Abstract
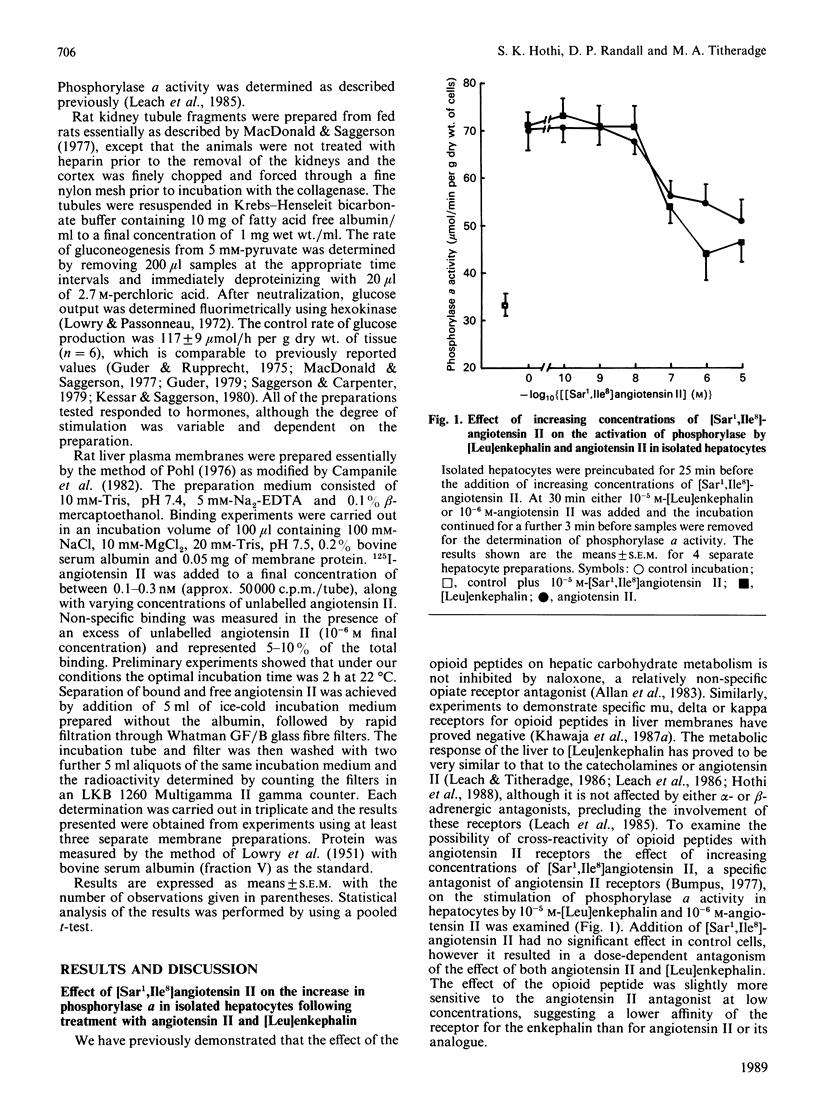
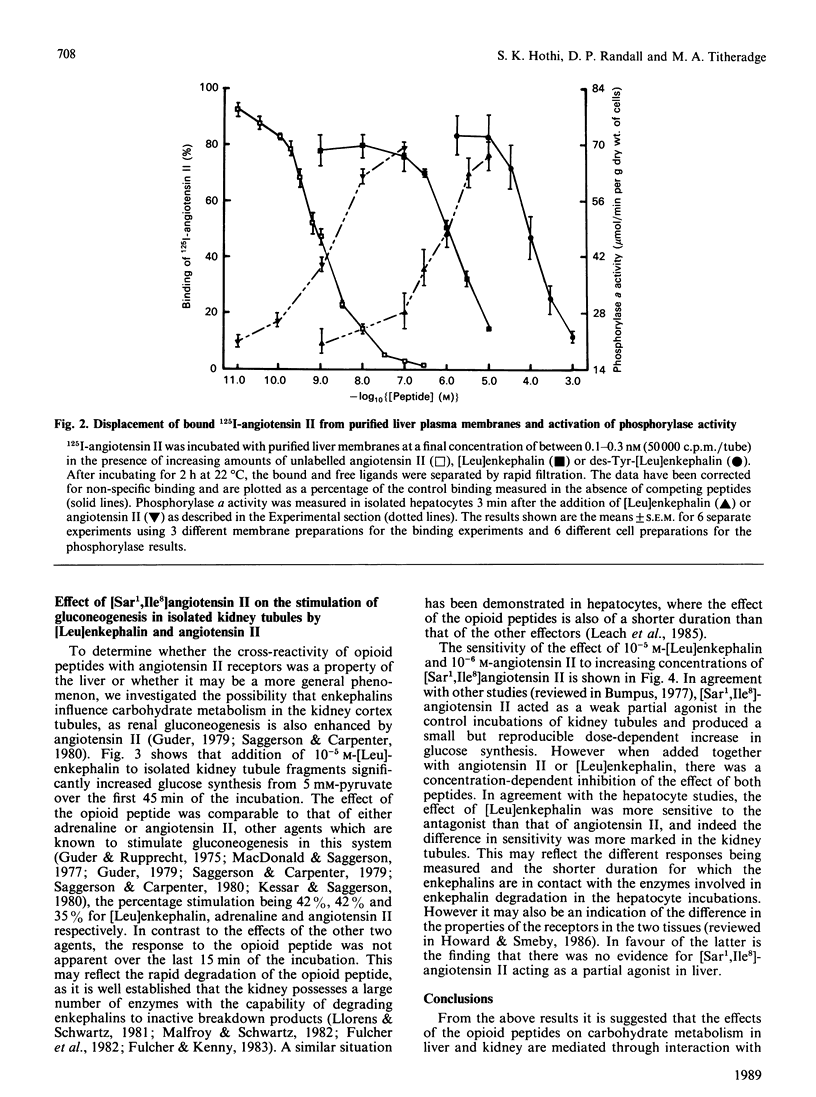
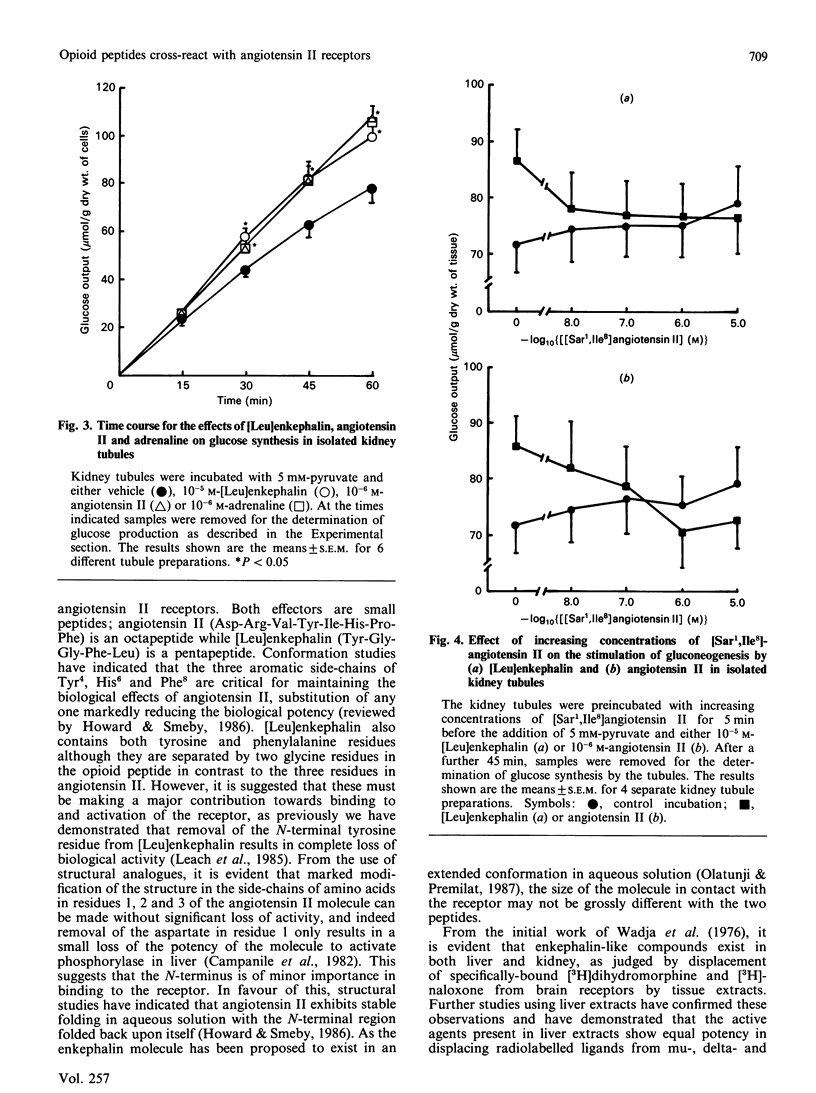
The possibility that the effects of [Leu]enkephalin in vitro on hepatic carbohydrate metabolism are mediated by interaction with angiotensin II receptors has been examined. Preincubation of hepatocytes with either the angiotensin II receptor antagonist [Sar1,Ile8]angiotensin II or 10 mM-dithiothreitol abolished the ability of both angiotensin II and [Leu]enkephalin to increase phosphorylase a in hepatocytes prepared from fed rats. Dithiothreitol had no effect on the stimulation of phosphorylase in the presence of glucagon or phenylephrine, although it also inhibited the response to vasopressin. [Leu]enkephalin displaced specifically bound 125I-labelled angiotensin II from hepatic plasma membranes over a concentration range of 10(-7)-10(-5) M. This correlated with the dose-response required to stimulate phosphorylase activity in intact hepatocytes and suggests that the effects of the opioid peptides on carbohydrate metabolism in liver are the result of cross-reactivity of the peptides with angiotensin II receptors. Addition of 10(-5) M-[Leu]enkephalin to isolated kidney tubule fragments stimulated gluconeogenesis from 5 mM-pyruvate, the magnitude of stimulation being comparable to that by either angiotensin II or adrenaline. This effect of the opioid peptide was also abolished by pretreatment of the tubules with [Sar1,Ile8]angiotensin II, suggesting that the ability of [Leu]enkephalin to interact with angiotensin II receptors is not restricted to the liver, but may occur in other tissues where both receptors occur together.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allan E. H., Green I. C., Titheradge M. A. The stimulation of glycogenolysis and gluconeogenesis in isolated hepatocytes by opioid peptides. Biochem J. 1983 Nov 15;216(2):507–510. doi: 10.1042/bj2160507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berry M. N., Friend D. S. High-yield preparation of isolated rat liver parenchymal cells: a biochemical and fine structural study. J Cell Biol. 1969 Dec;43(3):506–520. doi: 10.1083/jcb.43.3.506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blume A. J., Boone G., Lichtshtein D. Regulation of the neuroblastoma x glioma hybrid opiate receptors by Na+ and guanine nucleotides. Adv Exp Med Biol. 1979;116:163–174. doi: 10.1007/978-1-4684-3503-0_9. [DOI] [PubMed] [Google Scholar]
