Abstract
Metabolomics has been used extensively to capture the exposome. We investigated whether prospectively measured metabolites provided predictive power beyond well-established risk factors among 758 women with adjudicated cancers [n = 577 breast (BC) and n = 181 colorectal (CRC)] and n = 758 controls with available specimens (collected mean 7.2 years prior to diagnosis) in the Women’s Health Initiative Bone Mineral Density subcohort. Fasting samples were analyzed by LC-MS/MS and lipidomics in serum, plus GC-MS and NMR in 24 h urine. For feature selection, we applied LASSO regression and Super Learner algorithms. Prediction models were subsequently derived using logistic regression and Super Learner procedures, with performance assessed using cross-validation (CV). For BC, metabolites did not increase predictive performance over established risk factors (CV-AUCs~0.57). For CRC, prediction increased with the addition of metabolites (median CV-AUC across platforms increased from ~0.54 to ~0.60). Metabolites related to energy metabolism: adenosine, 2-hydroxyglutarate, N-acetyl-glycine, taurine, threonine, LPC (FA20:3), acetate, and glycerate; protein metabolism: histidine, leucic acid, isoleucine, N-acetyl-glutamate, allantoin, N-acetyl-neuraminate, hydroxyproline, and uracil; and dietary/microbial metabolites: myo-inositol, trimethylamine-N-oxide, and 7-methylguanine, consistently contributed to CRC prediction. Energy metabolism may play a key role in the development of CRC and may be evident prior to disease development.
Keywords: breast cancer, colorectal cancer, metabolite predictors, dietary biomarkers, metabolomics
1. Introduction
Breast cancer (BC) and colorectal cancer (CRC) are the first and third highest incident cancers in women in the US, respectively [1]. Substantial evidence outlined in the Third Expert Report of the World Cancer Research Fund (WCRF)/American Institute for Cancer Research (AICR) continuous update project supports the premise that dietary patterns and lifestyle factors significantly influence the risk of these cancers [2]. The Expert Report emphasizes the importance of maintaining a healthy weight, engaging in regular physical activity, adopting a diet high in fruits, vegetables, whole grains, and dietary fiber, and reducing intakes of red meat, animal fats, and refined carbohydrates [2,3,4,5]. Further, evidence suggests that even moderate alcohol consumption can contribute to an increased risk of post-menopausal BC and CRC [6].
Diet is a complex mixture of nutrients, bioactives, additives, and other components that can contribute to the risk of cancer [7]. Some chemicals, such as heterocyclic amines and polycyclic aromatic hydrocarbons formed when meat or fish are cooked at high temperatures, may be directly carcinogenic [8]. Other nutrients, such as saturated fat or added sugars, may be linked with cancer risk indirectly through alterations in various signaling pathways, such as insulin or inflammation [9,10,11]. Saturated fat may also contribute to excess caloric intake and weight gain [12], while foods rich in fermentable fiber may lead to beneficial gut microbial community structure [13,14]. These exposures along with phenotypic information can be captured with high-dimensional tools applied to blood or urine, such as metabolomics. Metabolomics is the comprehensive, qualitative, and quantitative study of the small molecules in an organism and includes both aqueous and lipid metabolites [15]. The metabolome reflects both endogenous processes, as well as diet and other environmental exposures. Thus, it provides a sensitive approach for testing and tracing the involvement of altered biological pathways and networks associated with chronic diseases, such as cancer. Although metabolomics has been used extensively to search for biomarkers of early cancer detection [16,17,18], metabolomic profiles are now being used as risk markers associated with environmental exposures [19,20].
In this study, our aims were to find potential prediagnostic serum and urine metabolite predictors of BC and CRC using multiple metabolomics platforms that provided predictive power above and beyond well-established risk factors within the Women’s Health Initiative (WHI) Bone Mineral Density (BMD) subcohort. Specifically, comparing several variable selection and prediction models, we assessed the competitive performance for BC and CRC prediction using metabolites compared to prediction models with only demographic, clinical, and lifestyle covariates, and assessed whether metabolites improved the prediction performance when added to these well-established risk factors. Also, comparing the results across variable selection and prediction approaches provides an evaluation of the robustness of the selected metabolites and their prediction performance. These analyses may provide novel metabolite–cancer associations and mechanisms, particularly for diet-related metabolites.
2. Materials and Methods
2.1. Women’s Health Initiative
The WHI recruited 161,808 post-menopausal women from 40 clinical centers nationwide between 1 October 1993 and 21 December 1998 [21]. All women were 50–79 years old when they were enrolled in at least one of three clinical trials (CT; n = 68,132) or an observational study (OS; n = 93,676). The three WHI CTs were a randomized controlled clinical trial of menopausal hormone therapy, of low-fat dietary modification, and of calcium/vitamin D supplementation. The WHI BMD subcohort included all participants at three clinical centers (Birmingham, AL; Pittsburgh, PA; and Tucson, AZ, with satellite in Phoenix, AZ) (n = 11,020) chosen to maximize racial and ethnic diversity. All women provided core questionnaires including medical history, reproductive history, family history, medication use, dietary intake, and personal habits [21].
2.2. Case and Control Selection
Cases and controls for this analysis were selected from the WHI BMD subcohort. The eligible sample was restricted to women who had sufficient serum (300 µL) and urine (550 µL) samples from the same time point, prior to and closest to BC or CRC case diagnosis date, and required to have no missing covariate data (n = 10,451). Clinical outcomes were reported biannually in the CT until 2005 through the trial periods, then annually, and annually in the OS. An initial report of invasive cancer during cohort follow-up was confirmed by a review of medical records and pathology reports by physician adjudicators.
The cases were defined as earliest incident invasive BC or CRC so that the biospecimen collection would be comparatively proximate. Each of the 758 case women was matched 1-to-1 to a control woman, disease free at the case occurrence follow-up time, based on age (within 2 years; Table 1), WHI enrollment date (within 2 months to control for follow-up duration), and self-identified race or ethnicity; the closest match was selected based on criteria to minimize an overall distance measure [22]. In total, 54% of the selected sample were in the OS, 34% in the dietary modification (DM) trial, and 12% in the hormone trials (HT) (but not in the DM trial). Our final population included n = 758 adjudicated cancers (577 invasive breast and 181 colorectal) and n = 758 controls.
Table 1.
Demographic, clinical, and lifestyle characteristics of the breast (BC) and colorectal cancer (CRC) cases and controls in the WHI Bone Mineral Density subcohort 1.
| Characteristics | BC Cases (n = 577) |
CRC Cases (n = 181) |
Controls (n = 758) |
|---|---|---|---|
| Demographic factors | |||
| Age (yrs) | 62; [56, 68] | 64; [58, 69] | 63; [57, 68] |
| Body Mass Index (kg/m)2 | 27.73; [24.45, 32.45] * | 28.05; [24.9, 31.98] * | 27.16; [24.19, 31.18] |
| Waist circumference (cm) | 86; [77, 96] * | 87; [78, 99] * | 84; [76, 93] * |
| Self-reported race or ethnicity | |||
| Alaska Native or American Indian | <10 * | <10 * | <10 * |
| Asian or Pacific Islander | <10 * | <10 * | <10 * |
| Hispanic or Latina | 25 (4%) | 10 (6%) | 35 (5%) |
| Non-Hispanic Black or African American | 67 (12%) | 26 (14%) | 93 (12%) |
| White | 477 (83) | 139 (77%) | 616 (81) |
| Unknown | <10 | <10 | <10 |
| Education | |||
| Less than high school | 37 (6%) | 15 (8%) | 41 (5%) |
| High school or GED | 120 (21%) | 43 (24%) | 166 (22%) |
| School after high school | 209 (36%) | 74 (41%) | 284 (38%) |
| College degree or higher | 207 (36%) | 48 (27%) | 267 (35%) |
| Unknown | <10 | <10 | 0 |
| Income | |||
| <$20,000 | 104 (18%) | 56 (31%) | 165 (22%) |
| $20,000–$35,000 | 171 (30%) | 47 (26%) | 232 (31%) |
| $35,000–$50,000 | 100 (17%) | 37 (20%) | 132 (17%) |
| $50,000–$75,000 | 85 (15%) | 21 (12%) | 134 (18%) |
| >$75,000 | 81 (14%) | 13 (7%) | 95 (13%) |
| Unknown | 36 (6%) | <10 | 0 |
| Lifestyle factors | |||
| Alcohol intake (svgs/wk) | 0.42; [0, 1.81] * | 0.21; [0, 2.73] * | 0.21; [0, 1.37] |
| Total calcium (mg/d) | 1024.1; [647.5, 1557.2] * | 1029.3; [711.8, 1532.0] * | 973.8; [649.7, 1530.9] * |
| Total folate (mcg/d) | 635.47; [420.94, 881.23] * | 619.21; [448.25, 861.41] * | 593.64; [419.06, 838.82] * |
| Red or processed meat (svgs/d) | 1.87; [1.04, 2.95] * | 1.85; [1.1, 3.31] * | 1.83; [1.07, 3] * |
| Energy expenditure (MET-hours/wk) | 7.08; [1.5, 16.75] * | 7.25; [1.38, 16.42] * | 7; [1.5, 15.5] * |
| History of smoking (current yes/no) | 45 (7.84%) * | 16 (8.94%)* | 47 (6.2%) |
| Any supplement use | 257 (44.54%) * | 71 (39.23%) | 328 (43.27%) |
| Uses anti-diabetes medication | 24 (4.16%) | 16 (8.84%) | 35 (4.62%) |
| Uses anti-hypertensive medication | 185 (32.06%) | 68 (37.57%) | 231 (30.47%) |
| Uses anti-lipid medication | 42 (7.28%) | 16 (8.84%) | 50 (6.6%) |
| Uses NSAIDs | 196 (33.97%) | 58 (32.04%) | 262 (34.56%) |
| Clinical risk factors | |||
| Gail 5-year risk score | 1.9; [1.16, 2.22] | 1.7; [1.1, 1.92] | 1.68; [1.15, 1.95] |
