Abstract
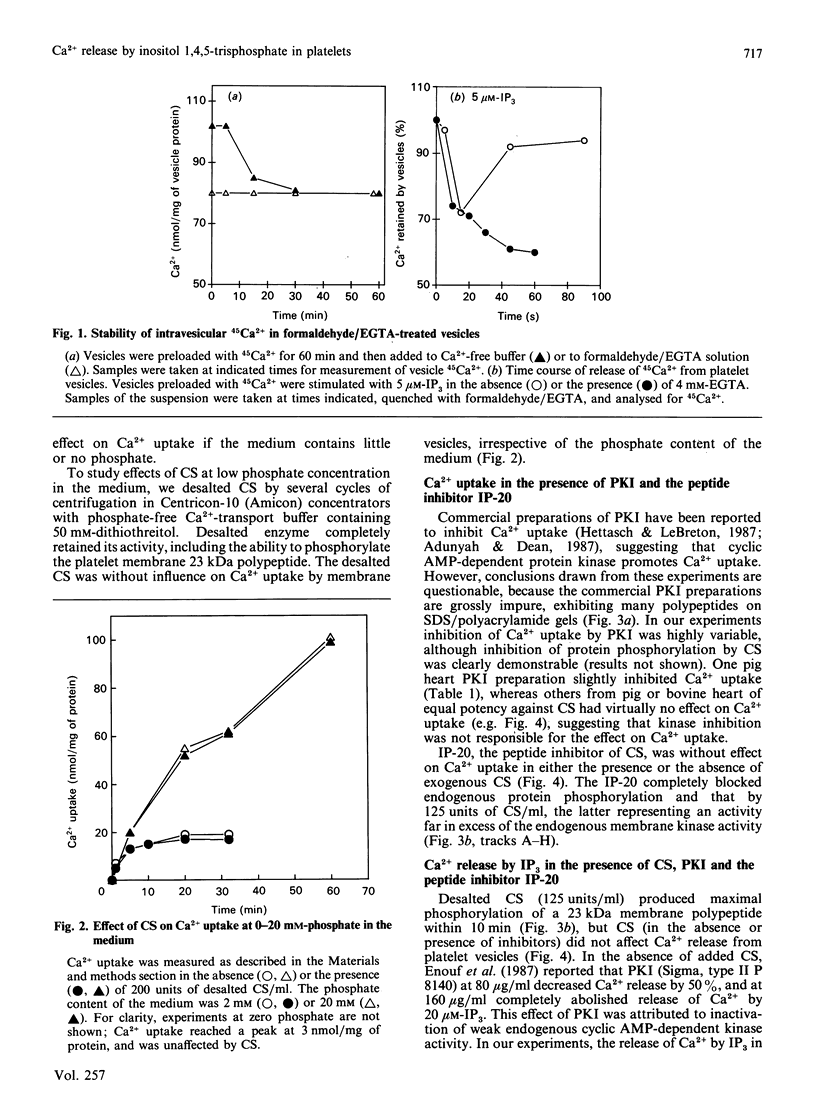
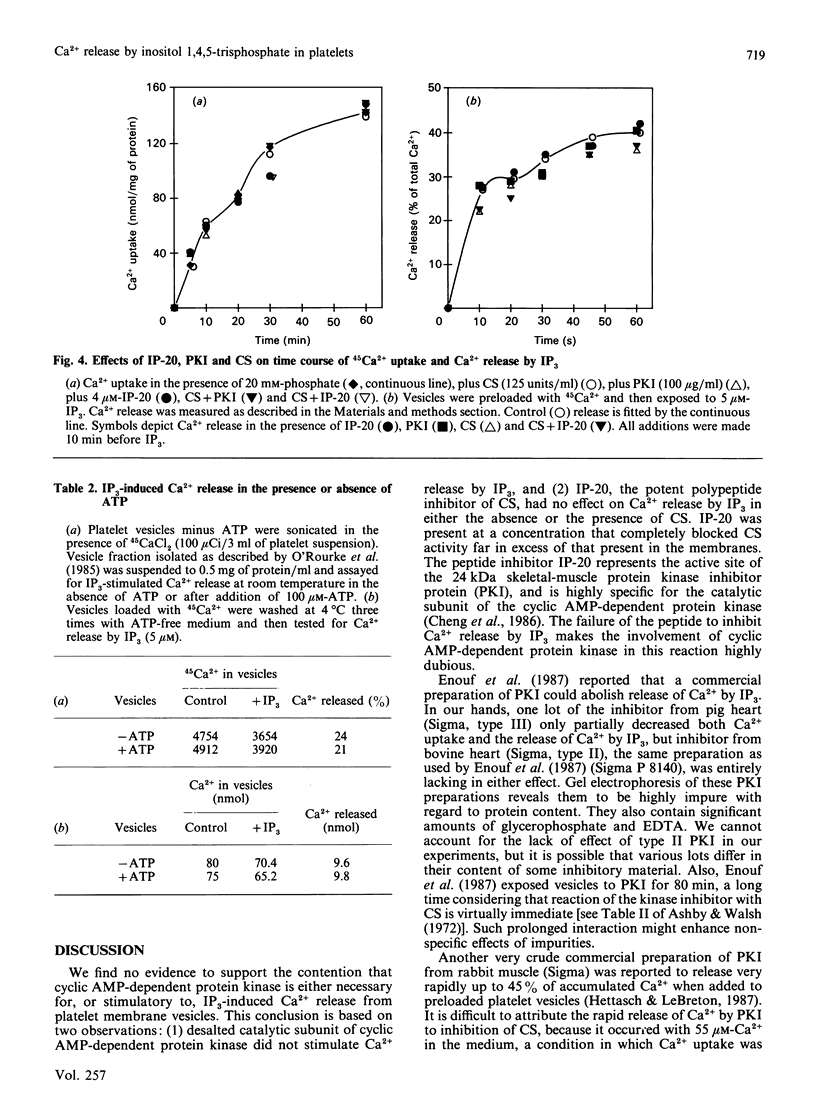
In contrast with previous reports, it was found that membrane-protein phosphorylation by the catalytic subunit (CS) of cyclic AMP-dependent protein kinase had no effect on Ca2+ uptake into platelet membrane vesicles or on subsequent Ca2+ release by inositol 1,4,5-trisphosphate (IP3). Furthermore, IP-20, a highly potent synthetic peptide inhibitor of CS, which totally abolished membrane protein phosphorylation by endogenous or exogenous CS, also had no effect on either Ca2+ uptake or release by IP3. Commercial preparations of protein kinase inhibitor protein (PKI) usually had no effect, but one preparation partially inhibited Ca2+ uptake, which is attributable to the gross impurity of the commercial PKI preparation. IP3-induced release of Ca2+ was also unaffected by the absence of ATP from the medium, supporting the conclusion that Ca2+ release by IP3 does not require the phosphorylation of membrane protein.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adunyah S. E., Dean W. L. Ca2+ transport in human platelet membranes. Kinetics of active transport and passive release. J Biol Chem. 1986 Mar 5;261(7):3122–3127. [PubMed] [Google Scholar]
- Adunyah S. E., Dean W. L. Effects of sulfhydryl reagents and other inhibitors on Ca2+ transport and inositol trisphosphate-induced Ca2+ release from human platelet membranes. J Biol Chem. 1986 Oct 5;261(28):13071–13075. [PubMed] [Google Scholar]
- Adunyah S. E., Dean W. L. Inositol triphosphate-induced Ca2+ release from human platelet membranes. Biochem Biophys Res Commun. 1985 May 16;128(3):1274–1280. doi: 10.1016/0006-291x(85)91078-2. [DOI] [PubMed] [Google Scholar]
- Adunyah S. E., Dean W. L. Regulation of human platelet membrane Ca2+ transport by cAMP- and calmodulin-dependent phosphorylation. Biochim Biophys Acta. 1987 Oct 1;930(3):401–409. doi: 10.1016/0167-4889(87)90013-9. [DOI] [PubMed] [Google Scholar]
- Ashby C. D., Walsh D. A. Characterization of the interaction of a protein inhibitor with adenosine 3',5'-monophosphate-dependent protein kinases. I. Interaction with the catalytic subunit of the protein kinase. J Biol Chem. 1972 Oct 25;247(20):6637–6642. [PubMed] [Google Scholar]
