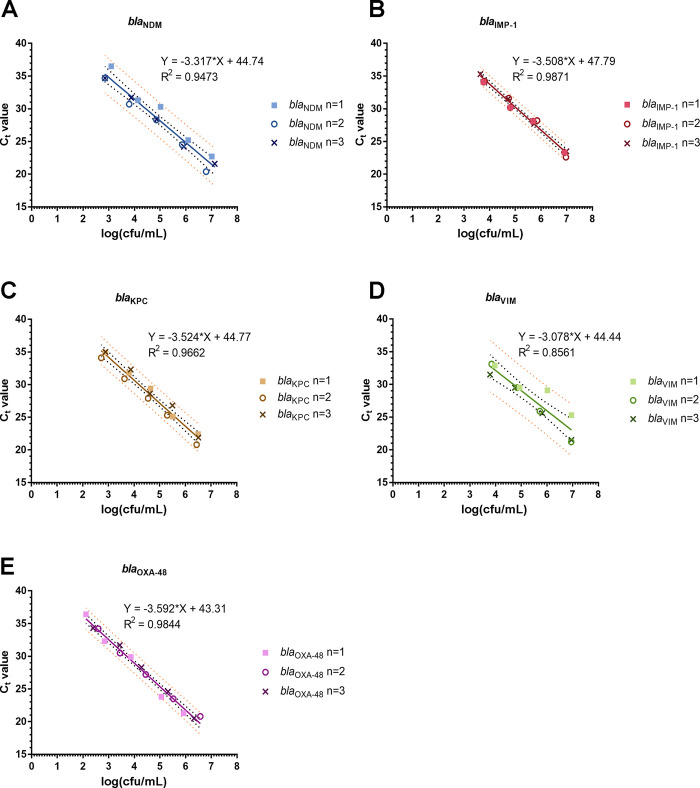
A linear regression model was utilised to examine the correlation between Ct values and bacterial counts (Fig 1, Table 1). For each estimated concentration, only Ct values that were recorded on GeneXpert for all independent repeats were included in the analysis. The data points of all genes exhibited a good fit to a linear model with R2 values ranging between 0.8561 (blaVIM) and 0.9871 (blaIMP-1).
Fig 1. Standard curves of Ct values plotted against bacteria plate counts.
(A-E) Linear regression lines of Ct values against log(cfu/mL) for stool samples spiked with NDM-1-producing E. cloacae, IMP-1-producing P. aeruginosa, KPC-2-producing E. coli, VIM-2-producing P. aeruginosa, and OXA-48-type-producing E. coli respectively. Black dotted lines denote 95% confidence interval while orange dotted lines denote 95% prediction interval.
Table 1. Values used to plot standard curve.
| CP Gene | n = 1 | n = 2 | n = 3 | |||
|---|---|---|---|---|---|---|
| Ct value | log(CFU/mL) | Ct value | log(CFU/mL) | Ct value | log(CFU/mL) | |
| bla NDM | 36.5 | 3.093422 | 34.7 | 2.847161 | 34.7 | 2.853293 |
| bla NDM | 31.3 | 4.132473 | 30.7 | 3.792392 | 31.7 | 3.869232 |
| bla NDM | 30.3 | 5.01424 | 28.3 | 4.849215 | 28.4 | 4.861335 |
| bla NDM | 25.2 | 6.088727 | 24.5 | 5.857332 | 24.2 | 5.922552 |
| bla NDM | 22.7 | 7.011429 | 20.4 | 6.79005 | 21.6 | 7.126023 |
| bla IMP-1 | 34.1 | 3.765917 | 34.2 | 3.808436 | 35.3 | 3.64015 |
| bla IMP-1 | 30.2 | 4.801632 | 31.6 | 4.742987 | 31.5 | 4.721536 |
| bla IMP-1 | 28.1 | 5.69314 | 28.2 | 5.845098 | 27.7 | 5.729705 |
| bla IMP-1 | 23.3 | 6.913814 | 22.6 | 6.971585 | 23.5 | 6.99417 |
| bla KPC | 34.9 | 2.882714 | 34.1 | 2.718778 | 35 | 2.884607 |
| bla KPC | 31.8 | 3.80618 | 30.9 | 3.626682 | 32.3 | 3.871184 |
| bla KPC | 29.4 | 4.64673 | 27.9 | 4.548185 | 28.6 | 4.619789 |
| bla KPC | 25.1 | 5.50965 | 25.3 | 5.293731 | 26.8 | 5.496007 |
| bla KPC | 22.4 | 6.496007 | 20.8 | 6.436693 | 21.9 | 6.50515 |
| bla VIM | 32.9 | 3.970037 | 33.1 | 3.843025 | 31.5 | 3.78533 |
| bla VIM | 29.5 | 4.934498 | 29.5 | 4.838849 | 29.5 | 4.750765 |
| bla VIM | 29.1 | 6.018423 | 25.9 | 5.718778 | 25.6 | 5.817345 |