- Bumpus F. M. Mechanisms and sites of action of newer angiotensin agonists and antagonists in terms of activity and receptor. Fed Proc. 1977 Jul;36(8):2128–2132. [PubMed] [Google Scholar]
- Campanile C. P., Crane J. K., Peach M. J., Garrison J. C. The hepatic angiotensin II receptor. I. Characterization of the membrane-binding site and correlation with physiological response in hepatocytes. J Biol Chem. 1982 May 10;257(9):4951–4958. [PubMed] [Google Scholar]
- Cooper D. M., Londos C., Gill D. L., Rodbell M. Opiate receptor-mediated inhibition of adenylate cyclase in rat striatal plasma membranes. J Neurochem. 1982 Apr;38(4):1164–1167. doi: 10.1111/j.1471-4159.1982.tb05365.x. [DOI] [PubMed] [Google Scholar]
- Dave J. R., Rubinstein N., Eskay R. L. Evidence that beta-endorphin binds to specific receptors in rat peripheral tissues and stimulates the adenylate cyclase-adenosine 3',5'-monophosphate system. Endocrinology. 1985 Oct;117(4):1389–1396. doi: 10.1210/endo-117-4-1389. [DOI] [PubMed] [Google Scholar]
- Fulcher I. S., Kenny A. J. Proteins of the kidney microvillar membrane. The amphipathic forms of endopeptidase purified from pig kidneys. Biochem J. 1983 Jun 1;211(3):743–753. doi: 10.1042/bj2110743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fulcher I. S., Matsas R., Turner A. J., Kenny A. J. Kidney neutral endopeptidase and the hydrolysis of enkephalin by synaptic membranes show similar sensitivity to inhibitors. Biochem J. 1982 May 1;203(2):519–522. doi: 10.1042/bj2030519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guder W. G., Rupprecht A. Metabolism of isolated kidney tubules. Independent actions of catecholamines on renal cyclic adenosine 3':5'-monophosphate levels and gluconeogenesis. Eur J Biochem. 1975 Mar 17;52(2):283–290. doi: 10.1111/j.1432-1033.1975.tb03996.x. [DOI] [PubMed] [Google Scholar]
- Guder W. G. Stimulation of renal gluconeogenesis by angiotensin II. Biochim Biophys Acta. 1979 May 16;584(3):507–519. doi: 10.1016/0304-4165(79)90123-5. [DOI] [PubMed] [Google Scholar]