- Authi K. S., Crawford N. Inositol 1,4,5-trisphosphate-induced release of sequestered Ca2+ from highly purified human platelet intracellular membranes. Biochem J. 1985 Aug 15;230(1):247–253. doi: 10.1042/bj2300247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bushfield M., McNicol A., MacIntyre D. E. Inhibition of platelet-activating-factor-induced human platelet activation by prostaglandin D2. Differential sensitivity of platelet transduction processes and functional responses to inhibition by cyclic AMP. Biochem J. 1985 Nov 15;232(1):267–271. doi: 10.1042/bj2320267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng H. C., Kemp B. E., Pearson R. B., Smith A. J., Misconi L., Van Patten S. M., Walsh D. A. A potent synthetic peptide inhibitor of the cAMP-dependent protein kinase. J Biol Chem. 1986 Jan 25;261(3):989–992. [PubMed] [Google Scholar]
- Enouf J., Giraud F., Bredoux R., Bourdeau N., Levy-Toledano S. Possible role of a cAMP-dependent phosphorylation in the calcium release mediated by inositol 1,4,5-trisphosphate in human platelet membrane vesicles. Biochim Biophys Acta. 1987 Apr 2;928(1):76–82. doi: 10.1016/0167-4889(87)90087-5. [DOI] [PubMed] [Google Scholar]
- Feinstein M. B., Egan J. J., Sha'afi R. I., White J. The cytoplasmic concentration of free calcium in platelets is controlled by stimulators of cyclic AMP production (PGD2, PGE1, forskolin). Biochem Biophys Res Commun. 1983 Jun 15;113(2):598–604. doi: 10.1016/0006-291x(83)91768-0. [DOI] [PubMed] [Google Scholar]
- Hack N., Croset M., Crawford N. Studies on the bivalent-cation-activated ATPase activities of highly purified human platelet surface and intracellular membranes. Biochem J. 1986 Feb 1;233(3):661–668. doi: 10.1042/bj2330661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halenda S. P., Volpi M., Zavoico G. B., Sha'afi R. I., Feinstein M. B. Effects of thrombin, phorbol myristate acetate and prostaglandin D2 on 40-41 kDa protein that is ADP ribosylated by pertussis toxin in platelets. FEBS Lett. 1986 Aug 18;204(2):341–346. doi: 10.1016/0014-5793(86)80840-7. [DOI] [PubMed] [Google Scholar]
- Hettasch J. M., Le Breton G. C. Modulation of Ca2+ fluxes in isolated platelet vesicles: effects of cAMP-dependent protein kinase and protein kinase inhibitor on Ca2+ sequestration and release. Biochim Biophys Acta. 1987 Oct 22;931(1):49–58. doi: 10.1016/0167-4889(87)90049-8. [DOI] [PubMed] [Google Scholar]
- Knight D. E., Scrutton M. C. Cyclic nucleotides control a system which regulates Ca2+ sensitivity of platelet secretion. Nature. 1984 May 3;309(5963):66–68. doi: 10.1038/309066a0. [DOI] [PubMed] [Google Scholar]
