Abstract
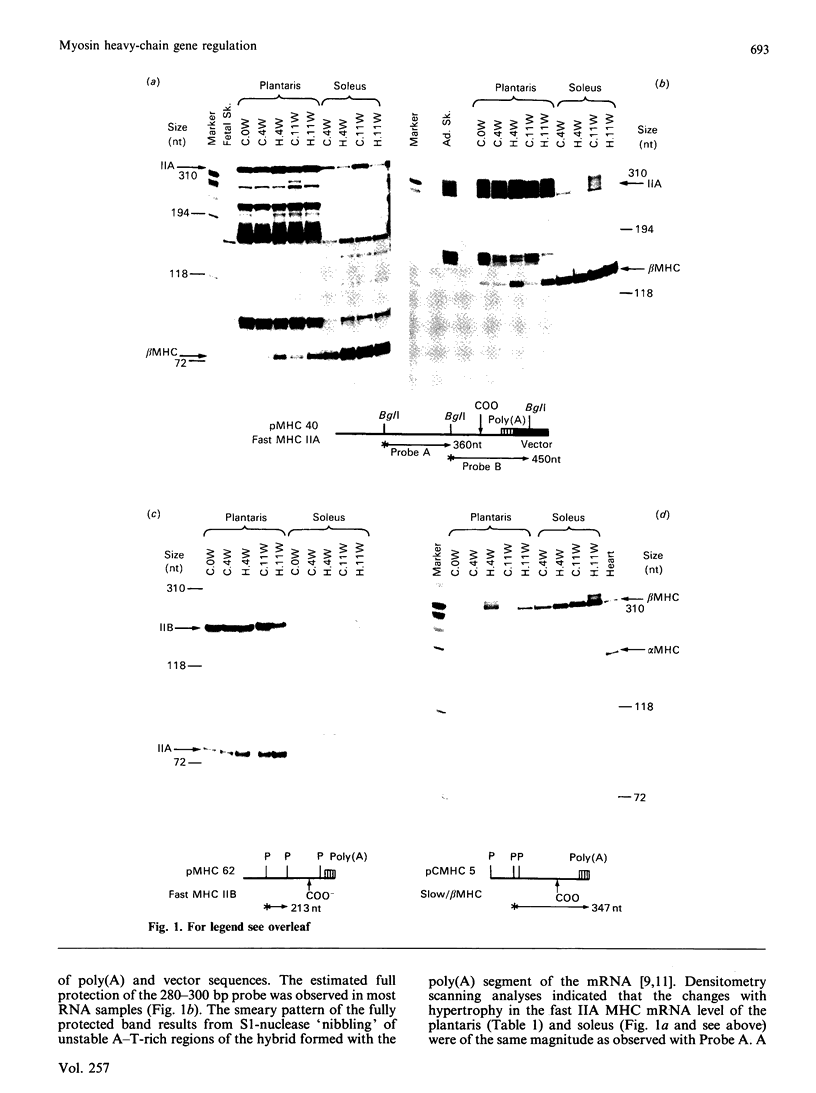
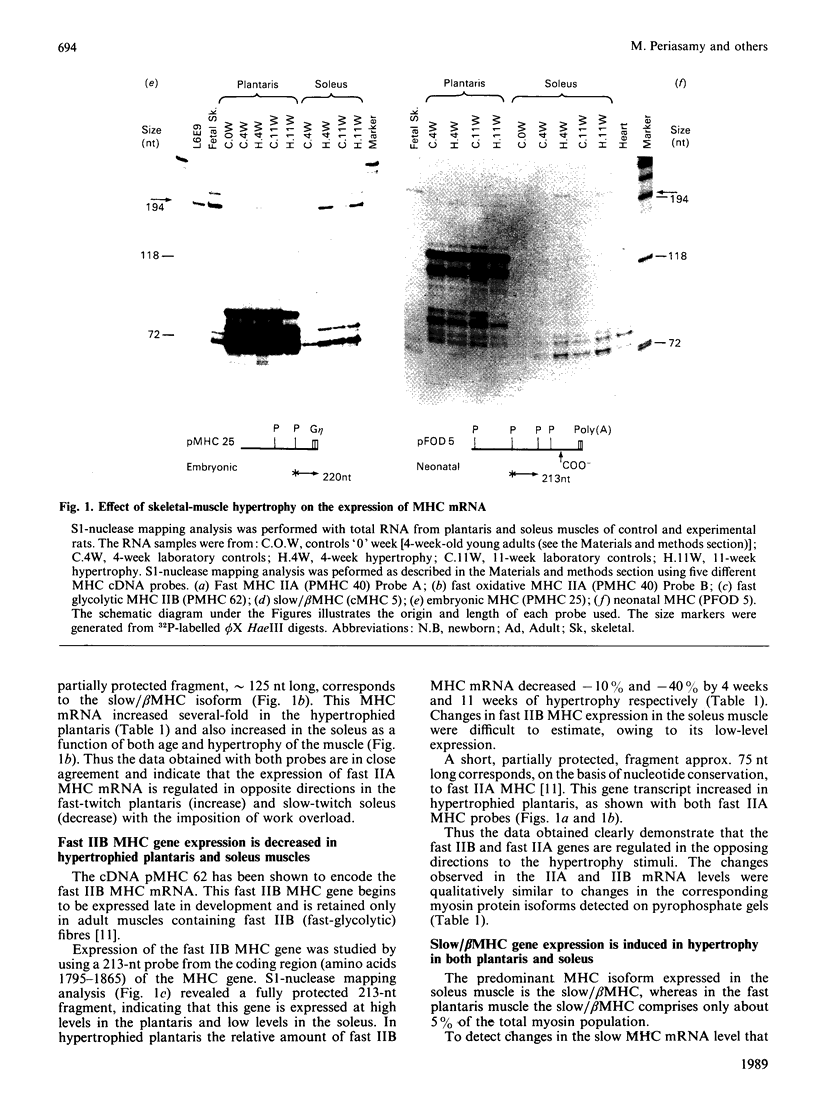
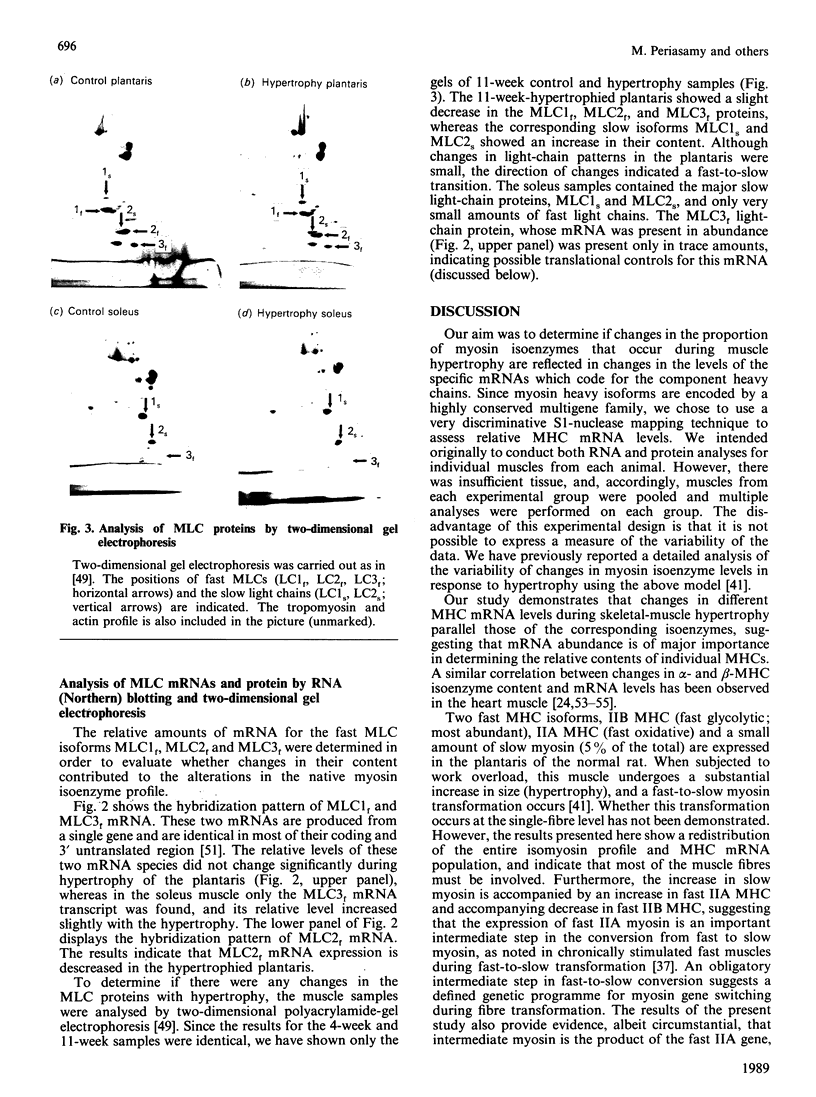
Changes in the myosin phenotype of differentiated muscle are a prominent feature of the adaptation of the tissue to a variety of physiological stimuli. In the present study the molecular basis of changes in the proportion of myosin isoenzymes in rat skeletal muscle which occur during compensatory hypertrophy caused by the combined removal of synergist muscles and spontaneous running exercise was investigated. The relative amounts of sarcomeric myosin heavy (MHC)- and light (MLC)-chain mRNAs in the plantaris (fast) and soleus (slow) muscles from rats was assessed with cDNA probes specific for different MHC and MLC genes. Changes in the proportion of specific MHC mRNA levels were in the same direction as, and of similar magnitude to, changes in the proportion of myosin isoenzymes encoded for by the mRNAs. No significant changes in the proportion of MLC proteins or mRNA were detected. However, high levels of MLC3 mRNA were measured in both normal and hypertrophied soleus muscles which contained only trace amounts of MLC3 protein. Small amounts of embryonic and neonatal MHC mRNAs were induced in both muscles during hypertrophy. We conclude that the change in the pattern of myosin isoenzymes during skeletal-muscle adaptation to work overload is a consequence of changes in specific MHC mRNA levels.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baldwin K. M., Valdez V., Herrick R. E., MacIntosh A. M., Roy R. R. Biochemical properties of overloaded fast-twitch skeletal muscle. J Appl Physiol Respir Environ Exerc Physiol. 1982 Feb;52(2):467–472. doi: 10.1152/jappl.1982.52.2.467. [DOI] [PubMed] [Google Scholar]
- Bandman E. Myosin isoenzyme transitions in muscle development, maturation, and disease. Int Rev Cytol. 1985;97:97–131. doi: 10.1016/s0074-7696(08)62349-9. [DOI] [PubMed] [Google Scholar]
- Berk A. J., Sharp P. A. Sizing and mapping of early adenovirus mRNAs by gel electrophoresis of S1 endonuclease-digested hybrids. Cell. 1977 Nov;12(3):721–732. doi: 10.1016/0092-8674(77)90272-0. [DOI] [PubMed] [Google Scholar]
- Brown W. E., Salmons S., Whalen R. G. The sequential replacement of myosin subunit isoforms during muscle type transformation induced by long term electrical stimulation. J Biol Chem. 1983 Dec 10;258(23):14686–14692. [PubMed] [Google Scholar]
