Abstract
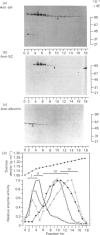
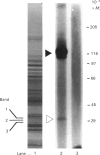
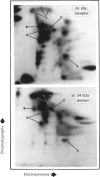
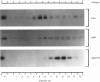
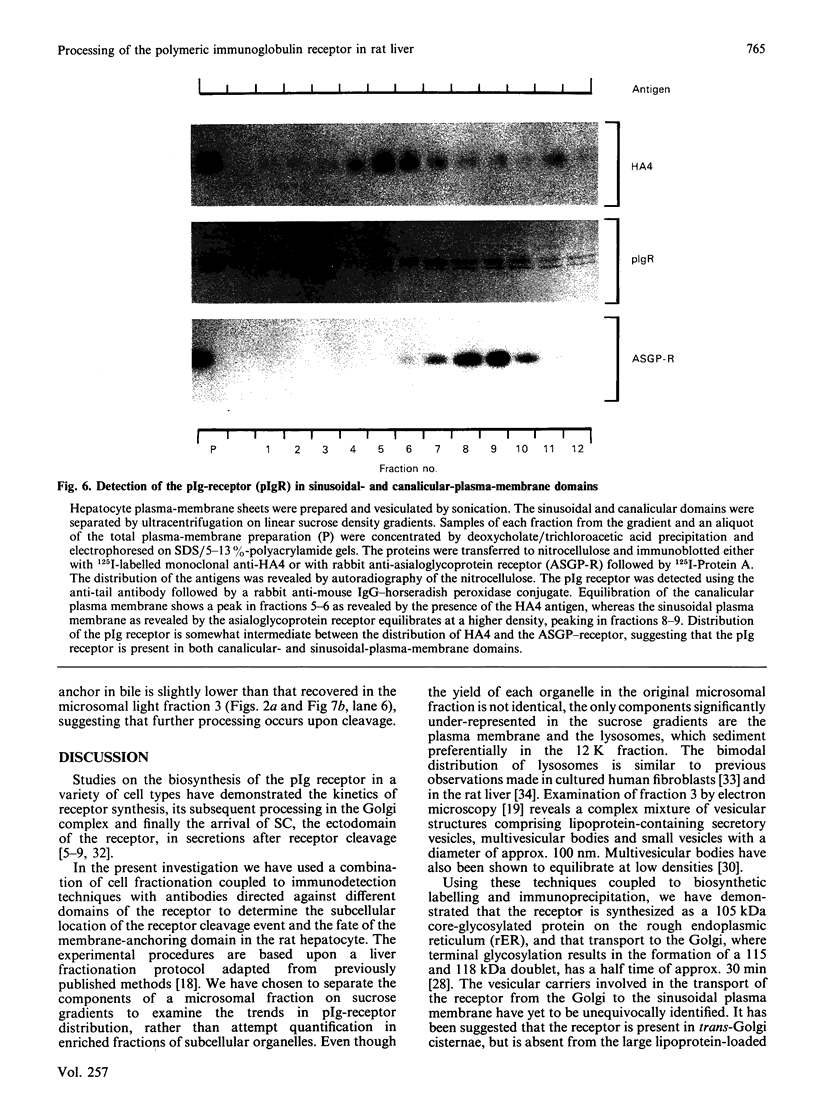
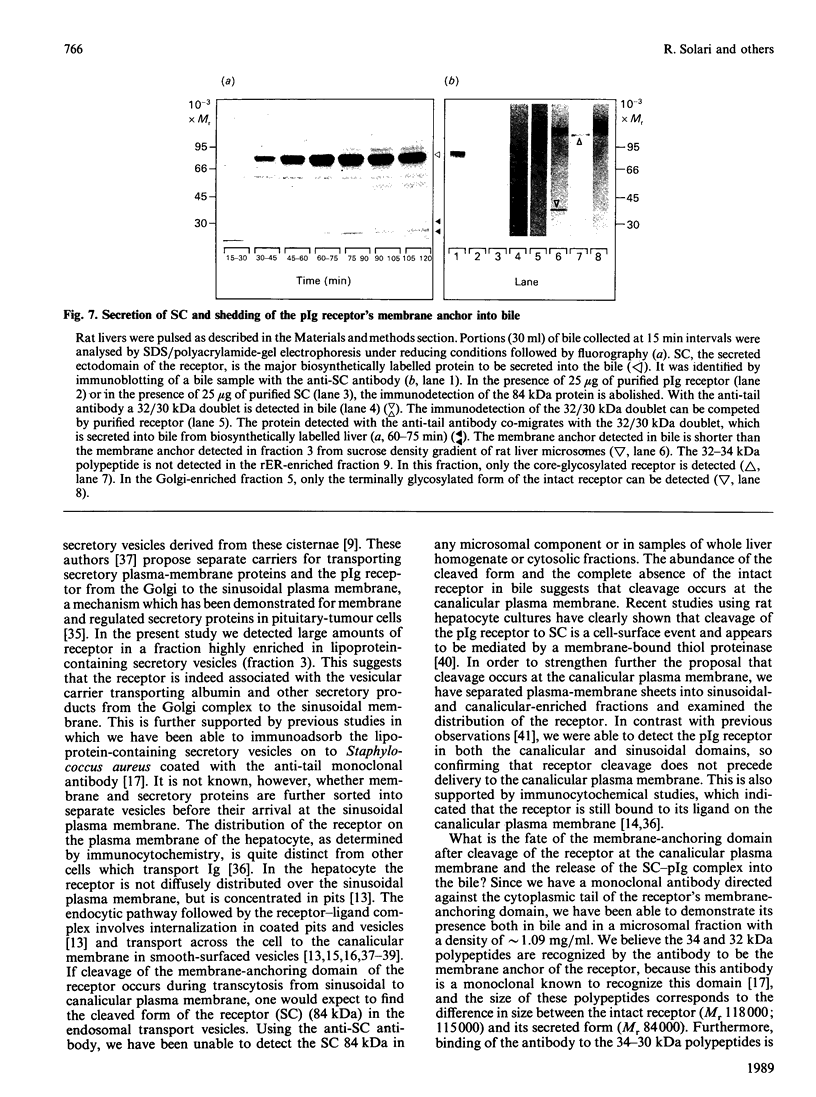
Transcytosis of polymeric immunoglobulin (pIg) across glandular and mucosal epithelia is mediated by a member of the immunoglobulin supergene family, the pIg receptor. During transcellular routing, the receptor is cleaved and its ectoplasmic domain, known as secretory component (SC), is released into secretions bound to pIg. Using receptor-domain-specific antibodies, we have combined cell fractionation and immunoblotting of rat liver to examine the cellular routing of the receptor, the cellular location of the cleavage event and the fate of the anchor domain. Cleavage is a late event in receptor processing. It appears to occur at the canalicular plasma membrane, since intact receptor is present in this membrane domain and no SC is detected in whole liver homogenate or in cell fractions. The membrane anchor remaining after cleavage can be recovered in bile, as well as in a low-density fraction obtained after equilibrium centrifugation of liver (microsomal fractions) on sucrose density gradients. These data suggest that the membrane-anchor domain may be internalized as well as secreted together with SC into bile.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bartles J. R., Braiterman L. T., Hubbard A. L. Endogenous and exogenous domain markers of the rat hepatocyte plasma membrane. J Cell Biol. 1985 Apr;100(4):1126–1138. doi: 10.1083/jcb.100.4.1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartles J. R., Feracci H. M., Stieger B., Hubbard A. L. Biogenesis of the rat hepatocyte plasma membrane in vivo: comparison of the pathways taken by apical and basolateral proteins using subcellular fractionation. J Cell Biol. 1987 Sep;105(3):1241–1251. doi: 10.1083/jcb.105.3.1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birbeck M. S., Cartwright P., Hall J. G., Orlans E., Peppard J. The transport by hepatocytes of immunoglobulin A from blood to bile visualized by autoradiography and electron microscopy. Immunology. 1979 Jun;37(2):477–484. [PMC free article] [PubMed] [Google Scholar]
- Brandtzaeg P. Transport models for secretory IgA and secretory IgM. Clin Exp Immunol. 1981 May;44(2):221–232. [PMC free article] [PubMed] [Google Scholar]