- Gunther S. Characterization of angiotensin II receptor subtypes in rat liver. J Biol Chem. 1984 Jun 25;259(12):7622–7629. [PubMed] [Google Scholar]
- Hothi S. K., Leach R. P., Titheradge M. A. Comparison of the effects of [leucine]enkephalin and angiotensin on hepatic carbohydrate and cyclic nucleotide metabolism. Biochem J. 1988 Feb 1;249(3):669–676. doi: 10.1042/bj2490669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessar P., Saggerson E. D. Evidence that catecholamines stimulate renal gluconeogenesis through an alpha 1-type of adrenoceptor. Biochem J. 1980 Jul 15;190(1):119–123. doi: 10.1042/bj1900119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Leach R. P., Allan E. H., Titheradge M. A. The stimulation of glycogenolysis in isolated hepatocytes by opioid peptides. Biochem J. 1985 Apr 1;227(1):191–197. doi: 10.1042/bj2270191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leach R. P., Shears S. B., Kirk C. J., Titheradge M. A. Changes in free cytosolic calcium and accumulation of inositol phosphates in isolated hepatocytes by [Leu]enkephalin. Biochem J. 1986 Sep 1;238(2):537–542. doi: 10.1042/bj2380537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leach R. P., Titheradge M. A. The stimulation of glycogenolysis in isolated hepatocytes by opioid peptides. Biochem J. 1986 Sep 1;238(2):531–535. doi: 10.1042/bj2380531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leslie R. D., Eff C., Barnett A. H., Spiliopoulos A. J., Pyke D. A., Stubbs W. A., Alberti K. G. Opiate receptors and the metabolic response to intravenous glucose. Diabete Metab. 1982 Sep;8(3):235–239. [PubMed] [Google Scholar]
- Leslie R. D., Pyke D. A., Stubbs W. A. Sensitivity to enkephalin as a cause of non-insulin dependent diabetes. Lancet. 1979 Feb 17;1(8112):341–343. doi: 10.1016/s0140-6736(79)92887-3. [DOI] [PubMed] [Google Scholar]
- Llorens C., Schwartz J. C. Enkephalinase activity in rat peripheral organs. Eur J Pharmacol. 1981 Jan 5;69(1):113–116. doi: 10.1016/0014-2999(81)90609-9. [DOI] [PubMed] [Google Scholar]