- Buller A. J., Mommaerts W. F., Seraydarian K. Enzymic properties of myosin in fast and slow twitch muscles of the cat following cross-innervation. J Physiol. 1969 Dec;205(3):581–597. doi: 10.1113/jphysiol.1969.sp008984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler-Browne G. S., Whalen R. G. Myosin isozyme transitions occurring during the postnatal development of the rat soleus muscle. Dev Biol. 1984 Apr;102(2):324–334. doi: 10.1016/0012-1606(84)90197-0. [DOI] [PubMed] [Google Scholar]
- Bárány M., Close R. I. The transformation of myosin in cross-innervated rat muscles. J Physiol. 1971 Mar;213(2):455–474. doi: 10.1113/jphysiol.1971.sp009393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dillmann W. H. Diabetes mellitus induces changes in cardiac myosin of the rat. Diabetes. 1980 Jul;29(7):579–582. doi: 10.2337/diab.29.7.579. [DOI] [PubMed] [Google Scholar]
- Everett A. W., Sinha A. M., Umeda P. K., Jakovcic S., Rabinowitz M., Zak R. Regulation of myosin synthesis by thyroid hormone: relative change in the alpha- and beta-myosin heavy chain mRNA levels in rabbit heart. Biochemistry. 1984 Apr 10;23(8):1596–1599. doi: 10.1021/bi00303a002. [DOI] [PubMed] [Google Scholar]
- Flink I. L., Rader J. H., Morkin E. Thyroid hormone stimulates synthesis of a cardiac myosin isozyme. Comparison of the two-two-dimensional electrophoretic patterns of the cyanogen bromide peptides of cardiac myosin heavy chains from euthyroid and thyrotoxic rabbits. J Biol Chem. 1979 Apr 25;254(8):3105–3110. [PubMed] [Google Scholar]
- Garfinkel L. I., Periasamy M., Nadal-Ginard B. Cloning and characterization of cDNA sequences corresponding to myosin light chains 1, 2, and 3, troponin-C, troponin-T, alpha-tropomyosin, and alpha-actin. J Biol Chem. 1982 Sep 25;257(18):11078–11086. [PubMed] [Google Scholar]
- Gauthier G. F., Burke R. E., Lowey S., Hobbs A. W. Myosin isozymes in normal and cross-reinnervated cat skeletal muscle fibers. J Cell Biol. 1983 Sep;97(3):756–771. doi: 10.1083/jcb.97.3.756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gauthier G. F., Lowey S., Benfield P. A., Hobbs A. W. Distribution and properties of myosin isozymes in developing avian and mammalian skeletal muscle fibers. J Cell Biol. 1982 Feb;92(2):471–484. doi: 10.1083/jcb.92.2.471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green H. J., Klug G. A., Reichmann H., Seedorf U., Wiehrer W., Pette D. Exercise-induced fibre type transitions with regard to myosin, parvalbumin, and sarcoplasmic reticulum in muscles of the rat. Pflugers Arch. 1984 Apr;400(4):432–438. doi: 10.1007/BF00587545. [DOI] [PubMed] [Google Scholar]