| bla VIM | 25.3 | 6.952631 | 21.2 | 6.946125 | 21.5 | 6.955848 |
| bla OXA-48 | 36.4 | 2.113943 | 34.2 | 2.598426 | 34.3 | 2.39794 |
| bla OXA-48 | 32.3 | 2.845098 | 30.5 | 3.441957 | 31.7 | 3.436693 |
| bla OXA-48 | 29.9 | 3.865301 | 27.2 | 4.447158 | 28.3 | 4.278754 |
| bla OXA-48 | 23.8 | 5.054358 | 23.5 | 5.518514 | 24.6 | 5.31527 |
| bla OXA-48 | 21.3 | 5.920819 | 20.8 | 6.572097 | 20.5 | 6.322219 |
Samples for validation of standard curve
The standard curves were validated using selected stool samples obtained from an ongoing study investigating the household transmission of CPO in Singapore. The samples used for validation were collected between 7th March and 13th July 2022 and only carbapenamase-positive samples were used. Among 133 total patient stool samples, seven blaNDM, one blaIMP-1 and eleven blaOXA-48-type samples were used for validation.
Evaluation of standard curve
Bacterial counts of stool samples were quantified by culture and compared to estimated values determined from the standard curve (Fig 1). Ct values, Carba-R estimated bacterial counts and colony counts determined from traditional culture are summarised in Table 2. Delta values, defined as the absolute difference between Carba-R estimated log(cfu/mL) and colony count log(cfu/mL), were also calculated. A delta value of 0.71 log(cfu/mL) was observed for the sole blaIMP-1 sample, while a maximum delta value of 1.36 log(cfu/mL) and 1.56 log(cfu/mL) was observed for blaNDM and blaOXA-48-type, respectively (Table 2). Statistical analysis was only performed for blaNDM and blaOXA-48-type samples as only one blaIMP-1 sample and no blaKPC and blaVIM samples were received during the study period. The average delta value for blaNDM and blaOXA-48-type was 0.56 log(cfu/mL) (95% CI 0.24–0.88) and 0.80 log(cfu/mL) (95% CI 0.53–1.07), respectively (S2 Table), suggesting that the standard curve of blaNDM was able to predict bacterial loads more accurately than that of blaOXA-48-type. However, due to the small sample size, the accuracy of the standard curves cannot be conclusively defined.
Table 2. Summary of Ct values, Carba-R estimated and colony counted bacterial loads of clinical samples.