| Family history of cancer | |||
| Yes | 80 (14%) | 30 (17%) | 124 (16%) |
| No | 453 (79%) | 139 (77%) | 617 (8%) |
| Unknown | 44 (8%) | 12 (7%) | 17 (2%) |
| Personal history of cancer | |||
| Yes | 27 (5%) | 13 (7%) | 39 (5.2%) |
| No | 547 (95%) | 168 (93%) | 719 (95%) |
| Unknown | <10 | 0 | 0 |
| History of colonoscopy | |||
| Yes | 253 (44%) | 71 (39%) | 355 (47%) |
| No | 249 (43%) | 90 (50%) | 401 (53%) |
| Unknown | 75 (13%) | 20 (11%) | <10 |
| History of colon polyp removal | |||
| Yes | 39 (7%) | 11 (6%) | 73 (10%) |
| No | 454 (79%) | 145 (80%) | 677 (89%) |
| Unknown | 84 (15%) | 25 (14%) | <10 |
| History of treated diabetes | |||
| Yes | 27 (5%) | 19 (11%) | 40 (5%) |
| No | 549 (95%) | 162 (90%) | 717 (95%) |
| Unknown | <10 | 0 | <10 |
| History of treated hypertension | |||
| Yes | 144 (25%) | 55 (30%) | 186 (25%) |
| No | 356 (62%) | 104 (57%) | 568 (75%) |
| Unknown | 77 (13%) | 22 (12%) | <10 |
| Had at least one term pregnancy | 510 (88%) | 158 (87%) | 676 (89%) |
| Post-menopausal hormone therapy use | |||
| Never | 286 (50%) | 97 (54%) | 331 (44%) |
| Past | 75 (13%) | 30 (17%) | 125 (16%) |
| Current estrogen alone | 117 (20%) | 36 (20%) | 189 (25%) |
| Current estrogen and progestin | 98 (17%) | 18 (10%) | 113 (15%) |
| Study variables | |||
| WHI enrollment date | |||
| Baseline | 108 (19%) | 44 (24%) | 152 (20%) |
| Year 1 | 220 (38%) | 65 (36%) | 285 (38%) |
| Year 3 | 243 (42%) | 68 (38%) | 311 (41%) |
| Year 6 | <10 | <10 | <10 |
| Year 9 | 0 | <10 | <10 |
| Calcium / Vitamin D (CaD) trial arm | |||
| Not randomized to CaD | 462 (80%) | 147 (81%) | 560 (74%) |
| Control arm | 54 (9%) | 19 (11%) | 104 (14%) |
| Intervention arm | 61 (11%) | 15 (8%) | 94 (12%) |
| Dietary Modification (DM) trial arm | |||
| Not randomized to DM | 384 (67%) | 122 (67%) | 488 (64%) |
| Control arm | 118 (20%) | 27 (15%) | 163 (22%) |
| Intervention arm | 75 (13%) | 32 (18%) | 107 (14%) |
| Hormone therapy (HT) trial arm | |||
| Not randomized to HT | 495 (86%) | 144 (80%) | 641 (85%) |
| Estrogen-only control arm | 19 (3%) | 13 (7%) | 27 (4%) |
| Estrogen-only intervention arm | 15 (3%) | 11 (6%) | 34 (4%) |
| Estrogen and progestin control arm | 16 (3%) | <10 | 30 (4%) |
| Estrogen and progestin intervention arm | 32 (6%) | <10 | 26 (3%) |
1 For continuous variables, the summaries displayed are: median; inter-quartile range. For binary and categorical variables, the summaries displayed are: count (proportion). * Denotes variables with a nonzero proportion of missing data. Overall, the proportion of missing data ranged from 0% to 11%, with most variables having less than 3% missing data. MET: metabolic equivalent hours per week of recreational physical activity; NSAIDs: non-steroidal anti-inflammatory drugs.
2.3. Metabololite Profiling
2.3.1. Measurement of Serum Metabolites
Targeted LC-MS: Serum samples were analyzed by targeted LC-MS/MS using liquid chromatography coupled to a Sciex Triple Quad 6500+ Triple Quadrupole mass spectrometer equipped with an ESI ionization source as described previously [23]. The instrument was attached to two Shimadzu UPLC pumps, and the pumps were connected to an auto-sampler in parallel so that chromatography separation could be performed using two analytical hydrophilic interaction liquid chromatography (HILIC) columns independently, one for positive ionization mode and the other for negative ionization mode. Identical columns (Waters XBridge BEH Amide XP) were used for both separations, and the samples were injected for each column separately. While one column was performing separation and MS data acquisition in ESI+ ionization mode, the other column was equilibrated and readied for analysis in ESI mode. The LC-MS system was controlled using AB Sciex Analyst 1.6.3 software. Serum metabolites were extracted using methanol in a 1:2 (v/v) ratio, dried, and reconstituted in HILIC solvent. MS data acquisition was performed in multiple reaction monitoring (MRM) mode. Measured MS peaks were integrated using AB Sciex MultiQuant 3.0.3 software. A total of 304 metabolites were targeted (see Supplemental Table S4), of which 150 were detected with less than 20% missing values. A total of 304 metabolites were targeted, of which 150 were detected with less than 20% missing values.
Lipidomics: Separately, serum lipid metabolites were measured using the Sciex QTRAP 5500 Lipidyzer platform including the SelexION differential mobility spectrometry (DMS) method [24,25,26]. Serum lipids were extracted using dichloromethane/methanol, dried under nitrogen, and the samples reconstituted in 100 μL of 10 mM ammonium acetate in dichlormethane:methanol (50:50). Lipids were analyzed in multiple reaction monitoring in both positive and negative ionization modes, with and without DMS. The method targeted lipids in 13 major lipid classes: cholesterol ester (CE), ceramides (CER), diacylglycerol (DAG), dihydroceramides (DCER), free fatty acids (FFA), hexosylceramides (HCER), lactosylceramide (LCER), lysophosphatidylcholine (LPC), lysophosphatidylethanolamine (LPE), phosphatidylcholine (PC), phosphatidylethanolamine (PE), sphingomyelin (SM), and triacylglycerol (TAG; see Supplemental Table S4). Absolute concentrations of lipids were obtained based on 54 isotope-labeled internal standards. A total of 1070 lipids were targeted, of which 687 lipids that had less than 20% missing values were measured.
2.3.2. Measurements of Urine Metabolites
NMR spectroscopy: Metabolite profiles from 24 h urine samples were analyzed by NMR spectroscopy using a Bruker Avance III 800 MHz NMR spectrometer. Each sample (300 mL) was mixed with 300 mL phosphate buffer in D2O (pH = 7.4) containing an internal standard, 3-(trimethylsilyl)propionic acid-2,2,3,3-d4 sodium salt (TSP). Data were acquired at 298 K using a one-dimensional pulse sequence with suppression of the residual water signal using presaturation. Spectral width, time domain points, relaxation delay, and number of transients were 10,000 Hz, 32,768, 2 s, and 64, respectively. The raw data were Fourier transformed after zero filling by a factor of two and multiplied using an exponential window function with a line broadening of 0.5 Hz. The resulting spectra were phase and baseline corrected and referenced to the internal standard, TSP. Metabolite peaks were identified using databases and relative concentrations for 59 metabolites were obtained (Supplemental Table S4). None of the metabolites had missing values.
GC-MS analysis: Urine metabolites were also analyzed by untargeted gas chromatography–mass spectrometry (GC-MS) method using an Agilent 7890A/5875C instrument [27]. Urine samples were treated with urease and methoxime prior to derivatization of metabolites using MSTFA (N-Methyl-N-trimethylsilyltrifluoroacetamide) containing 1% TMCS (2,2,2-Trifluoro-N-methyl-N-(trimethylsilyl)-acetamide, chlorotrimethylsilane). We measured a total of 267 metabolites, 107 of which were identified (MSI level 1 or 2; Supplemental Table S4), with none having greater than 20% missing values. Overall, more than 1000 metabolites were identified from serum and urine samples using these four complementary analytical platforms.
2.4. Metabolite Quality Controls (QC)
Analysis protocols used multiple layers of QC samples as well as isotope-labeled or unlabeled internal standards to assess instrument stability/performance during the analysis and help with normalization and metabolite quantitation. Different types of QCs used included: (a) unblinded instrument QC samples (commercially obtained pooled human serum from Innovative Research, Inc. (Novi, MI, USA)) run every 10 samples and at the beginning and end of each batch of samples; (b) blinded, pooled study samples (5% for urine; 10% for serum) interspersed with the biological study samples (3 QCs/batch of 27 study samples), used to normalize batches of samples over the run; (c) 17 split-sample blinded duplicates of study samples also interspersed with study serum and urine samples, used to calculate reported median metabolite coefficient of variation (CV) values; (d) isotope-labeled internal standards for targeted analysis of aqueous metabolite (n = 33) and lipids (n = 54) in serum, which enabled absolute concentration determination and ensured evaluation of instrument stability and data quality; (e) internal standard, TSP, used to assess the spectral quality, calibrate spectra, and help with data normalization of urine NMR spectra; and (f) FAME (fatty acid methyl esters) of different fatty acid chain lengths for retention time indexing and myristic acid-d27 for help with metabolite identification and data normalization, respectively. Median CVs of blinded pooled study QC samples for the four different platforms (two for serum analysis and two for urine analysis) across the samples were 2.9% for global NMR from 24 h urine, 6.4% for targeted lipidomics, 20.7% for targeted LC-MS/MS, and 45.4% for global GC-MS.
2.5. Statistical Analysis
2.5.1. Participant Data
From the originally collected participant data, we selected a base set of covariates (age, chronologic time of visit, and race or ethnicity) and all demographic, clinical, and lifestyle covariates that were adjusted for in Prentice et al. [28]. Some categorical variables—including race or ethnicity, education level, and income—were recoded as binary variables. The base set of variables listed above and other demographic, clinical, and lifestyle covariates considered are summarized in Table 1. We considered all identified metabolites from the four metabolomics platforms with less than 20% missing data. For each outcome, we used all cases corresponding to that outcome and all controls (i.e., all 758 controls were used in predicting both outcomes), which has been shown to improve prediction performance [29].