- Gregory P., Low R. B., Stirewalt W. S. Changes in skeletal-muscle myosin isoenzymes with hypertrophy and exercise. Biochem J. 1986 Aug 15;238(1):55–63. doi: 10.1042/bj2380055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gustafson T. A., Markham B. E., Morkin E. Effects of thyroid hormone on alpha-actin and myosin heavy chain gene expression in cardiac and skeletal muscles of the rat: measurement of mRNA content using synthetic oligonucleotide probes. Circ Res. 1986 Aug;59(2):194–201. doi: 10.1161/01.res.59.2.194. [DOI] [PubMed] [Google Scholar]
- Hoh J. Y., McGrath P. A., White R. I. Electrophoretic analysis of multiple forms of myosin in fast-twitch and slow-twitch muscles of the chick. Biochem J. 1976 Jul 1;157(1):87–95. doi: 10.1042/bj1570087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ianuzzo D., Patel P., Chen V., O'Brien P., Williams C. Thyroidal trophic influence on skeletal muscle myosin. Nature. 1977 Nov 3;270(5632):74–76. doi: 10.1038/270074a0. [DOI] [PubMed] [Google Scholar]
- Izumo S., Lompré A. M., Matsuoka R., Koren G., Schwartz K., Nadal-Ginard B., Mahdavi V. Myosin heavy chain messenger RNA and protein isoform transitions during cardiac hypertrophy. Interaction between hemodynamic and thyroid hormone-induced signals. J Clin Invest. 1987 Mar;79(3):970–977. doi: 10.1172/JCI112908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izumo S., Nadal-Ginard B., Mahdavi V. All members of the MHC multigene family respond to thyroid hormone in a highly tissue-specific manner. Science. 1986 Feb 7;231(4738):597–600. doi: 10.1126/science.3945800. [DOI] [PubMed] [Google Scholar]
- Izumo S., Nadal-Ginard B., Mahdavi V. Protooncogene induction and reprogramming of cardiac gene expression produced by pressure overload. Proc Natl Acad Sci U S A. 1988 Jan;85(2):339–343. doi: 10.1073/pnas.85.2.339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jolesz F., Sreter F. A. Development, innervation, and activity-pattern induced changes in skeletal muscle. Annu Rev Physiol. 1981;43:531–552. doi: 10.1146/annurev.ph.43.030181.002531. [DOI] [PubMed] [Google Scholar]