| Sample no. | CP Gene | Ct value | Carba-R estimated (cfu/mL) | Colony Count (cfu/mL) | Delta valuea |
|---|---|---|---|---|---|
| 1 | bla IMP-1 | 27.60 | 5.76 | 5.04 | 0.71 |
| 2 | bla NDM | 27.20 | 5.29 | 4.60 | 0.69 |
| 3 | bla NDM | 20.80 | 7.22 | 6.56 | 0.66 |
| 4 | bla NDM | 30.00 | 4.44 | 4.33 | 0.11 |
| 5 | bla NDM | 22.30 | 6.77 | 6.28 | 0.49 |
| 6 | bla NDM | 23.30 | 6.46 | 6.43 | 0.032 |
| 7 | bla NDM | 33.60 | 3.36 | 4.72 | 1.36 |
| 8 | bla NDM | 29.00 | 4.75 | 5.33 | 0.58 |
| 9 | bla OXA-48 | 24.70 | 5.18 | 5.85 | 0.67 |
| 10 | bla OXA-48 | 29.10 | 3.96 | 2.51 | 1.45 |
| 11 | bla OXA-48-type b | 22.40 | 5.82 | 4.83 | 0.99 |
| 12 | bla OXA-48 | 31.40 | 3.32 | 3.15 | 0.17 |
| 13 | bla OXA-48 | 22.40 | 5.82 | 4.99 | 0.84 |
| 14 | bla OXA-48 | 21.10 | 6.18 | 5.97 | 0.21 |
| 15 | bla OXA-48 | 22.90 | 5.68 | 4.12 | 1.56 |
| 16 | bla OXA-48 | 25.60 | 4.93 | 5.88 | 0.95 |
| 17 | bla OXA-48-like | 30.70 | 3.51 | 4.31 | 0.80 |
| 18 | bla OXA-48 | 28.10 | 4.23 | 3.40 | 0.83 |
| 19 | bla OXA-48 | 25.40 | 4.99 | 4.66 | 0.33 |
a Absolute difference between colony-counted bacterial load and Carba-R estimated values determined from standard curves
b blaOXA-48-type includes blaOXA-48 and blaOXA-48-like CP genes
Discussion
The GeneXpert platform has been widely reported for its sensitivity and specificity [1–4], and would be a good test to correlate Ct values and bacterial loads. Here, using spiked stool samples, standard curves were generated to estimate bacterial loads based on Ct values measured by GeneXpert. We were able to estimate bacterial counts for blaNDM and blaOXA-48-type samples with errors of 0.56 log(cfu/mL) (95% CI 0.24–0.88) and 0.80 log(cfu/mL) (95% CI 0.53–1.07), respectively. We could not validate the other three genes as blaIMP-1-positive stool samples were rare, while there were no blaKPC-positive and blaVIM-positive samples during the study period. We noted a lower LOD across all genes compared to what Yee and colleagues have shown, despite using a similar preparation method. This could be attributed to differences in the methods as to how the stool samples were spiked [1].
To our knowledge, besides Burillo’s group [7], there are no studies evaluating the performance of the Carba-R assay for predicting bacterial or gene loads. Burillo et al. showed that based on Ct values, they could determine if the bacterial load in a bronchial sample was ≥105 cfu/mL (Ct ≤ 24.7), ≥104 cfu/mL (24.7 < Ct ≤ 26.9), or <104 cfu/mL (Ct > 26.9) [7]. Similarly, our study shows the correlation of Ct values and bacterial loads, but in stool samples. Additionally, in a previous study by Ko et. al. to evaluate the diagnostic performance of the Carba-R assay using rectal swabs, a regression line was generated to compare GeneXpert Ct values and cultures of carbapenemase resistant organisms [4]. However, as the purpose of Ko’s group’s study was not to estimate bacterial loads, only one line was plotted for all five genes [4]. Here, we show that there are slight differences in Carba-R assay’s analytical sensitivity for detecting each gene. Notably, in accordance with the higher LOD obtained for blaIMP and blaVIM, each Ct value also corresponded with a higher bacterial count compared to blaNDM, blaKPC and blaOXA. While there are few studies that verify the LOD of each gene target, higher LODs for blaIMP-1 and blaVIM have been reported [4, 8]. This may reflect lower copy numbers of these two genes as compared to blaKPC, blaNDM and blaOXA-48-type. This should be taken into account when the standard curve is used for bacterial load estimation (Fig 1 and S1 Table). If implemented, the inclusion of a variety of isolates should be considered to improve the robustness of the standard curve. We also noted that the standard curve of blaNDM was able to predict bacterial loads more accurately than that of blaOXA-48-type. Of eleven blaOXA-48-type-containing stool samples, three had Carba-R estimated loads that were greater than 1 log(cfu/mL) above the bacterial counts from culture. OXA-48-type enzymes are known for being weakly hydrolysing, especially so if the OXA-48-type producing isolate do not produce ESBL [9, 10]. This can hinder growth on selective media and may have led to an underestimation of the true bacterial load in the three aforementioned samples. Again, this highlights the limitations of culture-only methods for detection and quantification of CPO.