2.5.2. Imputing Missing Data
While the base set of covariates were measured on all participants in this study, there were missing data in some of the demographic, clinical, and lifestyle variables [BMI < 1%, waist circumference < 1%, smoking history < 1%, energy expenditure 6%, and intake of alcohol, calcium, folate, and red and processed meat missing for fewer than 3% of participants]. We used multiple imputation via chained equations [30] to perform imputations. We ignored the outcome in all imputation models to simplify our procedure for assessing prediction performance described below (see, e.g., [31]). For metabolomics variables, those with more than 20% missing values were removed toward ensuring robust results. For the remaining variables, half of the minimum nonzero value was used to impute the values that were below detection limits. For each platform, we created one set of multiple imputed datasets consisting of only the metabolites and base set of variables, and a second set of multiple imputed datasets consisting of the metabolites and all risk factor variables (including the base set of variables). More detail on the imputation procedure is provided in the Supplemental Methods.
After each platform-specific imputation step was complete, we further processed the data following a similar specification to Zheng et al. [32]. In particular, outliers were truncated to within three times the interquartile range of the first and third quartile. For LC-MS and GC-MS metabolites, we normalized the data within each imputation round and batch using local polynomial regression fitting (in the R package loess) with tuning parameter set to 0.75 among quality-control samples.
To minimize the effect of possible correlated variables on our results and to study the utility of different platforms, we first considered each measurement platform (NMR, LC-MS, GC-MS, and Lipidyzer) separately. Then, for each platform, we performed analyses based on metabolomics alone, and established risk factors + metabolomics, with the base set of covariates (age, chronologic time of visit, and race or ethnicity) always included in each analysis. In a sensitivity analysis, we pooled the metabolites from all platforms together.
2.5.3. Algorithms Used for Selecting a Set of Metabolites
In all analyses, we adjusted for the base set of variables to account for the sampling design [33]. We report adjusted analyses using only the metabolites and using the metabolites plus other established risk factors. For a given platform and set of adjustment covariates, to evaluate the robustness of variable selection and prediction performance, we applied three algorithms to select a set of metabolites and covariates to use in the final risk-prediction algorithm, described below. The first algorithm performed no variable selection (i.e., allowed all metabolites and covariates into the final prediction algorithm). The second was lasso regression [34] implemented in the R package glmnet, with tuning parameters selected using ten-fold cross-validation. We forced the base set of covariates into all lasso models to ensure proper adjustment for these variables. We then selected all variables with a nonzero estimated coefficient.
The final procedure used the Super Learner [35] implemented in the R package SuperLearner [36]. The Super Learner is a particular implementation of stacking models [37]; in this algorithm, a library of candidate learners is fit to the data, and cross-validation is used to create the convex combination of these candidate learners that minimizes a cross-validated loss criterion. In these analyses, we used the non-negative log-likelihood loss function. The resulting convex combination has both finite-sample and asymptotic guarantees on its performance [35]. Our candidate library consisted of elastic net regression [38], boosted trees [39], and random forests [40]. The R implementations of these algorithms and the tuning parameters used are provided in Supplemental Table S1. To perform variable selection using the Super Learner, we first computed a variable importance measure for each candidate algorithm: an estimated coefficient for the elastic net and a decrease in Gini impurity for both trees and forests. We then ranked the variables from most to least important by the algorithm-specific metrics and combined the ranks using the convex weights of the Super Learner. We then selected variables with weighted rank (weights based on the Super Learner; see the Supplemental Methods) in the top 20. This ensures that algorithms with high weight in the Super Learner ensemble—implying that the algorithm has favorable cross-validated performance—have a large influence in selecting variables.
2.5.4. Assessing Prediction Performance
After selecting a set of metabolites and covariates, we addressed the performance of these variables in predicting either BC or CRC. We fit two final prediction algorithms for each platform. The first was a simple logistic regression. The second was the Super Learner, using the same approach as described above. This resulted in four procedures based on variable selection: variable selection with the lasso, followed by either logistic regression (denoted lasso + GLM below) or the Super Learner (denoted lasso + SL) for prediction; and variable selection with the Super Learner, followed by logistic regression (denoted SL + GLM) or the Super Learner (denoted SL + SL) for prediction. We compared these four approaches with two that did not use variable selection: the Super Learner with all variables (denoted SL) and the Super Learner with all variables that used a library of candidate learners augmented with variable selection algorithms [denoted SL (with screens)]. Further details on these procedures are provided in the Supplemental Methods.
Assessing prediction performance of sets of selected variables was complicated by the fact that these variables were not determined a priori [41]. We used cross-validation to assess the performance of a combined procedure for variable selection and prediction using the selected variables, whereby the selected variables and prediction algorithm were determined on training data and prediction performance was evaluated on independent data. We repeated this cross-validated procedure 100 times for each platform and set of adjustment variables (base set of variables only or all risk factor variables). We measured prediction performance using the cross-validated area under the receiver operating characteristic curve (CV-AUC). Detail on this cross-validated procedure is provided in Supplemental Methods.
2.5.5. Final Selection of Metabolites
We obtained a final set of selected variables from each platform by applying the variable selection procedure to the full set of observations for each imputed dataset; our final set consisted of those metabolites and covariates that were selected in over 70% of the individual imputed datasets. For each platform and set of adjustment covariates, we then took the union of the sets resulting from the two variable selection procedures. Our final set of metabolites was a further union of the platform-specific selected sets, while the final set of adjustment covariates was the unique covariates selected in any of the platform-specific analyses (Supplemental Figures S1 and S2).
2.5.6. Post Hoc Sensitivity Analyses
Prior studies within WHI cohorts suggest an interaction between HT use and insulin such that associations between obesity-related measures, i.e., BMI, adipokines, levels of insulin, etc., and both BC and CRC were only observed among non-HT users [42,43]. It has been proposed that oral HT exposes the liver to a large dose of estrogen, leading to altered hepatic protein synthesis. Because HT could potentially alter metabolites associated with BC and CRC in our analysis, we conducted a sensitivity analysis for both cancer outcomes, excluding women randomized to the active arms of the HT or who reported current HT use at baseline.
3. Results
Characteristics of the WHI BMD participants stratified by BC and CRC cases and controls are given in Table 1. The mean time between blood draw and cancer diagnosis was 7.2 years (IQR 2.4–11.6 years).
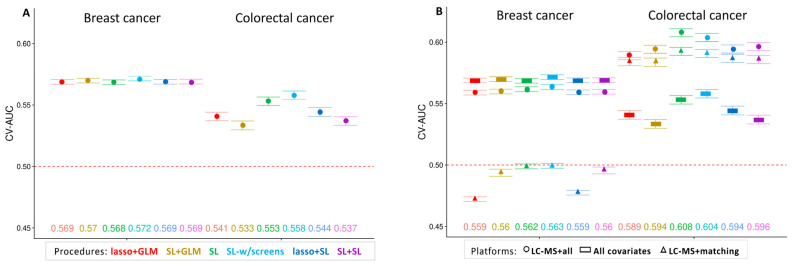
Of the four metabolomics platforms, the greatest prediction potential was observed with LC-MS, which targeted water-soluble metabolites in serum. We present the cross-validated performance of each procedure for predicting BC and CRC using the LC-MS platform in Figure 1. In the left panel, we see that the base set of covariates, forced into all prediction models, and demographic, clinical, and lifestyle variables alone were moderately predictive of BC, and similar across all six prediction procedures, with a maximum CV-AUC of 0.572 from the SL (with screens) procedure. Performance for predicting CRC based on addition of risk covariates was similar (absolute difference 0.014), at a maximum CV-AUC of 0.558. In the right panel, we overlay the prediction performance using the metabolites and the prediction performance using all covariates and the metabolites. Metabolites alone were not good predictors of BC (CV-AUCs at or below 0.5), and the prediction performance of risk covariates plus metabolites was similar to that of the covariates alone, without performance improvement. In contrast, for CRC, prediction performance was improved for all six algorithms when using metabolites alone or metabolites plus risk covariates, with a maximum CV-AUC of 0.593 based on metabolites alone and 0.608 combining metabolites and clinical variables from the SL algorithm with no variable selection.
Figure 1.
Cross-validated area under the receiver operating characteristic curve (CV-AUC) averaged over 100 Monte Carlo replications of each variable selection + regression procedure for predicting breast cancer and colorectal cancer, with 95% confidence intervals (CIs). (panel A) an analysis using only the covariates. (panel B) analyses using covariates only (circles), LC-MS metabolites + base set of covariates (triangles), and LC-MS metabolites + all covariates (squares). Point estimates of CV-AUC are provided at the bottom of each panel (on the right-hand panel, the point estimates correspond to the LC-MS metabolites + all covariates analysis).
Results for the remaining platforms tended to also be consistent across the six algorithms. GC-MS and Lipidyzer-detected metabolites provided little to no additional prediction performance for either BC or CRC over the clinical variables. NMR-detected metabolites tended not to increase prediction performance for BC over the clinical variables; for prediction of CRC, these metabolites had a performance comparable to the risk covariates and also led to a slight increase in prediction performance when added to the risk covariates. The full set of results are presented in Supplemental Tables S2 and S3.
In Table 2, we present the selected risk covariates and metabolites for predicting both BC and CRC, as well as the estimated proportion of variation explained (PEV) by each metabolite. Several risk covariates shown to be predictive of BC (including Gail 5-year risk score) or CRC (at least one colonoscopy or colon polyp removed) were selected, lending validation of our selection results. For individual metabolites, the PEVs were similar and in the range of 0.21 to 0.25, suggesting that many of the metabolites do not differentiate prediction performance alone, but can result in differential prediction performance together. Glycerate explained the most variability (PEV = 0.25) in CRC (adjusted for all risk factor variables). Metabolites selected for CRC, along with function, are given in Table 3.
Table 2.