- Kennedy J. M., Eisenberg B. R., Reid S. K., Sweeney L. J., Zak R. Nascent muscle fiber appearance in overloaded chicken slow-tonic muscle. Am J Anat. 1988 Feb;181(2):203–215. doi: 10.1002/aja.1001810209. [DOI] [PubMed] [Google Scholar]
- Leinwand L. A., Saez L., McNally E., Nadal-Ginard B. Isolation and characterization of human myosin heavy chain genes. Proc Natl Acad Sci U S A. 1983 Jun;80(12):3716–3720. doi: 10.1073/pnas.80.12.3716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lompré A. M., Nadal-Ginard B., Mahdavi V. Expression of the cardiac ventricular alpha- and beta-myosin heavy chain genes is developmentally and hormonally regulated. J Biol Chem. 1984 May 25;259(10):6437–6446. [PubMed] [Google Scholar]
- Mahdavi V., Chambers A. P., Nadal-Ginard B. Cardiac alpha- and beta-myosin heavy chain genes are organized in tandem. Proc Natl Acad Sci U S A. 1984 May;81(9):2626–2630. doi: 10.1073/pnas.81.9.2626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahdavi V., Periasamy M., Nadal-Ginard B. Molecular characterization of two myosin heavy chain genes expressed in the adult heart. Nature. 1982 Jun 24;297(5868):659–664. doi: 10.1038/297659a0. [DOI] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- Mondon C. E., Dolkas C. B., Reaven G. M. Site of enhanced insulin sensitivity in exercise-trained rats at rest. Am J Physiol. 1980 Sep;239(3):E169–E177. doi: 10.1152/ajpendo.1980.239.3.E169. [DOI] [PubMed] [Google Scholar]
- Nagai R., Pritzl N., Low R. B., Stirewalt W. S., Zak R., Alpert N. R., Litten R. Z. Myosin isozyme synthesis and mRNA levels in pressure-overloaded rabbit hearts. Circ Res. 1987 May;60(5):692–699. doi: 10.1161/01.res.60.5.692. [DOI] [PubMed] [Google Scholar]
- Nguyen H. T., Gubits R. M., Wydro R. M., Nadal-Ginard B. Sarcomeric myosin heavy chain is coded by a highly conserved multigene family. Proc Natl Acad Sci U S A. 1982 Sep;79(17):5230–5234. doi: 10.1073/pnas.79.17.5230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noble E. G., Dabrowski B. L., Ianuzzo C. D. Myosin transformation in hypertrophied rat muscle. Pflugers Arch. 1983 Mar 1;396(3):260–262. doi: 10.1007/BF00587864. [DOI] [PubMed] [Google Scholar]