- MacDonald D. W., Saggerson E. D. Hormonal control of gluconeogenesis in tubule fragments from renal cortex of fed rats. Effects of alpha-adrenergic stimuli, glucagon, theophylline and papaverine. Biochem J. 1977 Oct 15;168(1):33–42. doi: 10.1042/bj1680033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malfroy B., Schwartz J. C. Properties of "enkephalinase" from rat kidney: comparison of dipeptidyl-carboxypeptidase and endopeptidase activities. Biochem Biophys Res Commun. 1982 May 31;106(2):276–285. doi: 10.1016/0006-291x(82)91106-8. [DOI] [PubMed] [Google Scholar]
- Matsumura M., Fukushima T., Saito H., Saito S. In vivo and in vitro effects of beta-endorphin on glucose metabolism in the rat. Horm Metab Res. 1984 Jan;16(1):27–31. doi: 10.1055/s-2007-1014686. [DOI] [PubMed] [Google Scholar]
- Olatunji O. L., Premilat S. Solvent dependent conformational statistics of enkephalin and angiotensin II. Int J Pept Protein Res. 1987 Jan;29(1):1–8. doi: 10.1111/j.1399-3011.1987.tb02224.x. [DOI] [PubMed] [Google Scholar]
- Saggerson E. D., Carpenter C. A. Effect of compound D-600 (methoxyverapamil) on gluconeogenesis and on acceleration of the process by alpha-adrenergic stimuli in rat kidney tubules. Biochem J. 1980 Aug 15;190(2):283–291. doi: 10.1042/bj1900283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saggerson E. D., Carpenter C. A. Ouabain and K+ removal blocks alpha-adrenergic stimulation of gluconeogenesis in tubule fragments from fed rats. FEBS Lett. 1979 Oct 1;106(1):189–192. doi: 10.1016/0014-5793(79)80725-5. [DOI] [PubMed] [Google Scholar]
- Sharma S. K., Nirenberg M., Klee W. A. Morphine receptors as regulators of adenylate cyclase activity. Proc Natl Acad Sci U S A. 1975 Feb;72(2):590–594. doi: 10.1073/pnas.72.2.590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stubbs W. A., Delitala G., Jones A., Jeffcoate W. J., Edwards C. R., Ratter S. J., Besser G. M., Bloom S. R., Alberti K. G. Hormonal and metabolic responses to an enkephalin analogue in normal man. Lancet. 1978 Dec 9;2(8102):1225–1227. doi: 10.1016/s0140-6736(78)92100-1. [DOI] [PubMed] [Google Scholar]
- Werther G. A., Joffe S., Artal R., Sperling M. A. Opiate modulation of glucose turnover in dogs. Metabolism. 1985 Feb;34(2):136–140. doi: 10.1016/0026-0495(85)90122-2. [DOI] [PubMed] [Google Scholar]