Metabolites selected with proportion of explained variation for predicting breast cancer and colorectal cancer 1.
| Metabolites Selected | Proportion of Explained Variation 2 | Direction of Coefficient for Metabolites 3 |
|---|---|---|
| Breast Cancer | All covariates + metabolites: 0.27 | |
| Serum: | ||
| LC-MS | ||
| Azelaic acid | 0.23 | − |
| Choline | 0.23 | + |
| Cysteinyl glycine | 0.23 | − |
| Ethanolamine | 0.23 | + |
| Gamma tocopherol | 0.23 | + |
| Hippuric acid | 0.23 | − |
| Isovaleryl carnitine | 0.23 | + |
| N-isovaleryl glycine | 0.23 | − |
| Sucrose | 0.23 | − |
| Trimethylamine-N-oxide | 0.23 | + |
| Valine | 0.23 | + |
| Xylose | 0.23 | − |
| Lipidyzer 4 | ||
| Cholesteryl ester (CE 12:0) | 0.23 | − |
| Cholesteryl ester (CE 20:0) | 0.23 | − |
| Diacylglycerol (DAG 14:1) | 0.24 | + |
| Free fatty Acid (FFA 18:4) | 0.23 | − |
| Free fatty Acid (FFA 20:2) | 0.23 | + |
| Hexosylceramide (HCER 22:0) | 0.23 | + |
| Hexosylceramide (HCER 22:0) | 0.23 | − |
| Phosphatidylcholine (PC 18:1) | 0.23 | + |
| Phosphatidylcholine (PC 18:2) | 0.23 | + |
| Phosphatidylcholine (PC 16:0/18:2) | 0.24 | − |
| Phosphatidylethanolamine (PE 18:2) | 0.23 | + |
| Triacylglycerol (TAG 12:0) | 0.23 | − |
| Triacylglycerol (TAG 16:0) | 0.23 | − |
| Triacylglycerol (TAG 18:0) | 0.23 | − |
| Triacylglycerol (TAG 47:0/15:0) | 0.23 | − |
| Triacylglycerol (TAG 48:4/18:2) | 0.23 | − |
| Triacylglycerol (TAG 50:0/16:0) | 0.23 | + |
| Triacylglycerol (TAG 50:2/18:2) | 0.23 | − |
| Triacylglycerol (TAG 50:5/18:3) | 0.24 | − |
| Triacylglycerol (TAG 52:2/18:2) | 0.24 | + |
| Triacylglycerol (TAG 55:4/18:1) | 0.23 | − |
| Urine | ||
| NMR | ||
| Dimethylamine | 0.23 | − |
| Propanediol | 0.23 | − |
| Formate | 0.23 | + |
| Sucrose | 0.23 | − |
| Taurine | 0.23 | + |
| Uracil | 0.23 | − |
| Trimethylamine-N-oxide | 0.23 | − |
| 2-Hydroxyisobutyrate | 0.23 | + |
| 2-Oxoglutarate | 0.23 | − |
| GC-MS | ||
| Unknown 73.012.10 5 | 0.23 | − |
| Unknown 73.014.49 5 | 0.23 | + |
| Unknown 73.016.52 5 | 0.23 | + |
| Colorectal Cancer | All covariates + metabolites: 0.31 | |
| Serum | ||
| LC-MS | ||
| Adenosine | 0.23 | − |
| Leucic Acid | 0.21 | + |
| Glycerate | 0.25 | + |
| Myo-inositol | 0.22 | + |
| N-Acetyl-glutamate | 0.22 | − |
| N-Acetyl-glycine | 0.23 | + |
| N-Acetylneuraminate | 0.22 | + |
| 2-Hydroxyglutarate | 0.22 | + |
| Hydroxyproline | 0.21 | + |
| 7-Methylguanine | 0.22 | + |
| Lipidyzer 4 | ||
| Lysophosphatidylcholine (LPC 20:3) | 0.22 | − |
| Urine | ||
| NMR | ||
| Acetate | 0.21 | + |
| Allantoin | 0.21 | − |
| Histidine | 0.22 | − |
| Isoleucine | 0.21 | + |
| Taurine | 0.22 | + |
| Threonine | 0.21 | + |
| Trimethylamine-N-oxide | 0.21 | + |
| Uracil | 0.22 | − |
| GC-MS | ||
| Unknown 103 17.03 5 | 0.21 | − |
| Unknown 285 22.41 5 | 0.22 | + |
| Unknown 57 9.58 5 | 0.22 | + |
| Unknown 73 10.76 5 | 0.21 | − |
| Unknown 73 17.66 5 | 0.21 | + |
1 All variables listed below were selected by either the lasso or SL selection procedure in the corresponding platform-specific analysis. The base set of covariates (forced into all models) were age, WHI enrollment date, and self-reported race or ethnicity. Selected covariates for breast cancer: education level, income, alcohol intake, current smoking, total folate intake, Gail 5-year risk, family history of CRC, prior removal of ≤1 colon polyp, currently using estrogen, waist circumference, BMI (kg/m2), randomized to CaD or HT, date of sample draw visit. Selected covariates for colorectal cancer: age, self-reported race/ethnicity, education, income, alcohol intake, total folate intake, waist circumference, BMI (kg/m2), ≥1 colonoscopy, prior removal of ≥1 colon polyp, sample draw visit, randomized to DM control arm. 2 The proportion of explained variation (PEV) was estimated by first creating a dataset with only the selected metabolites and covariates for each outcome. Then, we used cross-validation to fit a logistic regression on each set of training data and predict on the test data; the PEV is defined as the correlation between the observed outcomes and the predictions. 3 Positive direction of the estimated coefficient from the multiple logistic regression model implies higher odds of being a case; negative direction implies lower odds of being a case. 4 In CE, X:A; FFA, X:A; DAG, X:A/Y:B; HCER, X:A; PC, X:A/Y:B; PE, X:A/Y:B; and LPC, X:A, X and Y indicate the number of carbon atoms and A and B indicate the number of double bonds in the fatty acid chains. Lipids without both A and B represent the sum of all fatty acids in that class. For example, DAG (14:1) equals the sum of all diacylglycerol, i.e., summing all DAG (x/14:1) and DAG (14:1/x). 5 Values represent mass at retention time of the unknown metabolites, i.e., 73 12.10 indicates a mass of 73 at 12.10 min. In TAG, X:A/Y:B, X indicates the total number of carbon atoms and A indicates the total number of double bonds in the three fatty acid chains, and Y indicates the number of carbon atoms and B indicates the number of double bonds in one of the fatty acid chains.
Table 3.
Metabolite predictors of colorectal cancer derived across all platforms and prediction algorithms, and sensitivity analyses, along with class and function 1.
| Metabolite | Class | Function/Relevance |
|---|---|---|
| 2-Hydroxyglutarate | Hydroxy acid | TCA intermediate; inhibitor of alpha-keto dehydrogenases, including histone demethylases; considered an oncometabolite |
| N-Acetyl-glycine | Alpha amino acid | Lipid signaling mediator |
| Taurine | Sulfur-containing amino acid |
Metabolism of fats, bile acids |
| Threonine | Amino acid | Metabolism of fats |
| TAG (53:2/FA18:1) | Triglyceride | Fat storage; energy |
| LPC (FA20:3) | Lysophosphatidyl choline | Cholesterol metabolism |
| CE (FA20) | Cholesteryl ester | Cholesterol metabolism |
| Acetate | Short chain fatty acid | Microbial metabolite; acetylation reactions, energy metabolism |
| Glycerate | Sugar acid | Generation of ATP |
| Adenosine | Nucleoside | Energy transfer, component of RNA/DNA |
| Hypoxanthine | Purine | Metabolism of adenosine |
| Uracil | Nucleic acid | Component of RNA |
| 7-Methylguanine | Purine | Component of RNA/DNA; potential biomarker of chicken |
| Histidine | Amino acid | Protein synthesis, histamine and carnosine biosynthesis, scavenger of ROS |
| Leucic acid | Hydroxy fatty acid | Leucine (branched-chain amino acid) metabolite; accelerates lipid peroxidation, oxidative stress |
| Isoleucine | Branched-chain amino acid | Protein metabolism, hemoglobin production, glucose control, immunity |
| N-Acetyl-glutamate | Alpha amino acid | Involved in the urea cycle |
| Allantoin | Imidazoles | Microbial metabolite; uric acid metabolite; found in dairy |
| N-Acetyl-neuraminate | Amino sugar | Component of glycoproteins and mucins involved in immunity |
| Hydroxyproline | Amino acid | Component of collagen; biomarker of meat |
| Myo-inositol | Sugar alcohol | Component of phosphatidylinositol; increases insulin sensitivity; biomarker of whole grains |
| Trimethylamine-N-oxide | Amine oxide | Microbial metabolite formed from choline, betaine, and carnitine; associated with cardiovascular disease; biomarker of fish and red meat |
1 Class and function ascertained from PubChem or Human Metabolome Database; unnamed metabolites not included. TAG: triacylglyceride; LPC: lysophosphatidyl choline; CE: cholesterol ester.
To assess the effect of performing variable selection and estimating prediction performance based on each platform separately, we performed a sensitivity analysis. In this analysis, we pooled the metabolites from each platform together after imputation but before the variable selection and prediction performance analysis. Here, we only fit the lasso + GLM algorithm since we observed similar performance across procedures in the primary analysis. Estimated prediction performance based on the pooled set of metabolites was similar to that observed for the lasso + GLM algorithm for the LC-MS metabolites: CV-AUCs of 0.554 (BC) and 0.58 (CRC). In Table 4, we present the set of selected risk covariates and metabolites, along with the estimated PEV. Many metabolites selected from this sensitivity analysis were also selected in the platform-specific analyses, more so for CRC than BC, which had few metabolites selected in the pooled analysis. The estimated PEV was also similar for most metabolites, with glycerate, which was positively associated with CRC, again providing the largest PEV.
Table 4.
