Abstract
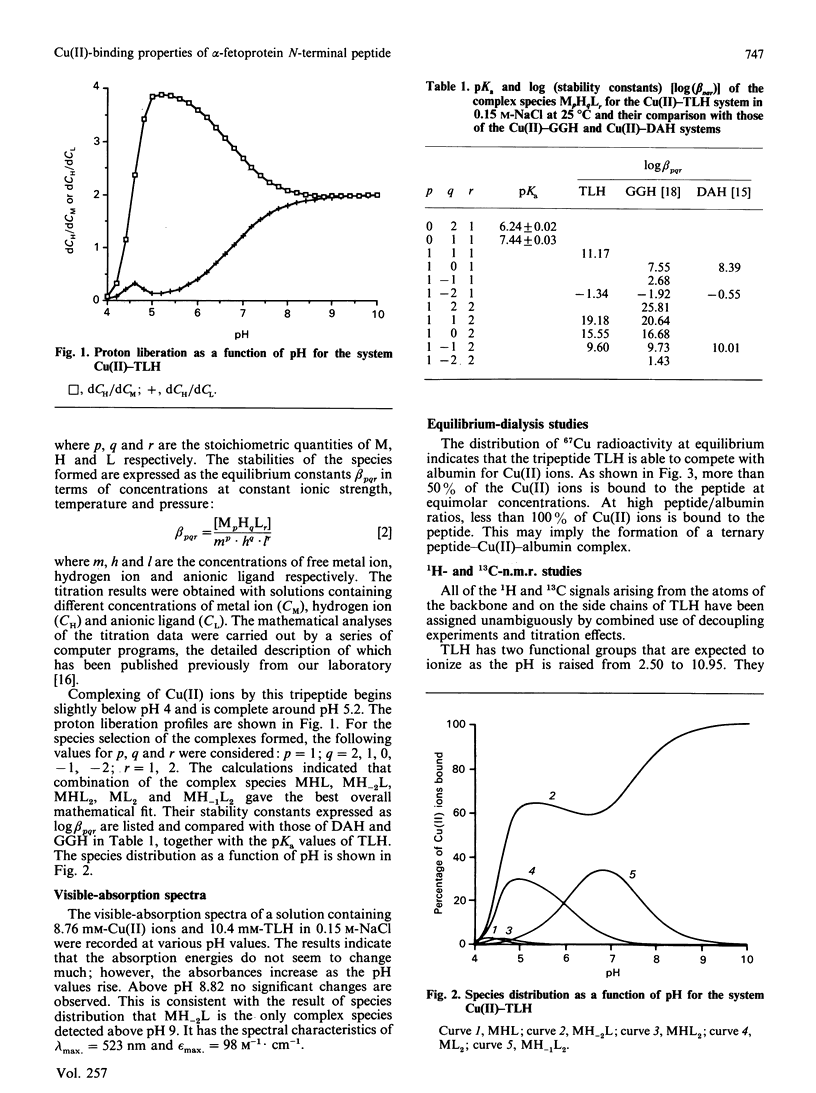
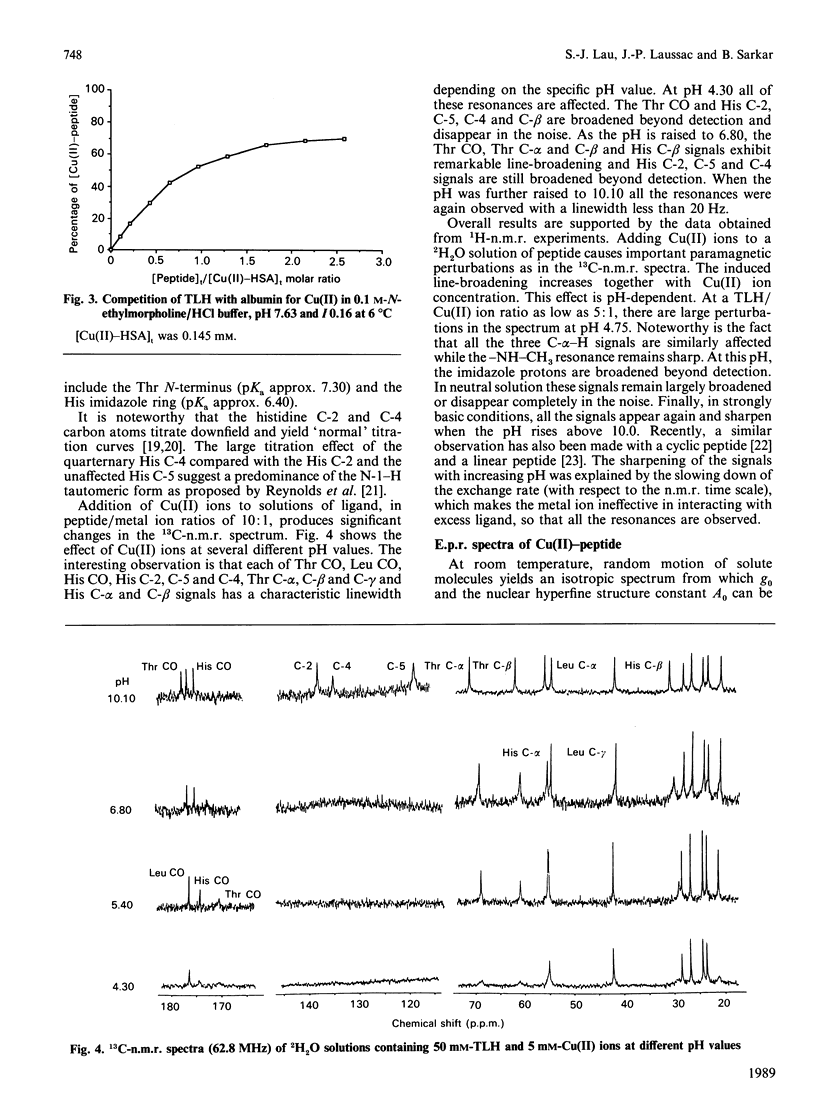
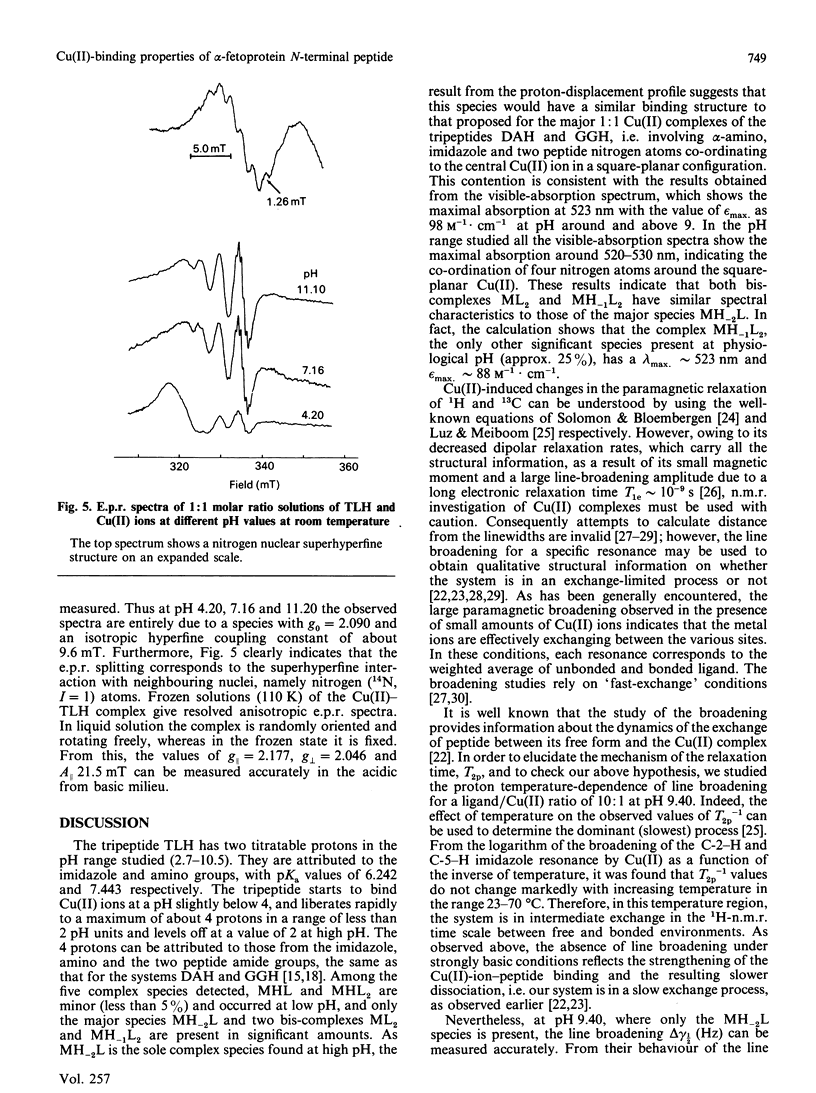
The N-terminal native sequence tripeptide of alpha-fetoprotein, L-threonyl-L-leucyl-L-histidine N-methylamide, was synthesized and its interaction with Cu(II) ions was investigated by potentiometric titration at 25 degrees C in 0.15 M-NaCl and by visible-absorption, e.p.r. and n.m.r. spectroscopy. Analyses of the results in the pH range 4-10 indicated the presence of multiple complex species in solution: MHL, MH-2L, MHL2, ML2 and MH-1L2, where M, H and L represent metal ion, proton and ligand anion respectively. Only the species MH-2L and MH-1L2 are present in significant amounts at physiological pH. The results of the visible-absorption spectroscopy are consistent with the findings of species distribution that MH-2L is the major complex species detected above physiological pH that has the spectral characteristics of lambda max. = 523 nm and epsilon max. = 98 M-1.cm-1. The nine superhyperfine lines in e.p.r. spectra of the major species MH-2L strongly support the co-ordination of four nitrogen atoms by Cu(II). Both 1H- and 13C-n.m.r. studies suggest that the species MH-2L is a square-planar complex. The results from the equilibrium-dialysis experiments showed that this peptide is able to compete with albumin for Cu(II) ions. At equimolar concentrations of albumin and the peptide, about 52% of the Cu(II) was bound to the peptide. The possibility that alpha-fetoprotein plays an important role as the Cu(II)-transport protein in fetal life is discussed.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abelev G. I. Alpha-fetoprotein in ontogenesis and its association with malignant tumors. Adv Cancer Res. 1971;14:295–358. doi: 10.1016/s0065-230x(08)60523-0. [DOI] [PubMed] [Google Scholar]