- Käser-Glanzmann R., Gerber E., Lüscher E. F. Regulation of the intracellular calcium level in human blood platelets: cyclic adenosine 3',5'-monophosphate dependent phosphorylation of a 22,000 dalton component in isolated Ca2+-accumulating vesicles. Biochim Biophys Acta. 1979 Dec 12;558(3):344–347. doi: 10.1016/0005-2736(79)90271-2. [DOI] [PubMed] [Google Scholar]
- Käser-Glanzmann R., Jakäbovä M., George J. N., Lüscher E. F. Stimulation of calcium uptake in platelet membrane vesicles by adenosine 3',5'-cyclic monophosphate and protein kinase. Biochim Biophys Acta. 1977 May 2;466(3):429–440. doi: 10.1016/0005-2736(77)90336-4. [DOI] [PubMed] [Google Scholar]
- Le Peuch C. J., Le Peuch D. A., Katz S., Demaille J. G., Hincke M. T., Bredoux R., Enouf J., Levy-Toledano S., Caen J. Regulation of calcium accumulation and efflux from platelet vesicles. Possible role for cyclic-AMP-dependent phosphorylation and calmodulin. Biochim Biophys Acta. 1983 Jun 23;731(3):456–464. doi: 10.1016/0005-2736(83)90041-x. [DOI] [PubMed] [Google Scholar]
- Lerea K. M., Glomset J. A., Krebs E. G. Agents that elevate cAMP levels in platelets decrease thrombin binding. J Biol Chem. 1987 Jan 5;262(1):282–288. [PubMed] [Google Scholar]
- Meyer T., Holowka D., Stryer L. Highly cooperative opening of calcium channels by inositol 1,4,5-trisphosphate. Science. 1988 Apr 29;240(4852):653–656. doi: 10.1126/science.2452482. [DOI] [PubMed] [Google Scholar]
- O'Rourke F. A., Halenda S. P., Zavoico G. B., Feinstein M. B. Inositol 1,4,5-trisphosphate releases Ca2+ from a Ca2+-transporting membrane vesicle fraction derived from human platelets. J Biol Chem. 1985 Jan 25;260(2):956–962. [PubMed] [Google Scholar]
- Szmigielski A., Guidotti A., Costa E. Endogenous protein kinase inhibitors. Purification, characterization, and distribution in different tissues. J Biol Chem. 1977 Jun 10;252(11):3848–3853. [PubMed] [Google Scholar]
- Watson S. P., McConnell R. T., Lapetina E. G. The rapid formation of inositol phosphates in human platelets by thrombin is inhibited by prostacyclin. J Biol Chem. 1984 Nov 10;259(21):13199–13203. [PubMed] [Google Scholar]
- Yamanishi J., Kawahara Y., Fukuzaki H. Effect of cyclic AMP on cytoplasmic free calcium in human platelets stimulated by thrombin: direct measurement with quin2. Thromb Res. 1983 Oct 15;32(2):183–188. doi: 10.1016/0049-3848(83)90029-4. [DOI] [PubMed] [Google Scholar]