Patients with intestinal colonisation of pathogens, notably Klebsiella pneumoniae, are at higher risk of developing clinical infections [11, 12]. This applies to CPO as well; patients who developed CPO infections were associated with higher relative load of KPC or OXA-48 producing bacteria in the gut [12–14]. The use of GeneXpert Ct values could facilitate quicker identification of patients at higher risk of clinical infections for early treatment interventions.
Heterogeneity in infectious disease dynamics, where a small proportion of individuals is responsible for a large proportion of transmission events [15], has been described for several pathogens including E. coli [16] and K. pneumoniae [17]. Infectiousness is most simply and frequently measured by bacterial load in samples such as feces [15, 18]. Multiple studies have shown that patients with higher bacterial loads were more likely to be capable of contaminating the environment and the personal protective equipment of nursing staff [19–22], which are known to be a reservoir and source of transmission of CPOs [23–27]. A recent study in a mouse model suggested that individuals who shed higher loads of bacteria are the main contributors to host-to-host transmission events [17]. Early identification and cohorting of colonized patients and nursing staff is one of the only, if not the only, ways of preventing nosocomial CPO outbreaks [28, 29], and infection control protocols that do not reach the individuals with high bacterial loads may be inadequate [15, 30]. However, cohorting is costly and often difficult to implement [26]. When resources are limited, the ability to estimate bacterial loads based on GeneXpert Ct values could allow for prioritisation of infection prevention strategies, such as decolonisation interventions and cohorting to minimize environmental contamination. Aside from clinical benefits, this would also serve as a more efficient method to estimate bacterial or gene loads for load dynamics studies in future research.
This study has several limitations. When generating a standard curve for each carbapenemase gene, the negative stool samples were spiked with a single organism. Further large-scale studies with more diverse clinical samples would ensure greater representation and account for potential variation between bacterial strains and species. In Singapore, CPE isolates collected from 2010 to 2015 across multiple hospitals revealed that the most prevalent carbapenemase genes were blaKPC, followed by blaNDM, blaOXA48-type, and blaIMP [31]. For the duration of the current study, subject recruitment from other hospitals was still a work in progress; however, once implemented, would help to address the current lack of blaKPC samples and allow a better representation of the carbapenemase gene distribution in Singapore. The standard curve was also generated based on the assumption that the CPO carries a single copy gene. While isolates carrying multiple carbapenemase gene copies have been reported [32, 33], the overall frequency of these cases is unknown. This should not be a major limitation as it was previously shown that gene loads were linearly correlated to their host strains’ abundance [12]. The applicability of the curve for predicting infection risk will also not be undermined as higher carbepenemase gene loads are also associated with higher risk of infection [13, 34]. Moreover, quantification of plasmid copy numbers requires DNA from pure cultures which require multiple days to obtain [35]. In times of large outbreaks, such methods may be too labour- and time-intensive, and assays with a short turnaround time for identification of high risk patients at the cost of a reasonable amount of accuracy may be favourable. However, in a non-outbreak setting with less time constraints, more comprehensive methods to determine bacterial loads may be employed instead.
Where appropriate validation is performed for the population of interest, this method enables the estimation of bacterial loads in the same turnaround time as the GeneXpert Carba-R assay without the need for traditional methods. Traditionally, to quantify the relative load of CPO or carbapenemase genes, the ratio of cultured CPO to total viable aerobic bacteria or the 2-ΔΔCt method, respectively, had to be used [12, 13, 36]. Using our standard curve, the same could be achieved within an hour, allowing research decisions to be made rapidly. Additionally, this assay could aid studies like bacterial load dynamics and correlation of bacterial loads to persistent CPO carriage, which could potentially be applied in clinical settings in the future.
Supporting information
All stool-amies suspensions were adjusted to 1x dilution.