- Deitcher D. L., Neutra M. R., Mostov K. E. Functional expression of the polymeric immunoglobulin receptor from cloned cDNA in fibroblasts. J Cell Biol. 1986 Mar;102(3):911–919. doi: 10.1083/jcb.102.3.911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn W. A., Hubbard A. L. Receptor-mediated endocytosis of epidermal growth factor by hepatocytes in the perfused rat liver: ligand and receptor dynamics. J Cell Biol. 1984 Jun;98(6):2148–2159. doi: 10.1083/jcb.98.6.2148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GREENWOOD F. C., HUNTER W. M., GLOVER J. S. THE PREPARATION OF I-131-LABELLED HUMAN GROWTH HORMONE OF HIGH SPECIFIC RADIOACTIVITY. Biochem J. 1963 Oct;89:114–123. doi: 10.1042/bj0890114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geuze H. J., Slot J. W., Strous G. J., Peppard J., von Figura K., Hasilik A., Schwartz A. L. Intracellular receptor sorting during endocytosis: comparative immunoelectron microscopy of multiple receptors in rat liver. Cell. 1984 May;37(1):195–204. doi: 10.1016/0092-8674(84)90315-5. [DOI] [PubMed] [Google Scholar]
- Gumbiner B., Kelly R. B. Two distinct intracellular pathways transport secretory and membrane glycoproteins to the surface of pituitary tumor cells. Cell. 1982 Jan;28(1):51–59. doi: 10.1016/0092-8674(82)90374-9. [DOI] [PubMed] [Google Scholar]
- Hoppe C. A., Connolly T. P., Hubbard A. L. Transcellular transport of polymeric IgA in the rat hepatocyte: biochemical and morphological characterization of the transport pathway. J Cell Biol. 1985 Dec;101(6):2113–2123. doi: 10.1083/jcb.101.6.2113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hornick C. A., Hamilton R. L., Spaziani E., Enders G. H., Havel R. J. Isolation and characterization of multivesicular bodies from rat hepatocytes: an organelle distinct from secretory vesicles of the Golgi apparatus. J Cell Biol. 1985 May;100(5):1558–1569. doi: 10.1083/jcb.100.5.1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubbard A. L., Bartles J. R., Braiterman L. T. Identification of rat hepatocyte plasma membrane proteins using monoclonal antibodies. J Cell Biol. 1985 Apr;100(4):1115–1125. doi: 10.1083/jcb.100.4.1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kühn L. C., Kraehenbuhl J. P. The membrane receptor for polymeric immunoglobulin is structurally related to secretory component. Isolation and characterization of membrane secretory component from rabbit liver and mammary gland. J Biol Chem. 1981 Dec 10;256(23):12490–12495. [PubMed] [Google Scholar]
- Meier P. J., Sztul E. S., Reuben A., Boyer J. L. Structural and functional polarity of canalicular and basolateral plasma membrane vesicles isolated in high yield from rat liver. J Cell Biol. 1984 Mar;98(3):991–1000. doi: 10.1083/jcb.98.3.991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mostov K. E., Blobel G. A transmembrane precursor of secretory component. The receptor for transcellular transport of polymeric immunoglobulins. J Biol Chem. 1982 Oct 10;257(19):11816–11821. [PubMed] [Google Scholar]