- Aoyagi Y., Ikenaka T., Ichida F. Comparative chemical structures of human alpha-fetoproteins from fetal serum and from ascites fluid of a patient with hepatoma. Cancer Res. 1977 Oct;37(10):3663–3667. [PubMed] [Google Scholar]
- Aoyagi Y., Ikenaka T., Ichida F. Copper(II)-binding ability of human alpha-fetoprotein. Cancer Res. 1978 Oct;38(10):3483–3486. [PubMed] [Google Scholar]
- Beattie J. K., Fensom D. J., Freeman H. C. A reinterpretation of paramagnetic line broadening in the nuclear magnetic resonance spectra of amino acids and peptides. I. The copper(II)--glycine system. J Am Chem Soc. 1976 Jan 21;98(2):500–507. doi: 10.1021/ja00418a029. [DOI] [PubMed] [Google Scholar]
- Gitlin D., Boesman M. Fetus-specific serum proteins in several mammals and their relation to human alpha-fetoprotein. Comp Biochem Physiol. 1967 May;21(2):327–336. doi: 10.1016/0010-406x(67)90793-1. [DOI] [PubMed] [Google Scholar]
- Gitlin D., Boesman M. Serum alpha-fetoprotein, albumin, and gamma-G-globulin in the human conceptus. J Clin Invest. 1966 Nov;45(11):1826–1838. doi: 10.1172/JCI105486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grigorova A. M., Cittanova N., Jayle M. F. Physico-chemical analogies of rat alpha-fetoprotein and rat serum albumin. Biochimie. 1977;59(2):217–220. doi: 10.1016/s0300-9084(77)80292-7. [DOI] [PubMed] [Google Scholar]
- Iyer K. S., Lau S. J., Laurie S. H., Sarkar B. Synthesis of the native copper(II)-transport site of human serum albumin and its copper(II)-binding properties. Biochem J. 1978 Jan 1;169(1):61–69. doi: 10.1042/bj1690061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau S. J., Kruck T. P., Sarkar B. A peptide molecule mimicking the copper(II) transport site of human serum albumin. A comparative study between the synthetic site and albumin. J Biol Chem. 1974 Sep 25;249(18):5878–5884. [PubMed] [Google Scholar]