(TIF)
(DOCX)
(DOCX)
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This study was financially supported by the Singapore Ministry of Health's National Medical Research Council (NMRC; https://www.nmrc.gov.sg/) under its NMRC Center Grant Programme: Collaborative Solutions Targeting Antimicrobial Resistance Threats in Health Systems (CoSTAR-HS) in the form of grants (CG21APR2005 and CoSTAR-HS/ARGSeedFund/2022/04) received by OTN. This study was also financially supported by the the Singapore Ministry of Health's National Medical Research Council in the form of an NMRC Clinician Scientist Award (MOH-000276) received by OTN. This study was also financially supported by the Singapore Ministry of Health's National Medical Research Council in the form of a NMRC Clinician Scientist Individual Research Grant (MOH-CIRG18nov-0006) received by KM. No additional external funding was received for this study. The funder had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Yee R, Fisher S, Bergman Y, Chambers KK, Tamma PD, Carroll KC, et al. Combined selective culture and molecular methods for the detection of carbapenem-resistant organisms from fecal specimens. Eur J Clin Microbiol Infect Dis. 2021;40(11):2315–2321. doi: 10.1007/s10096-021-04281-8 [DOI] [PubMed] [Google Scholar]
- 2.Tato M, Ruiz-Garbajosa P, Traczewski M, Dodgson A, McEwan A, Humphries R, et al. Multisite Evaluation of Cepheid Xpert Carba-R Assay for Detection of Carbapenemase-Producing Organisms in Rectal Swabs. J Clin Microbiol. 2016;54(7):1814–1819. doi: 10.1128/JCM.00341-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Farfour E, Lomont A, Fihman V, Lecuru M, Hüssler S, Ouzani S, et al. Rapid and accurate eXDR screening: use Xpert Carba-R® with FecalSwab®. Diagn Microbiol Infect Dis. 2021;99(4):115279. [DOI] [PubMed] [Google Scholar]
- 4.Ko YJ, Kim J, Kim HN, Yoon SY, Lim CS, Lee CK. Diagnostic performance of the Xpert Carba-R assay for active surveillance of rectal carbapenemase-producing organisms in intensive care unit patients. Antimicrob Resist Infect Control. 2019;8:127. doi: 10.1186/s13756-019-0579-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Global burden of bacterial antimicrobial resistance in 2019: a systematic analysis. Lancet. 2022;399(10325):629–655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hoellinger B, Deboscker S, Danion F, Lavigne T, Severac F, Ruch Y, et al. Incidence and Time-to-Onset of Carbapenemase-Producing Enterobacterales (CPE) Infections in CPE Carriers: a Retrospective Cohort Study. Microbiol Spectr. 2022;10(6):e0186822. doi: 10.1128/spectrum.01868-22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Burillo A, Marín M, Cercenado E, Ruiz-Carrascoso G, Pérez-Granda MJ, Oteo J, et al. Evaluation of the Xpert Carba-R (Cepheid) Assay Using Contrived Bronchial Specimens from Patients with Suspicion of Ventilator-Associated Pneumonia for the Detection of Prevalent Carbapenemases. PLoS One. 2016;11(12):e0168473. doi: 10.1371/journal.pone.0168473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cury AP, Almeida Junior JN, Costa SF, Salomão MC, Boszczowski Í, Duarte AJS, et al. Diagnostic performance of the Xpert Carba-R™ assay directly from rectal swabs for active surveillance of carbapenemase-producing organisms in the largest Brazilian University Hospital. J Microbiol Methods. 2020;171:105884. [DOI] [PubMed] [Google Scholar]
- 9.Poirel L, Potron A, Nordmann P. OXA-48-like carbapenemases: the phantom menace. J Antimicrob Chemother. 2012;67(7):1597–1606. doi: 10.1093/jac/dks121 [DOI] [PubMed] [Google Scholar]