- Nudel U., Katcoff D., Carmon Y., Zevin-Sonkin D., Levi Z., Shaul Y., Shani M., Yaffe D. Identification of recombinant phages containing sequences from different rat myosin heavy chain genes. Nucleic Acids Res. 1980 May 24;8(10):2133–2146. doi: 10.1093/nar/8.10.2133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- Periasamy M., Strehler E. E., Garfinkel L. I., Gubits R. M., Ruiz-Opazo N., Nadal-Ginard B. Fast skeletal muscle myosin light chains 1 and 3 are produced from a single gene by a combined process of differential RNA transcription and splicing. J Biol Chem. 1984 Nov 10;259(21):13595–13604. [PubMed] [Google Scholar]
- Periasamy M., Wieczorek D. F., Nadal-Ginard B. Characterization of a developmentally regulated perinatal myosin heavy-chain gene expressed in skeletal muscle. J Biol Chem. 1984 Nov 10;259(21):13573–13578. [PubMed] [Google Scholar]
- Periasamy M., Wydro R. M., Strehler-Page M. A., Strehler E. E., Nadal-Ginard B. Characterization of cDNA and genomic sequences corresponding to an embryonic myosin heavy chain. J Biol Chem. 1985 Dec 15;260(29):15856–15862. [PubMed] [Google Scholar]
- Pette D., Vrbová G. Neural control of phenotypic expression in mammalian muscle fibers. Muscle Nerve. 1985 Oct;8(8):676–689. doi: 10.1002/mus.880080810. [DOI] [PubMed] [Google Scholar]
- Robbins J., Horan T., Gulick J., Kropp K. The chicken myosin heavy chain family. J Biol Chem. 1986 May 15;261(14):6606–6612. [PubMed] [Google Scholar]
- Roy R. K., Sarkar S. Correlation between the protein and mRNA levels for myosin light chains and tropomyosin subunits during chick fast muscle development in vivo. FEBS Lett. 1982 Nov 22;149(1):22–28. doi: 10.1016/0014-5793(82)81063-6. [DOI] [PubMed] [Google Scholar]
- Rubinstein N., Mabuchi K., Pepe F., Salmons S., Gergely J., Sreter F. Use of type-specific antimyosins to demonstrate the transformation of individual fibers in chronically stimulated rabbit fast muscles. J Cell Biol. 1978 Oct;79(1):252–261. doi: 10.1083/jcb.79.1.252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salmons S., Henriksson J. The adaptive response of skeletal muscle to increased use. Muscle Nerve. 1981 Mar-Apr;4(2):94–105. doi: 10.1002/mus.880040204. [DOI] [PubMed] [Google Scholar]
- Sinha A. M., Umeda P. K., Kavinsky C. J., Rajamanickam C., Hsu H. J., Jakovcic S., Rabinowitz M. Molecular cloning of mRNA sequences for cardiac alpha- and beta-form myosin heavy chains: expression in ventricles of normal, hypothyroid, and thyrotoxic rabbits. Proc Natl Acad Sci U S A. 1982 Oct;79(19):5847–5851. doi: 10.1073/pnas.79.19.5847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soeiro R., Birnboim H. C., Darnell J. E. Rapidly labeled HeLa cell nuclear RNA. II. Base composition and cellular localization of a heterogeneous RNA fraction. J Mol Biol. 1966 Aug;19(2):362–372. doi: 10.1016/s0022-2836(66)80010-4. [DOI] [PubMed] [Google Scholar]