Metabolites selected for predicting breast cancer and colorectal cancer in pooled analysis 1.
| Metabolites Selected | Proportion of Explained Variation 2 | Direction of Coefficient for Metabolites 3 |
|---|---|---|
| Breast Cancer | All covariates + metabolites: 0.27 | |
| Serum | ||
| LC-MS | ||
| Cystenyl-glycine | 0.22 | − |
| Ethanolamine | 0.21 | + |
| Sucrose | 0.22 | − |
| Lipidyzer 4 | ||
| Free fatty acid (FFA 20:2) | 0.22 | + |
| Phosphatidylcholine (PC 16:0/18:2) | 0.23 | + |
| Triacylglyceride (TAG 48:4/18:2) | 0.22 | − |
| Triacylglyceride (TAG 50:5/18:3) | 0.22 | − |
| Triacylglyceride (TAG 52:2/18:2) | 0.22 | + |
| Urine | ||
| NMR | ||
| Uracil | 0.22 | − |
| 2-Hydroxyisobutyrate | 0.22 | + |
| GC-MS | ||
| Unknown 73.0 14.49 5 | 0.21 | + |
| Unknown 73.0 12.10 5 | 0.22 | − |
| Colorectal Cancer | All covariates + metabolites: 0.33 | |
| Serum | ||
| LC-MS | ||
| Adenosine | 0.20 | − |
| Leucic acid | 0.18 | + |
| Glycerate | 0.23 | + |
| Hypoxanthine | 0.18 | + |
| Myoinositol | 0.19 | + |
| N-Acetylneuraminate | 0.19 | − |
| 2-Hydroxyglutarate | 0.19 | + |
| 7-Methylguanine | 0.19 | + |
| Lipidyzer | ||
| CE (FA20) | 0.17 | − |
| TAG (53:2/18:1) | 0.18 | + |
| Urine | ||
| NMR | ||
| Histidine | 0.18 | − |
| Taurine | 0.19 | + |
| Threonine | 0.17 | + |
| GC-MS | ||
| Unknown 103 17.03 5 | 0.18 | − |
| Unknown 285 22.41 5 | 0.18 | + |
| Unknown 57 9.58 5 | 0.18 | + |
| Unknown 73 10.76 5 | 0.18 | − |
| Unknown 73 17.27 5 | 0.17 | + |
1 All variables listed below were selected using the lasso for variable selection with all four platforms pooled together prior to variable selection. The base set of covariates (forced into all models) are age, WHI enrollment date, and self-reported race or ethnicity. Selected covariates for breast cancer: age, self-reported race/ethnicity, income, Gail 5-year risk score, waist circumference, sample draw visit, randomized to the CaD control arm. Selected covariates for colorectal cancer: age, self-reported race/ethnicity, income, education, waist circumference, sample draw visit. 2 The proportion of explained variation (PEV) was estimated by first creating a dataset with only the selected metabolites and covariates for each outcome. Then, we used cross-validation to fit a logistic regression on each set of training data and predict on the test data; the PEV is defined as the correlation between the observed outcomes and the predictions. 3 Positive direction of the estimated coefficient from the multiple logistic regression model implies higher odds of being a case; negative direction implies lower odds of being a case. 4 FFA: free fatty acid; FA: fatty acid; TAG: triacylglyceride; PC: phosphatidyl choline; CE: cholesterol ester. 5 Values represent mass at retention time of the unknown metabolites, i.e., 73 12.10 indicates a mass of 73 at 12.10 min. In post hoc analyses excluding women using HT, prediction performance in the subpopulation was modestly improved for CRC compared to the full population (CV-AUCs range from 0.622–0.637, while in the full population they range from 0.589–0.608). Prediction performance for BC was slightly decreased in the subpopulation compared to the full population (CV-AUCs range from 0.535–0.554, while in the full population they range from 0.559–0.563). Several LC-MS metabolites were selected in the subgroup analysis that were also selected in the whole-cohort analysis: cysteinyl glycine, N-isovaleryl glycine, and valine (BC); adenosine, leucic acid, glycerate, hydroxyproline, and 2-hydroxyglutarate (CRC). Additional metabolites selected in the subgroup analysis for CRC included adipic and 3-hydroxybutyric acids, involved in fatty acid metabolism; betaine, a marker of whole grains; glucuronate, found in gums and fermented beverages; and trigonelline, found in coffee.
4. Discussion
In this well-characterized cohort of post-menopausal women, we evaluated whether the addition of serum and urine metabolites from multiple platforms were equivalent to or provided improved prediction of BC and CRC, beyond well-established risk factors. For BC, risk covariates alone provided moderate predictive power, in the range of CV-AUC 0.57, with metabolites contributing no improvement. In fact, the highest CV-AUC using both risk covariates and metabolites was <0.56. Conversely, for CRC, the addition of metabolites, particularly serum aqueous species from the LC-MS platform, modestly improved prediction performance over risk covariates alone, from CV-AUC of 0.54 to 0.61. This improvement was consistent across various prediction algorithms and metabolite platforms and held whether we performed variable selection within each platform separately or after pooling all metabolites together.
While metabolites did not provide additional prediction power for BC in our analyses, of those that were selected, a large proportion were lipids (22 of 43 named metabolites) or metabolites related to lipid metabolism. Associations between lipids and BC align with accumulating evidence associating excess adiposity, especially after menopause, with increased BC risk [2,5,44]. Obesity is associated with systemic inflammation, insulin resistance, altered steroid metabolism, and other metabolic derangements—factors mechanistically linked to carcinogenesis [44,45,46]. However, few lipids were selected in sensitivity analyses where all metabolites were pooled across all platforms. Moreover, variables, such as alcohol intake, waist circumference and BMI, current estrogen use, and Gail 5-year risk score, were superior to metabolites in predicting BC.
In contrast, mainly aqueous and urinary metabolites were selected in predictive models for CRC, with few if any lipids. Twenty-two different named metabolites were selected in the various prediction algorithms that contributed to CRC prediction, the majority consistently selected across procedures. Several were related to energy metabolism, including adenosine, 2-hydroxyglutarate, and glycerate, with additional metabolites, N-acetyl-glycine, taurine, threonine, and lysophosphatidyl choline [LPC (FA20:3)] related to fatty acid metabolism in particular. These metabolites suggest altered metabolism, a hallmark of cancer. An even larger proportion of metabolites were involved in protein metabolism. Histidine, N-acetyl-glutamate, and allantoin were inversely associated with CRC. As has been previously reported in two other large prospective cohorts, higher circulating histidine, even up to 10 years prior to diagnosis, was associated with reduced risk of CRC [47]. N-acetyl-glutamate functions as a cofactor in ureagenesis, converting nitrogen from protein to urea acids such as allantoin [48]. Other amino acids and peptides were positively associated with CRC, potentially reflecting higher protein intakes. For example, trimethylamine N-oxide (TMAO) is elevated in blood after consumption of fish or foods rich in choline and carnitine, such as red meat, eggs, and dairy products, which can be converted to trimethylamine by gut microbes [49], and subsequently to TMAO by hepatic enzymes in the liver. This metabolite has also been previously linked with CRC [50,51]. The branched-chain amino acids isoleucine and leucic acid, a metabolite of leucine, hydroxyproline, methylguanine, and n-acetyl-neuraminate, are all animal protein derived metabolites. Higher intakes of animal protein, especially red and processed meat, are a known risk factor for CRC [52,53]. In addition to generation of ATP, adenosine, along with the purine 7-methylguanine and pyrimidine uracil, are involved in DNA and RNA synthesis as well as participating as signaling molecules. Lastly, myo-inositol is a biomarker found in whole grains. These metabolites as a group are highly representative of dietary exposures and support conclusions from the WCRF/AICR Third Expert Report indicating probable or convincing evidence for several dietary components contributing to CRC risk, i.e., red and process meat, heme-containing foods in general, and low intake of fruits and non-starchy vegetables, but less so for BC risk, with strong evidence limited to alcohol intake [2].
While metabolite biomarkers have historically been used for cancer detection, studies are now using pre-diagnostic metabolites to examine environmental exposure and cancer risk. To date, 10 studies have focused on BC, with varying metabolite signatures [19,54,55,56,57,58,59,60,61,62,63]. These studies, including information on population, sample size, follow-up time, and analytic platforms have been extensively detailed in His et al. [19]. Most studies report significant associations with one or more metabolites, most commonly specific lipid species and amino acids. However as has been noted, except for steroids, there is little metabolite overlap across studies, including our own, making it very difficult to conduct comparisons [19,60].
Similar work in large prospective cohorts using pre-diagnostic samples has been conducted in the context of CRC. A case-control study nested within two Shanghai cohorts identified several serum phosphatyidylcholines and phosphatidylethanolamines that were inversely associated with CRC, suggesting that dysregulation of glycerophospholipids may contribute to CRC [64]. In the Prostate, Lung, Colorectal, and Ovarian Cancer Screening Trial, an inverse association was reported between leucyl-leucine, a metabolite representing incomplete protein catabolism, and CRC risk after eight years of follow-up, although the association did not remain significant after adjusting for multiple comparisons [65]. In the European Prospective Investigation into Cancer and Nutrition, concentrations of two lipid species—hydroxysphingomyelin C22:2 and acylakyl-phosphatidylcholine C34:3—were significantly inversely associated with CRC risk using a targeted metabolomics approach [66], with nine additional features, including two potentially annotated ceramides, reported in a follow-up analysis using untargeted lipidomics [67]. Investigators also identified a metabolite signature of greater body size, i.e., BMI, waist circumference, and waist-to-hip ratio, associated with a CRC. These metabolites were mainly related to amino acids and lipids, some of which were reversible with weight loss in a small subset of participants in a weight-loss pilot intervention [68]. In a multicenter study, a panel of 17 urine metabolites separated CRC patients from controls, with two providing good prediction in post hoc analyses (AUC of 0.86): diacetylspermine and kynurenine [69]. Another untargeted metabolomics approach was employed in the Cancer Prevention Study II Nutrition Cohort, where six named metabolites were related to CRC risk, including guanidinoacetate, 2′-I-methylcytidien, vanillylmandelate, bilirubin (E,E), N-palmitoylglycine, and 3-methylxanthine [70]. Finally, using untargeted metabolomics on plasma obtained up to 26 years prior to diagnosis in the Northern Sweden Health and Disease Study, seven features were found to be associated with CRC risk, two of which were identified as pyroglutamic acid, an amino acid derivative, and hydroxytigecyline, an antibiotic metabolite [71]. In both of the latter two studies, efforts to replicate previous findings in prospective cohorts were unsuccessful, except for 3-hydroxybutyric acid [71]. While the majority of metabolites selected in our predictive analyses for CRC were also novel, histidine, TMAO, and hydroxy proline were previously reported in other studies [47,50,51,69].