- Mostov K. E., Deitcher D. L. Polymeric immunoglobulin receptor expressed in MDCK cells transcytoses IgA. Cell. 1986 Aug 15;46(4):613–621. doi: 10.1016/0092-8674(86)90887-1. [DOI] [PubMed] [Google Scholar]
- Mostov K. E., Friedlander M., Blobel G. The receptor for transepithelial transport of IgA and IgM contains multiple immunoglobulin-like domains. Nature. 1984 Mar 1;308(5954):37–43. doi: 10.1038/308037a0. [DOI] [PubMed] [Google Scholar]
- Mostov K. E., Kraehenbuhl J. P., Blobel G. Receptor-mediated transcellular transport of immunoglobulin: synthesis of secretory component as multiple and larger transmembrane forms. Proc Natl Acad Sci U S A. 1980 Dec;77(12):7257–7261. doi: 10.1073/pnas.77.12.7257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mostov K. E., de Bruyn Kops A., Deitcher D. L. Deletion of the cytoplasmic domain of the polymeric immunoglobulin receptor prevents basolateral localization and endocytosis. Cell. 1986 Nov 7;47(3):359–364. doi: 10.1016/0092-8674(86)90592-1. [DOI] [PubMed] [Google Scholar]
- Mullock B. M., Hinton R. H., Dobrota M., Peppard J., Orlans E. Distribution of secretory component in hepatocytes and its mode of transfer into bile. Biochem J. 1980 Sep 15;190(3):819–826. doi: 10.1042/bj1900819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullock B. M., Hinton R. H., Dobrota M., Peppard J., Orlans E. Endocytic vesicles in liver carry polymeric IgA from serum to bile. Biochim Biophys Acta. 1979 Oct 18;587(3):381–391. doi: 10.1016/0304-4165(79)90442-2. [DOI] [PubMed] [Google Scholar]
- Mullock B. M., Jones R. S., Hinton R. H. Movement of endocytic shuttle vesicles from the sinusoidal to the bile canalicular face of hepatocytes does not depend on occupation of receptor sites. FEBS Lett. 1980 May 5;113(2):201–205. doi: 10.1016/0014-5793(80)80591-6. [DOI] [PubMed] [Google Scholar]
- Mullock B. M., Luzio J. P., Hinton R. H. Preparation of a low-density species of endocytic vesicle containing immunoglobulin A. Biochem J. 1983 Sep 15;214(3):823–827. doi: 10.1042/bj2140823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musil L. S., Baenziger J. U. Cleavage of membrane secretory component to soluble secretory component occurs on the cell surface of rat hepatocyte monolayers. J Cell Biol. 1987 Jun;104(6):1725–1733. doi: 10.1083/jcb.104.6.1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pertoft H., Wärmegård B., Hök M. Heterogeneity of lysosomes originating from rat liver parenchymal cells. Metabolic relationship of subpopulations separated by density-gradient centrifugation. Biochem J. 1978 Jul 15;174(1):309–317. doi: 10.1042/bj1740309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rome L. H., Garvin A. J., Allietta M. M., Neufeld E. F. Two species of lysosomal organelles in cultured human fibroblasts. Cell. 1979 May;17(1):143–153. doi: 10.1016/0092-8674(79)90302-7. [DOI] [PubMed] [Google Scholar]