- Tsika R. W., Herrick R. E., Baldwin K. M. Interaction of compensatory overload and hindlimb suspension on myosin isoform expression. J Appl Physiol (1985) 1987 Jun;62(6):2180–2186. doi: 10.1152/jappl.1987.62.6.2180. [DOI] [PubMed] [Google Scholar]
- Umeda P. K., Kavinsky C. J., Sinha A. M., Hsu H. J., Jakovcic S., Rabinowitz M. Cloned mRNA sequences for two types of embryonic myosin heavy chains from chick skeletal muscle. II. Expression during development using S1 nuclease mapping. J Biol Chem. 1983 Apr 25;258(8):5206–5214. [PubMed] [Google Scholar]
- Umeda P. K., Sinha A. M., Jakovcic S., Merten S., Hsu H. J., Subramanian K. N., Zak R., Rabinowitz M. Molecular cloning of two fast myosin heavy chain cDNAs from chicken embryo skeletal muscle. Proc Natl Acad Sci U S A. 1981 May;78(5):2843–2847. doi: 10.1073/pnas.78.5.2843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weeds A. G., Burridge K. Myosin from cross-reinnervated cat muscles. Evidence for reciprocal transformation of heavy chains. FEBS Lett. 1975 Sep 15;57(2):203–208. doi: 10.1016/0014-5793(75)80717-4. [DOI] [PubMed] [Google Scholar]
- Weydert A., Barton P., Harris A. J., Pinset C., Buckingham M. Developmental pattern of mouse skeletal myosin heavy chain gene transcripts in vivo and in vitro. Cell. 1987 Apr 10;49(1):121–129. doi: 10.1016/0092-8674(87)90762-8. [DOI] [PubMed] [Google Scholar]
- Whalen R. G., Sell S. M., Butler-Browne G. S., Schwartz K., Bouveret P., Pinset-Härstöm I. Three myosin heavy-chain isozymes appear sequentially in rat muscle development. Nature. 1981 Aug 27;292(5826):805–809. doi: 10.1038/292805a0. [DOI] [PubMed] [Google Scholar]
- Wieczorek D. F., Periasamy M., Butler-Browne G. S., Whalen R. G., Nadal-Ginard B. Co-expression of multiple myosin heavy chain genes, in addition to a tissue-specific one, in extraocular musculature. J Cell Biol. 1985 Aug;101(2):618–629. doi: 10.1083/jcb.101.2.618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wydro R. M., Nguyen H. T., Gubits R. M., Nadal-Ginard B. Characterization of sarcomeric myosin heavy chain genes. J Biol Chem. 1983 Jan 10;258(1):670–678. [PubMed] [Google Scholar]