The strengths of this study include a well-characterized cohort, the novel use of four different metabolomics platforms, inclusion of both pre-diagnostic serum and urine, and several variable selection and prediction algorithms, which all yielded similar results, lending confidence to our findings. Further, our results are comparable to those reported by Wang et al. [72], using an alternate statistical approach in this population [73]. Our study population comprised post-menopausal women and may not be generalizable to other populations. We did not adjust for medication use, which may alter metabolite concentrations [74]; however, sensitivity analyses excluding women randomized to HT or women reporting use of HT at baseline yielded similar prediction performance results for both cancer outcomes. Other limitations include those commonly associated with metabolomic studies. A single data point may not be sufficient to adequately capture environmental or dietary exposures, and different metabolites measured may represent either exogenous exposures or alterations in endogenous processes. Further, our detected metabolite coverage of all pathways is incomplete. Nonetheless, we identified a panel of metabolites that were associated with risk of CRC and are biologically plausible.
In summary, we report a panel of metabolites associated with CRC risk in a subset derived from a large prospective cohort of post-menopausal women. That we identified more pre-diagnostic metabolites predictive of CRC than BC may reflect a more established, comprehensive set of metabolites available for CRC. Even for CRC, however, it is worth noting that the predictive contributions of metabolites were modest relative to established risk factors alone, and the results are largely disparate across this and other studies. This may be due, in part, to differences in populations, analytic platforms, length of biospecimen storage times, and statistical approaches. Further studies with repeated sampling, pooled analyses, and expanded metabolite platforms in diverse populations are needed to strengthen the comparison of results across studies.
Acknowledgments
The authors acknowledge the following investigators in the WHI Program: Program Office: (National Heart, Lung, and Blood Institute, Bethesda, Maryland) Jacques Rossouw, Shari Ludlam, Joan McGowan, Leslie Ford, and Nancy Geller. Clinical Coordinating Center: (Fred Hutchinson Cancer Research Center, Seattle, WA, USA) Garnet Anderson, Ross Prentice, Andrea LaCroix, and Charles Kooperberg. Investigators and Academic Centers: (Brigham and Women’s Hospital, Harvard Medical School, Boston, MA, USA) JoAnn E. Manson; (MedStar Health Research Institute/Howard University, Washington, DC, USA) Barbara V. Howard; (Stanford Prevention Research Center, Stanford, CA, USA) Marcia L. Stefanick; (Ohio State University, Columbus, OH, USA) Rebecca Jackson; (University of Arizona, Tucson/Phoenix, AZ, USA) Cynthia A. Thomson; (University at Buffalo, Buffalo, NY, USA) Jean Wactawski-Wende; (University of Florida, Gainesville/Jacksonville, FL, USA) Marian Limacher; (University of Iowa, Iowa City/Davenport, IA, USA) Jennifer Robinson; (University of Pittsburgh, Pittsburgh, PA, USA) Lewis Kuller; (Wake Forest University School of Medicine, Winston-Salem, NC, USA) Sally Shumaker; (University of Nevada, Reno, NV, USA) Robert Brunner. Women’s Health Initiative Memory Study: (Wake Forest University School of Medicine, Winston-Salem, NC, USA) Mark Espeland. For a list of all the investigators who have contributed to WHI science, please visit: https://www.whi.org/doc/WHI-Investigator-Long-List.pdf, accessed on 20 July 2024. The views expressed are those of the authors and do not necessarily represent the views of the National Heart, Lung, and Blood Institute, the National Cancer Institute, the National Institutes of Health, or the U.S. Department of Health and Human Services.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/metabo14080463/s1, Supplemental Methods; Figure S1: Schematic showing the procedure taken to obtain the final sets of selected metabolites in Table 2. For each platform and set of covariates, we first perform lasso or Super Learner (SL) variable selection on each imputed dataset (for NMR, there were no missing data, so we only use the original dataset). Next, for both lasso and SL, we then determined the metabolites and covariates that were selected in at least 7 imputed datasets. Third, we combine the selected variables from the lasso and SL procedures. Our final set of variables pools across all platforms. Figure S2: Schematic showing the procedure taken to assess prediction performance. For a given platform and set of covariates, we randomly split the data 100 times into 5-fold cross-validation (CV) allocations. Then for each of the 100 allocations separately, we performed five-fold cross-validation to assess prediction performance: for each fold k in turn, we (i) perform variable selection on the folds other than k; (ii) train a prediction model on the folds other than k; (iii) assess prediction performance on fold k; and (iv) average the prediction performance across the five folds to obtain CV prediction performance. We then averaged the CV prediction performance across the 100 random allocations to obtain the overall CV prediction performance for each platform, set of covariates, and procedure. Table S1: Candidate learners in the Super Learner ensemble along with their R implementation, tuning parameter values, and description of the tuning parameters. All tuning parameters besides those listed here are set to their default values. In particular, the random forests are grown with 500 trees, a minimum node size of 5 for continuous outcomes and 1 for binary outcomes, and a subsampling fraction of 0.632; the boosted trees are grown with a maximum of 1000 trees, shrinkage rate of 0.1, and a minimum of 10 observations per node. Table S2: Cross-validated area under the receiver operating characteristic curve (CV-AUC) averaged over 100 Monte Carlo replications of each variable selection + regression procedure for predicting breast cancer, with 95% confidence intervals (CIs). An analysis with only covariates (base set + clinical) is provided for comparison. The library of candidate learners used in the Super Learner (SL) is provided in Table S1. Table S3: Cross-validated area under the receiver operating characteristic curve (CV-AUC) averaged over 100 Monte Carlo replications of each variable selection + regression procedure for predicting colorectal cancer, with 95% confidence intervals (CIs). An analysis with only covariates (base set + clinical) is provided for comparison. The library of candidate learners used in the Super Learner (SL) is provided in Table S1. A list of all metabolites targeted, and transitions for LC-MS is given in Table S4.
Author Contributions
Conceptualization, S.L.N., B.D.W., Y.H., D.R., R.L.P., M.L.N. and J.W.L.; Methodology, S.L.N., B.D.W., Y.H., D.R., R.L.P., M.L.N., J.W.L. and C.Z.; Formal Analysis, B.D.W., Y.H., C.Z. and S.L.N.; Investigation, S.L.N., B.D.W., Y.H., D.R., L.F.T., M.L.N. and J.W.L.; Data Curation, L.F.T., C.Z., H.P., G.A.N.G., D.D. and H.G.; Writing—Original Draft Preparation, S.L.N. and B.D.W.; Writing—Review & Editing, S.L.N., B.D.W., M.L.N., J.W.L., Y.H., G.A.N.G., D.R., L.F.T., C.Z., S.A.A.B., F.K.T. and H.D.S.; Visualization, B.D.W.; Project Administration, L.F.T., R.L.P., M.L.N. and J.W.L.; Funding Acquisition, M.L.N., J.W.L., R.L.P., S.L.N., Y.H., G.A.N.G., D.R., L.F.T., C.Z. and S.A.A.B. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Institutional Review Board of Fred Hutchinson Cancer Center (protocol code #6299 approved 31 December 2006 with continuation approval obtained 29 November 2023).
Informed Consent Statement
Written informed consent was obtained from all participants involved in the study.
Data Availability Statement
Data, codebook, analytic code used in this report may be accessed in a collaborative mode as described on the Women’s Health Initiative website (www.whi.org).
Conflicts of Interest
The authors declare no conflicts of interest.
Funding Statement
The WHI program is funded by the National Heart, Lung, and Blood Institute, National Institutes of Health, U.S. Department of Health and Human Services through 75N92021D00001, 75N92021D00002, 75N92021D00003, 75N92021D00004, 75N92021D00005. The NPAAS study is funded by NCI R01 CA119171. S.L.N., J.W.L., M.L.N., and D.R. are additionally funded by NIDDK P30 DK035816 (Nutrition and Obesity Research Center), NCI P30 CA015704, and NIH ORIP S10 OD021562 (D.R.) grants. Y.H. is additionally funded by NCI R01 CA277133.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Siegel R.L., Wagle N.S., Cercek A., Smith R.A., Jemal A. Colorectal cancer statistics, 2023. CA Cancer J. Clin. 2023;73:233–254. doi: 10.3322/caac.21772. [DOI] [PubMed] [Google Scholar]
- 2.American Institute for Cancer Research . Continuous Update Project Expert Report: Diet, Nutrition, Physical Activity and Cancer: A Global Perspective. World Cancer Research Fund; London, UK: 2018. [(accessed on 2 April 2023)]. Available online: www.dietandcancerreport.org. [Google Scholar]
- 3.Yusof A.S., Isa Z.M., Shah S.A. Dietary patterns and risk of colorectal cancer: A systematic review of cohort studies (2000–2011) Asian Pac. J. Cancer Prev. 2012;13:4713–4717. doi: 10.7314/APJCP.2012.13.9.4713. [DOI] [PubMed] [Google Scholar]