- Solari R., Kraehenbuhl J. P. Biosynthesis of the IgA antibody receptor: a model for the transepithelial sorting of a membrane glycoprotein. Cell. 1984 Jan;36(1):61–71. doi: 10.1016/0092-8674(84)90074-6. [DOI] [PubMed] [Google Scholar]
- Solari R., Kühn L., Kraehenbuhl J. P. Antibodies recognizing different domains of the polymeric immunoglobulin receptor. J Biol Chem. 1985 Jan 25;260(2):1141–1145. [PubMed] [Google Scholar]
- Solari R., Racine L., Tallichet C., Kraehenbuhl J. P. Distribution and processing of the polymeric immunoglobulin receptor in the rat hepatocyte: morphological and biochemical characterization of subcellular fractions. J Histochem Cytochem. 1986 Jan;34(1):17–23. doi: 10.1177/34.1.3941264. [DOI] [PubMed] [Google Scholar]
- Speicher D. W., Morrow J. S., Knowles W. J., Marchesi V. T. A structural model of human erythrocyte spectrin. Alignment of chemical and functional domains. J Biol Chem. 1982 Aug 10;257(15):9093–9101. [PubMed] [Google Scholar]
- Sztul E. S., Howell K. E., Palade G. E. Biogenesis of the polymeric IgA receptor in rat hepatocytes. I. Kinetic studies of its intracellular forms. J Cell Biol. 1985 Apr;100(4):1248–1254. doi: 10.1083/jcb.100.4.1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sztul E. S., Howell K. E., Palade G. E. Biogenesis of the polymeric IgA receptor in rat hepatocytes. II. Localization of its intracellular forms by cell fractionation studies. J Cell Biol. 1985 Apr;100(4):1255–1261. doi: 10.1083/jcb.100.4.1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sztul E. S., Howell K. E., Palade G. E. Intracellular and transcellular transport of secretory component and albumin in rat hepatocytes. J Cell Biol. 1983 Nov;97(5 Pt 1):1582–1591. doi: 10.1083/jcb.97.5.1582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi I., Nakane P. K., Brown W. R. Ultrastructural events in the translocation of polymeric IgA by rat hepatocytes. J Immunol. 1982 Mar;128(3):1181–1187. [PubMed] [Google Scholar]
- Wall D. A., Hubbard A. L. Receptor-mediated endocytosis of asialoglycoproteins by rat liver hepatocytes: biochemical characterization of the endosomal compartments. J Cell Biol. 1985 Dec;101(6):2104–2112. doi: 10.1083/jcb.101.6.2104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weibel E. R., Stäubli W., Gnägi H. R., Hess F. A. Correlated morphometric and biochemical studies on the liver cell. I. Morphometric model, stereologic methods, and normal morphometric data for rat liver. J Cell Biol. 1969 Jul;42(1):68–91. doi: 10.1083/jcb.42.1.68. [DOI] [PMC free article] [PubMed] [Google Scholar]