- 10.Bakthavatchalam YD, Anandan S, Veeraraghavan B. Laboratory Detection and Clinical Implication of Oxacillinase-48 like Carbapenemase: The Hidden Threat. J Glob Infect Dis. 2016;8(1):41–50. doi: 10.4103/0974-777X.176149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gorrie CL, Mirceta M, Wick RR, Edwards DJ, Thomson NR, Strugnell RA, et al. Gastrointestinal Carriage Is a Major Reservoir of Klebsiella pneumoniae Infection in Intensive Care Patients. Clin Infect Dis. 2017;65(2):208–215. doi: 10.1093/cid/cix270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lázaro-Perona F, Rodríguez-Tejedor M, Ruiz-Carrascoso G, Díaz-Pollán B, Loeches B, Ramos-Ramos JC, et al. Intestinal loads of OXA-48-producing Klebsiella pneumoniae in colonized patients determined from surveillance rectal swabs. Clin Microbiol Infect. 2021;27(8):1169.e1167-1169.e1112. doi: 10.1016/j.cmi.2020.09.054 [DOI] [PubMed] [Google Scholar]
- 13.Migliorini LB, Leaden L, de Sales RO, Correa NP, Marins MM, Koga PCM, et al. The Gastrointestinal Load of Carbapenem-Resistant Enterobacteriacea Is Associated With the Transition From Colonization to Infection by Klebsiella pneumoniae Isolates Harboring the bla(KPC) Gene. Front Cell Infect Microbiol. 2022;12:928578. doi: 10.3389/fcimb.2022.928578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shimasaki T, Seekatz A, Bassis C, Rhee Y, Yelin RD, Fogg L, et al. Increased Relative Abundance of Klebsiella pneumoniae Carbapenemase-producing Klebsiella pneumoniae Within the Gut Microbiota Is Associated With Risk of Bloodstream Infection in Long-term Acute Care Hospital Patients. Clin Infect Dis. 2019;68(12):2053–2059. doi: 10.1093/cid/ciy796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kempf F, La Ragione R, Chirullo B, Schouler C, Velge P. Super Shedding in Enteric Pathogens: A Review. Microorganisms. 2022;10(11). doi: 10.3390/microorganisms10112101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chase-Topping M, Gally D, Low C, Matthews L, Woolhouse M. Super-shedding and the link between human infection and livestock carriage of Escherichia coli O157. Nat Rev Microbiol. 2008;6(12):904–912. doi: 10.1038/nrmicro2029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Young TM, Bray AS, Nagpal RK, Caudell DL, Yadav H, Zafar MA. Animal Model To Study Klebsiella pneumoniae Gastrointestinal Colonization and Host-to-Host Transmission. Infect Immun. 2020;88(11). doi: 10.1128/IAI.00071-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Woolhouse M. Quantifying Transmission. Microbiol Spectr. 2017;5(4). doi: 10.1128/microbiolspec.MTBP-0005-2016 [DOI] [PubMed] [Google Scholar]
- 19.O’Hara LM, Calfee DP, Miller LG, Pineles L, Magder LS, Johnson JK, et al. Optimizing Contact Precautions to Curb the Spread of Antibiotic-resistant Bacteria in Hospitals: A Multicenter Cohort Study to Identify Patient Characteristics and Healthcare Personnel Interactions Associated With Transmission of Methicillin-resistant Staphylococcus aureus. Clin Infect Dis. 2019;69(Suppl 3):S171–s177. doi: 10.1093/cid/ciz621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.O’Hara LM, Nguyen MH, Calfee DP, Miller LG, Pineles L, Magder LS, et al. Risk factors for transmission of carbapenem-resistant Enterobacterales to healthcare personnel gloves and gowns in the USA. J Hosp Infect. 2021;109:58–64. doi: 10.1016/j.jhin.2020.12.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jackson SS, Harris AD, Magder LS, Stafford KA, Johnson JK, Miller LG, et al. Bacterial burden is associated with increased transmission to health care workers from patients colonized with vancomycin-resistant Enterococcus. Am J Infect Control. 2019;47(1):13–17. doi: 10.1016/j.ajic.2018.07.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lerner A, Adler A, Abu-Hanna J, Cohen Percia S, Kazma Matalon M, Carmeli Y. Spread of KPC-producing carbapenem-resistant Enterobacteriaceae: the importance of super-spreaders and rectal KPC concentration. Clin Microbiol Infect. 2015;21(5):470.e471-477. doi: 10.1016/j.cmi.2014.12.015 [DOI] [PubMed] [Google Scholar]