- 4.Xiao Y., Xia J., Li L., Ke Y., Cheng J., Xie Y., Chu W., Cheung P., Kim J.H., Colditz G.A., et al. Associations between dietary patterns and the risk of breast cancer: A systematic review and meta-analysis of observational studies. Breast Cancer Res. 2019;21:16. doi: 10.1186/s13058-019-1096-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Clinton S.K., Giovannucci E.L., Hursting S.D. The World Cancer Research Fund/American Institute for Cancer Research Third Expert Report on diet, nutrition, physical activity, and cancer: Impact and future directions. J. Nutr. 2020;150:663–671. doi: 10.1093/jn/nxz268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Anderson B.O., Berdzuli N., Ilbawi A., Kestel D., Kluge H.P., Krech R., Mikkelsen B., Neufeld M., Poznyak V., Rekve D., et al. Health and cancer risks associated with low levels of alcohol consumption. Lancet Public Health. 2023;8:e6–e7. doi: 10.1016/S2468-2667(22)00317-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Key T.J., Bradbury K.E., Perez-Cornago A., Sinha R., Tsilidis K.K., Tsugane S. Diet, nutrition, and cancer risk: What do we know and what is the way forward? BMJ. 2020;368:m511. doi: 10.1136/bmj.m511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cross A.J., Sinha R. Meat-related mutagens/carcinogens in the etiology of colorectal cancer. Environ. Mol. Mutagen. 2004;44:44–55. doi: 10.1002/em.20030. [DOI] [PubMed] [Google Scholar]
- 9.Mei J., Qian M., Hou Y., Liang M., Chen Y., Wang C., Zhang J. Association of saturated fatty acids with cancer risk: A systematic review and meta-analysis. Lipids Health Dis. 2024;23:32. doi: 10.1186/s12944-024-02025-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Talukdar J.R., Steen J.P., Goldenberg J.Z., Zhang Q., Vernooij R.W.M., Ge L., Zeraatkar D., Bala M.M., Ball G.D.C., Thabane L., et al. Saturated fat, the estimated absolute risk and certainty of risk for mortality and major cancer and cardiometabolic outcomes: An overview of systematic reviews. Syst. Rev. 2023;12:179. doi: 10.1186/s13643-023-02312-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Epner M., Yang P., Wagner R.W., Cohen L. Understanding the link between sugar and cancer: An examination of the preclinical and clinical evidence. Cancers. 2022;14:6042. doi: 10.3390/cancers14246042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hariri N., Gougeon R., Thibault L. A highly saturated fat-rich diet is more obesogenic than diets with lower saturated fat content. Nutr. Res. 2010;30:632–643. doi: 10.1016/j.nutres.2010.09.003. [DOI] [PubMed] [Google Scholar]
- 13.Zhang P. Influence of foods and nutrition on the gut microbiome and complications for intestinal health. Int. J. Mol. Sci. 2022;23:9588. doi: 10.3390/ijms23179588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Anderson S.M., Sears C.L. The role of the gut microbiome in cancer: A review, with special focus on colorectal neoplasia and clostridioides difficile. Clin. Infect. Dis. 2023;77((Suppl. 6)):S471–S478. doi: 10.1093/cid/ciad640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Putri S.P., Nakayama Y., Matsuda F., Uchikata T., Kobayashi S., Matsubara A., Fukusaki E. Current metabolomics: Practical applications. J. Biosci. Bioeng. 2013;115:579–589. doi: 10.1016/j.jbiosc.2012.12.007. [DOI] [PubMed] [Google Scholar]
- 16.Nannini G., Meoni G., Amedei A., Tenori L. Metabolomics profile in gastrointestinal cancers: Update and future perspectives. World J. Gastroenterol. 2020;26:2514–2532. doi: 10.3748/wjg.v26.i20.2514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cheung P.K., Ma M.H., Tse H.F., Yeung K.F., Tsang H.F., Chu M.K.M., Kan C.M., Cho W.C.S., Ng L.B.W., Chan L.W.C., et al. The applications of metabolomics in the molecular diagnostics of cancer. Expert Rev. Mol. Diagn. 2019;19:785–793. doi: 10.1080/14737159.2019.1656530. [DOI] [PubMed] [Google Scholar]
- 18.Yang L., Wang Y., Cai H., Wang S., Shen Y., Ke C. Application of metabolomics in the diagnosis of breast cancer: A systematic review. J. Cancer. 2020;11:2540–2551. doi: 10.7150/jca.37604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.His M., Gunter M.J., Keski-Rahkonen P., Rinaldi S. Application of metabolomics to epidemiologic studies of breast cancer: New perspectives for etiology and prevention. J. Clin. Oncol. 2023;42:103–115. doi: 10.1200/JCO.22.02754. [DOI] [PubMed] [Google Scholar]
- 20.Orsini A., Diquigiovanni C., Bonora E. Omics technologies improving breast cancer research and diagnostics. Int. J. Mol. Sci. 2023;24:12690. doi: 10.3390/ijms241612690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.The Women’s Health Initiative Study Group Design of the Women’s Health Initiative clinical trial and observational study. Control. Clin. Trials. 1998;19:61–109. doi: 10.1016/S0197-2456(97)00078-0. [DOI] [PubMed] [Google Scholar]
- 22.Bergstralh E.J., Kosanke J.L. Computerized Matching of Cases to Controls. Technical Report #56. Department of Health Sciences Research. Mayo Clinic; Rochester, MN, USA: 1995. [Google Scholar]
- 23.Kurup K., Matyi S., Giles C.B., Wren J.D., Jones K., Ericsson A., Raftery D., Wang L., Promislow D., Richardson A., et al. Calorie restriction prevents age-related changes in the intestinal microbiota. Aging. 2021;13:6298–6329. doi: 10.18632/aging.202753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hwangbo N., Zhang X., Raftery D., Gu H., Hu S.C., Montine T.J., Quinn J.F., Chung K.A., Hiller A.L., Wang D., et al. A Metabolomic Aging Clock Using Human Cerebrospinal Fluid. J. Gerontol. A Biol. Sci. Med. Sci. 2022;77:744–754. doi: 10.1093/gerona/glab212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hanson A.J., Banks W.A., Bettcher L.F., Pepin R., Raftery D., Craft S. Cerebrospinal fluid lipidomics: Effects of an intravenous triglyceride infusion and apoE status. Metabolomics. 2019;16:6. doi: 10.1007/s11306-019-1627-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ghorasaini M., Mohammed Y., Adamski J., Bettcher L., Bowden J.A., Cabruja M., Contrepois K., Ellenberger M., Gajera B., Haid M., et al. Cross-Laboratory Standardization of Preclinical Lipidomics Using Differential Mobility Spectrometry and Multiple Reaction Monitoring. Anal. Chem. 2021;93:16369–16378. doi: 10.1021/acs.analchem.1c02826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chan E.C., Pasikanti K.K., Nicholson J.K. Global urinary metabolic profiling procedures using gas chromatography-mass spectrometry. Nat. Protoc. 2011;6:1483–1499. doi: 10.1038/nprot.2011.375. [DOI] [PubMed] [Google Scholar]
- 28.Prentice R.L., Pettinger M., Neuhouser M.L., Tinker L.F., Huang Y., Zheng C., Manson J.E., Mossavar-Rahmani Y., Anderson G.L., Lampe J.W. Application of blood concentration biomarkers in nutritional epidemiology: Example of carotenoid and tocopherol intake in relation to chronic disease risk. Am. J. Clin. Nutr. 2019;10:1189–1196. doi: 10.1093/ajcn/nqy360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mansournia M.A., Jewell N.P., Greenland S. Case-control matching: Effects, misconceptions, and recommendations. Eur. J. Epidemiol. 2018;33:5–14. doi: 10.1007/s10654-017-0325-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.van Buuren S., Groothuis-Oudshoorn K. Mice: Multivariate Imputation by Chained Equations in R. J. Stat. Softw. 2011;45:1–67. doi: 10.18637/jss.v045.i03. [DOI] [Google Scholar]
- 31.Jaeger B.C., Cantor R., Sthanam V., Xie R., Kirklin J.K., Rudraraju R. Improving outcome predictions for patients receiving mechanical circulatory support by optimizing imputation of missing values. Circ. Cardiovasc. Qual. Outcomes. 2021;14:e007071. doi: 10.1161/CIRCOUTCOMES.120.007071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zheng C., Gowda G.A.N., Raftery D., Neuhouser M.L., Tinker L.F., Prentice R.L., Beresford S.A.A., Zhang Y., Bettcher L., Pepin R., et al. Evaluation of potential metabolomic-based biomarkers of protein, carbohydrate and fat intakes using a controlled feeding study. Eur. J. Nutr. 2021;60:4207–4218. doi: 10.1007/s00394-021-02577-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rubin D.B. The use of matched samling and regression adjustment to remove bias in observational studies. Biometrics. 1973;29:185–203. doi: 10.2307/2529685. [DOI] [Google Scholar]
- 34.Tibshirani R. Regression shrinkage and selection via the Lasso. J. R. Stat. Soc. Ser. B. 1996;58:267–288. doi: 10.1111/j.2517-6161.1996.tb02080.x. [DOI] [Google Scholar]
- 35.van der Laan M.J., Polley E.C., Hubbard A.E. Super learner. Stat. Appl. Genet. Mol. Biol. 2007;6 doi: 10.2202/1544-6115.1309. [DOI] [PubMed] [Google Scholar]
- 36.Polley E., LeDell E., Kennedy C., van der Laan M.J. SuperLearner: Super Learner Prediction. R Package, Version 2.0-26. 2023. [(accessed on 18 February 2022)]. Available online: https://github.com/ecpolley/SuperLearner.
- 37.Wolpert D.H. Stacked generalization. Neural Netw. 1992;5:241–259. doi: 10.1016/S0893-6080(05)80023-1. [DOI] [Google Scholar]
- 38.Zou H., Hastie T. Regularization and variable selection via the elastic net. J. R. Stat. Soc. Serial. B. 2005;67:301–320. doi: 10.1111/j.1467-9868.2005.00503.x. [DOI] [Google Scholar]
- 39.Friedman J.H. Greedy function approximation: A gradient boosting machine. Ann. Stat. 2001;29:1189–1232. doi: 10.1214/aos/1013203451. [DOI] [Google Scholar]
- 40.Breiman L. Random forests. Mach. Learn. 2001;45:5–32. doi: 10.1023/A:1010933404324. [DOI] [Google Scholar]
- 41.Leeb H., Potscher B.M. Model selection and inference: Facts and fiction. Econom. Theory. 2005;21:21–59. doi: 10.1017/S0266466605050036. [DOI] [Google Scholar]
- 42.Gunter M.J., Hoover D.R., Yu H., Wassertheil-Smoller S., Rohan T.E., Manson J.E., Li J., Ho G.Y., Xue X., Anderson G.L., et al. Insulin, insulin-like growth factor-I, and risk of breast cancer in postmenopausal women. J. Natl. Cancer Inst. 2009;101:48–60. doi: 10.1093/jnci/djn415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gunter M.J., Wang T., Cushman M., Xue X., Wassertheil-Smoller S., Strickler H.D., Rohan T.E., Manson J.E., McTiernan A., Kaplan R.C., et al. Circulating adipokines and inflammatory markers and postmenopausal breast cancer risk. J. Natl. Cancer Inst. 2015;107:djv169. doi: 10.1093/jnci/djv169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kolb R., Zhang W. Obesity and breast cancer: A case of inflamed adipose tissue. Cancers. 2020;12:1686. doi: 10.3390/cancers12061686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pati S., Irfan W., Jameel A., Ahmed S., Shahid R.K. Obesity and cancer: A current overview of epidemiology, pathogenesis, outcomes, and management. Cancers. 2023;15:485. doi: 10.3390/cancers15020485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Society A.C. Does Body Weight Affect Cancer Risk? [(accessed on 15 May 2024)]. Available online: https://www.cancer.org/cancer/risk-prevention/diet-physical-activity/body-weight-and-cancer-risk/effects.html.