- Laussac J. P., Haran R., Sarkar B. N.m.r. and e.p.r. investigation of the interaction of copper(II) and glycyl-L-histidyl-L-lysine, a growth-modulating tripeptide from plasma. Biochem J. 1983 Feb 1;209(2):533–539. doi: 10.1042/bj2090533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laussac J. P., Sarkar B. 13Carbon-nuclear magnetic resonance investigation of the Cu(II)-binding to the native sequence peptide representing the Cu(II)-transport site of human albumin. Evidence for the involvement of the beta-carboxyl side chain of aspartyl residue. J Biol Chem. 1980 Aug 25;255(16):7563–7568. [PubMed] [Google Scholar]
- Laussac J. P., Sarkar B. Characterization of the copper(II)- and nickel(II)-transport site of human serum albumin. Studies of copper(II) and nickel(II) binding to peptide 1-24 of human serum albumin by 13C and 1H NMR spectroscopy. Biochemistry. 1984 Jun 5;23(12):2832–2838. doi: 10.1021/bi00307a046. [DOI] [PubMed] [Google Scholar]
- Laussac J. P., Sarkar B. Isolation, purification and 13C- and 1H-n.m.r. assignments of peptide [1-24] of human serum albumin. Int J Pept Protein Res. 1985 Oct;26(4):425–438. doi: 10.1111/j.1399-3011.1985.tb01009.x. [DOI] [PubMed] [Google Scholar]
- Morinaga T., Sakai M., Wegmann T. G., Tamaoki T. Primary structures of human alpha-fetoprotein and its mRNA. Proc Natl Acad Sci U S A. 1983 Aug;80(15):4604–4608. doi: 10.1073/pnas.80.15.4604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peisach J., Blumberg W. E. Structural implications derived from the analysis of electron paramagnetic resonance spectra of natural and artificial copper proteins. Arch Biochem Biophys. 1974 Dec;165(2):691–708. doi: 10.1016/0003-9861(74)90298-7. [DOI] [PubMed] [Google Scholar]
- Peters T., Jr Serum albumin: recent progress in the understanding of its structure and biosynthesis. Clin Chem. 1977 Jan;23(1):5–12. [PubMed] [Google Scholar]