- 23.Blanco N O’Hara LM, Harris AD. Transmission pathways of multidrug-resistant organisms in the hospital setting: a scoping review. Infect Control Hosp Epidemiol. 2019;40(4):447–456. doi: 10.1017/ice.2018.359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.van Loon K, Voor In ’t Holt AF, Vos MC. A Systematic Review and Meta-analyses of the Clinical Epidemiology of Carbapenem-Resistant Enterobacteriaceae. Antimicrob Agents Chemother. 2018;62(1):e01730–01717. doi: 10.1128/AAC.01730-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Clarivet B, Grau D, Jumas-Bilak E, Jean-Pierre H, Pantel A, Parer S, et al. Persisting transmission of carbapenemase-producing Klebsiella pneumoniae due to an environmental reservoir in a university hospital, France, 2012 to 2014. Euro Surveill. 2016;21(17):30213. [DOI] [PubMed] [Google Scholar]
- 26.Hilliquin D, Lomont A, Zahar JR. Cohorting for preventing the nosocomial spread of Carbapenemase-Producing Enterobacterales, in non-epidemic settings: is it mandatory? J Hosp Infect. 2020:Epub ahead of print. doi: 10.1016/j.jhin.2020.04.022 [DOI] [PubMed] [Google Scholar]
- 27.Goodman KE, Simner PJ, Tamma PD, Milstone AM. Infection control implications of heterogeneous resistance mechanisms in carbapenem-resistant Enterobacteriaceae (CRE). Expert Rev Anti Infect Ther. 2016;14(1):95–108. doi: 10.1586/14787210.2016.1106940 [DOI] [PubMed] [Google Scholar]
- 28.Nordmann P. Carbapenemase-producing Enterobacteriaceae: overview of a major public health challenge. Med Mal Infect. 2014;44(2):51–56. doi: 10.1016/j.medmal.2013.11.007 [DOI] [PubMed] [Google Scholar]
- 29.Fournier S, Monteil C, Lepainteur M, Richard C, Brun-Buisson C, Jarlier V, et al. Long-term control of carbapenemase-producing Enterobacteriaceae at the scale of a large French multihospital institution: a nine-year experience, France, 2004 to 2012. Euro Surveill. 2014;19(19). doi: 10.2807/1560-7917.es2014.19.19.20802 [DOI] [PubMed] [Google Scholar]
- 30.Woolhouse ME, Dye C, Etard JF, Smith T, Charlwood JD, Garnett GP, et al. Heterogeneities in the transmission of infectious agents: implications for the design of control programs. Proc Natl Acad Sci U S A. 1997;94(1):338–342. doi: 10.1073/pnas.94.1.338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Marimuthu K, Venkatachalam I, Koh V, Harbarth S, Perencevich E, Cherng BPZ, et al. Whole genome sequencing reveals hidden transmission of carbapenemase-producing Enterobacterales. Nat Commun. 2022;13(1):3052. doi: 10.1038/s41467-022-30637-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Feng Y, Liu L, McNally A, Zong Z. Coexistence of three bla(KPC-2) genes on an IncF/IncR plasmid in ST11 Klebsiella pneumoniae. J Glob Antimicrob Resist. 2019;17:90–93. [DOI] [PubMed] [Google Scholar]
- 33.Abe R, Akeda Y, Sugawara Y, Matsumoto Y, Motooka D, Kawahara R, et al. Enhanced Carbapenem Resistance through Multimerization of Plasmids Carrying Carbapenemase Genes. mBio. 2021;12(3):e0018621. doi: 10.1128/mBio.00186-21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dahdouh E, Cendejas-Bueno E, Ruiz-Carrascoso G, Schüffelmann C, Lázaro-Perona F, Castro-Martínez M, et al. Intestinal loads of extended-spectrum beta-lactamase and Carbapenemase genes in critically ill pediatric patients. Front Cell Infect Microbiol. 2023;13:1180714. doi: 10.3389/fcimb.2023.1180714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jiang J, Chen L, Chen X, Li P, Xu X, Fowler VG, Jr., et al. Carbapenemase-Encoding Gene Copy Number Estimator (CCNE): a Tool for Carbapenemase Gene Copy Number Estimation. Microbiol Spectr. 2022;10(4):e0100022. doi: 10.1128/spectrum.01000-22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ramos-Ramos JC, Lázaro-Perona F, Arribas JR, García-Rodríguez J, Mingorance J, Ruiz-Carrascoso G, et al. Proof-of-concept trial of the combination of lactitol with Bifidobacterium bifidum and Lactobacillus acidophilus for the eradication of intestinal OXA-48-producing Enterobacteriaceae. Gut Pathog. 2020;12:15. doi: 10.1186/s13099-020-00354-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
All stool-amies suspensions were adjusted to 1x dilution.
(TIF)
(DOCX)
(DOCX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.