- 47.Rothwell J.A., Besevic J., Dimou N., Breeur M., Murphy N., Jenab M., Wedekind R., Viallon V., Ferrari P., Achaintre D., et al. Circulating amino acid levels and colorectal cancer risk in the European Prospective Investigation into Cancer and Nutrition and UK Biobank cohorts. BMC Med. 2023;21:80. doi: 10.1186/s12916-023-02739-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Caldovic L., Ah Mew N., Shi D., Morizono H., Yudkoff M., Tuchman M. N-acetylglutamate synthase: Structure, function and defects. Mol. Genet. Metab. 2010;100((Suppl. 1)):S13–S19. doi: 10.1016/j.ymgme.2010.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jalandra R., Dalal N., Yadav A.K., Verma D., Sharma M., Singh R., Khosla A., Kumar A., Solanki P.R. Emerging role of trimethylamine-N-oxide (TMAO) in colorectal cancer. Appl. Microbiol. Biotechnol. 2021;105:7651–7660. doi: 10.1007/s00253-021-11582-7. [DOI] [PubMed] [Google Scholar]
- 50.Bae S., Ulrich C.M., Neuhouser M.L., Malysheva O., Bailey L.B., Xiao L.R., Brown E.C., Cushing-Haugen K.L., Zheng Y.Y., Cheng T.Y.D., et al. Plasma choline metabolites and colorectal cancer risk in the Women’s Health Initiative Observational Study. Cancer Res. 2014;74:7442–7452. doi: 10.1158/0008-5472.CAN-14-1835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Byrd D.A., Zouiouich S., Karwa S., Li X.S., Wang Z., Sampson J.N., Loftfield E., Huang W.Y., Hazen S.L., Sinha R. Associations of serum trimethylamine N-oxide and its precursors with colorectal cancer risk in the Prostate, Lung, Colorectal, Ovarian Cancer Screening Trial Cohort. Cancer. 2024;130:1982–1990. doi: 10.1002/cncr.35219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chan D.S., Lau R., Aune D., Vieira R., Greenwood D.C., Kampman E., Norat T. Red and processed meat and colorectal cancer incidence: Meta-analysis of prospective studies. PLoS ONE. 2011;6:e20456. doi: 10.1371/journal.pone.0020456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Aune D., Chan D.S., Vieira A.R., Navarro Rosenblatt D.A., Vieira R., Greenwood D.C., Kampman E., Norat T. Red and processed meat intake and risk of colorectal adenomas: A systematic review and meta-analysis of epidemiological studies. Cancer Causes Control. 2013;24:611–627. doi: 10.1007/s10552-012-0139-z. [DOI] [PubMed] [Google Scholar]
- 54.Playdon M.C., Ziegler R.G., Sampson J.N., Stolzenberg-Solomon R., Thompson H.J., Irwin M.L., Mayne S.T., Hoover R.N., Moore S.C. Nutritional metabolomics and breast cancer risk in a prospective study. Am. J. Clin. Nutr. 2017;106:637–649. doi: 10.3945/ajcn.116.150912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Moore S.C., Mazzilli K.M., Sampson J.N., Matthews C.E., Carter B.D., Playdon M.C., Wang Y., Stevens V.L. A metabolomics analysis of postmenopausal breast cancer risk in the cancer prevention study II. Metabolites. 2021;11:95. doi: 10.3390/metabo11020095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.His M., Viallon V., Dossus L., Schmidt J.A., Travis R.C., Gunter M.J., Overvad K., Kyro C., Tjonneland A., Lecuyer L., et al. Lifestyle correlates of eight breast cancer-related metabolites: A cross-sectional study within the EPIC cohort. BMC Med. 2021;19:312. doi: 10.1186/s12916-021-02183-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Moore S.C., Playdon M.C., Sampson J.N., Hoover R.N., Trabert B., Matthews C.E., Ziegler R.G. A metabolomics analysis of body mass index and postmenopausal breast cancer risk. J. Natl. Cancer Inst. 2018;110:588–597. doi: 10.1093/jnci/djx244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.His M., Viallon V., Dossus L., Gicquiau A., Achaintre D., Scalbert A., Ferrari P., Romieu I., Onland-Moret N.C., Weiderpass E., et al. Prospective analysis of circulating metabolites and breast cancer in EPIC. BMC Med. 2019;17:178. doi: 10.1186/s12916-019-1408-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lecuyer L., Dalle C., Lefevre-Arbogast S., Micheau P., Lyan B., Rossary A., Demidem A., Petera M., Lagree M., Centeno D., et al. Diet-related metabolomic signature oflLong-termbBreast cancer risk using penalized regression: An exploratory study in the SU.VI.MAX cohort. Cancer Epidemiol. Biomark. Prev. 2020;29:396–405. doi: 10.1158/1055-9965.EPI-19-0900. [DOI] [PubMed] [Google Scholar]
- 60.Stevens V.L., Carter B.D., Jacobs E.J., McCullough M.L., Teras L.R., Wang Y. A prospective case-cohort analysis of plasma metabolites and breast cancer risk. Breast Cancer Res. 2023;25:5. doi: 10.1186/s13058-023-01602-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Jobard E., Dossus L., Baglietto L., Fornili M., Lecuyer L., Mancini F.R., Gunter M.J., Tredan O., Boutron-Ruault M.C., Elena-Herrmann B., et al. Investigation of circulating metabolites associated with breast cancer risk by untargeted metabolomics: A case-control study nested within the French E3N cohort. Br. J. Cancer. 2021;124:1734–1743. doi: 10.1038/s41416-021-01304-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kuhn T., Floegel A., Sookthai D., Johnson T., Rolle-Kampczyk U., Otto W., von Bergen M., Boeing H., Kaaks R. Higher plasma levels of lysophosphatidylcholine 18:0 are related to a lower risk of common cancers in a prospective metabolomics study. BMC Med. 2016;14:13. doi: 10.1186/s12916-016-0552-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Brantley K.D., Zeleznik O.A., Dickerman B.A., Balasubramanian R., Clish C.B., Avila-Pacheco J., Rosner B., Tamimi R.M., Eliassen A.H. A metabolomic analysis of adiposity measures and pre- and postmenopausal breast cancer risk in the Nurses’ Health Studies. Br. J. Cancer. 2022;127:1076–1085. doi: 10.1038/s41416-022-01873-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Shu X., Xiang Y.B., Rothman N., Yu D., Li H.L., Yang G., Cai H., Ma X., Lan Q., Gao Y.T., et al. Prospective study of blood metabolites associated with colorectal cancer risk. Int. J. Cancer. 2018;143:527–534. doi: 10.1002/ijc.31341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Cross A.J., Moore S.C., Boca S., Huang W.Y., Xiong X., Stolzenberg-Solomon R., Sinha R., Sampson J.N. A prospective study of serum metabolites and colorectal cancer risk. Cancer. 2014;120:3049–3057. doi: 10.1002/cncr.28799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Harewood R., Rothwell J.A., Besevic J., Viallon V., Achaintre D., Gicquiau A., Rinaldi S., Wedekind R., Prehn C., Adamski J., et al. Association between pre-diagnostic circulating lipid metabolites and colorectal cancer risk: A nested case-control study in the European Prospective Investigation into Cancer and Nutrition (EPIC) EBioMedicine. 2024;101:105024. doi: 10.1016/j.ebiom.2024.105024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Perttula K., Schiffman C., Edmands W.M.B., Petrick L., Grigoryan H., Cai X., Gunter M.J., Naccarati A., Polidoro S., Dudoit S., et al. Untargeted lipidomic features associated with colorectal cancer in a prospective cohort. BMC Cancer. 2018;18:996. doi: 10.1186/s12885-018-4894-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kliemann N., Viallon V., Murphy N., Beeken R.J., Rothwell J.A., Rinaldi S., Assi N., van Roekel E.H., Schmidt J.A., Borch K.B., et al. Metabolic signatures of greater body size and their associations with risk of colorectal and endometrial cancers in the European Prospective Investigation into Cancer and Nutrition. BMC Med. 2021;19:101. doi: 10.1186/s12916-021-01970-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Deng L., Ismond K., Liu Z., Constable J., Wang H., Alatise O.I., Weiser M.R., Kingham T.P., Chang D. Urinary Metabolomics to identify a unique biomarker panel for detecting colorectal cancer: A multicenter study. Cancer Epidemiol. Biomark. Prev. 2019;28:1283–1291. doi: 10.1158/1055-9965.EPI-18-1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.McCullough M.L., Hodge R.A., Campbell P.T., Stevens V.L., Wang Y. Pre-Diagnostic circulating metabolites and colorectal cancer risk in the Cancer Prevention Study-II Nutrition Cohort. Metabolites. 2021;11:156. doi: 10.3390/metabo11030156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Vidman L., Zheng R., Boden S., Ribbenstedt A., Gunter M.J., Palmqvist R., Harlid S., Brunius C., Van Guelpen B. Untargeted plasma metabolomics and risk of colorectal cancer—An analysis nested within a large-scale prospective cohort. Cancer Metab. 2023;11:17. doi: 10.1186/s40170-023-00319-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wang R., Dai R., Huang Y., Neuhouser M.L., Lampe J.W., Raftery D., Tabung F.K., Zheng C. Variable selection with FDR control for noisy data—An application to screening metabolites that are associated with breast and colorectal cancer. arXiv. 20232310.06696 [Google Scholar]
- 73.Foygel Barber R., Candes E.J. Controlling the false discovery rate via knockoffs. Ann. Stat. 2015;45:2055–2085. [Google Scholar]
- 74.Gunter M.J., Hoover D.R., Yu H., Wassertheil-Smoller S., Rohan T.E., Manson J.E., Howard B.V., Wylie-Rosett J., Anderson G.L., Ho G.Y., et al. Insulin, insulin-like growth factor-I, endogenous estradiol, and risk of colorectal cancer in postmenopausal women. Cancer Res. 2008;68:329–337. doi: 10.1158/0008-5472.CAN-07-2946. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data, codebook, analytic code used in this report may be accessed in a collaborative mode as described on the Women’s Health Initiative website (www.whi.org).