- Purves L. R., Branch W. R., Geddes E. W., Manso C., Portugal M. Serum alpha-feto-protein. VII. The range of apparent serum values in normal people, pregnant women, and primary liver cancer high risk populations. Cancer. 1973 Mar;31(3):578–587. doi: 10.1002/1097-0142(197303)31:3<578::aid-cncr2820310313>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- Rakhit G., Sarkar B. Electron spin resonance study of the copper(II) complexes of human and dog serum albumins abd some peptide analogs. J Inorg Biochem. 1981 Nov;15(3):233–241. doi: 10.1016/s0162-0134(00)80158-0. [DOI] [PubMed] [Google Scholar]
- Reynolds W. F., Peat I. R., Freedman M. H., Lyerla J. R., Jr Determination of the tautomeric form of the imidazole ring of L-histidine in basic solution by carbon-13 magnetic resonance spectroscopy. J Am Chem Soc. 1973 Jan 24;95(2):328–331. doi: 10.1021/ja00783a006. [DOI] [PubMed] [Google Scholar]
- Ruoslahti E., Pihko H., Seppälä M. Alpha-fetoprotein: immunochemical purification and chemical properties. Expression in normal state and in malignant and non-malignant liver disease. Transplant Rev. 1974;20(0):38–60. doi: 10.1111/j.1600-065x.1974.tb00140.x. [DOI] [PubMed] [Google Scholar]
- Ruoslahti E., Seppälä M. Studies of carcino-fetal proteins. 3. Development of a radioimmunoassay for -fetoprotein. Demonstration of -fetoprotein in serum of healthy human adults. Int J Cancer. 1971 Nov 15;8(3):374–383. doi: 10.1002/ijc.2910080304. [DOI] [PubMed] [Google Scholar]
- Ruoslahti E., Terry W. D. alpha foetoprotein and serum albumin show sequence homology. Nature. 1976 Apr 29;260(5554):804–805. doi: 10.1038/260804a0. [DOI] [PubMed] [Google Scholar]
- Van Furth R., Adinolfi M. In vitro synthesis of the foetal alpha 1-globulin in man. Nature. 1969 Jun 28;222(5200):1296–1299. doi: 10.1038/2221296a0. [DOI] [PubMed] [Google Scholar]


