Abstract
Lung cancer remains the number one cancer related mortality in the United States . While it is the third most diagnosed cancer, it is often found at an advanced stage. Survival rates for stage I lung cancer are above 70% while survival rates for stage IV lung cancer are less than 10% at five years. Methods to detect lung cancer at an earlier stage when it can be more effectively treated have been investigated for many years. These included regular chest x-rays (CXRs) and sputum samples. Unfortunately, these testing modalities did not show any benefit. This changed in 2011 when data from the National Lung Screening Trial were published. This landmark trial showed conclusively that a low-radiation dose chest computed tomography scan (LDCT) performed annually in patients with a heavy smoking history reduced lung cancer related mortality by 20%. These results have led to a nationwide effort to increase lung cancer screening. While the number of eligible patients that are being screened on a national level remains modest, significant efforts are being made at the state and local levels to increase awareness and to improve screening. These efforts have also targeted underserved areas and are focused on reducing disparities in access.
The need for a practical method to detect lung cancer at an early, curable stage has been recognized for many years. However, finding an effective screening tool has been difficult. In 1968, a randomized study in England examined the efficacy of twice yearly chest x-rays (CXRs) in 55,000 men from the general population. Unfortunately, no difference in lung cancer mortality was seen in this study.1 In the 1970s, three trials examined the usage of CXRs and sputum cytology in 20,000 patients with a heavy smoking history. Unfortunately, no difference was noted in lung cancer incidence or mortality.2 In 2011, a large, randomized trial involving 155,000 patients determined no benefit in the utility of annual CXRs in reducing lung cancer incidence or cancer related mortality.3
As technology evolved over time, computed tomography (CT) scans became more widely used. CT scans provided the image resolution necessary to detect lung cancers at an earlier stage versus a chest x-ray. Being able to detect lung cancer earlier, when treatment options could be curative, reduced cancer related mortality. In 2006, one of the first studies utilizing CTs as a screening tool was the International Early Lung Cancer Action Project (IELCAP). This international, non-randomized multicenter study screened 31,500 patients, including patients from Delaware, with annual CT scans. A total of 484 lung cancers were detected, 85% of which were stage I.4 Building upon this data, the National Lung Screening Trial (NLST) conclusively established CT scans as a screening tool in 2011. This landmark study examined 53,000 patients between the ages of 55 and 74 with at least a 30-pack year smoking history and who were either current smokers or had quit within 15 years. Patients were randomized to either a low-dose CT (LDCT) or CXR. This study showed a 20% reduction in lung cancer-related mortality in those who received CT screening.5 The Nelson Trial in Europe confirmed the findings of the NLST and reported a 26% reduction in lung cancer mortality.6 Since then, the American Cancer Society has broadened the guidelines to include patients between the ages of 50 to 80 who have at least a 20-pack year smoking history.7 Patients also need to have a shared decision-making discussion with a qualified health professional prior to CT screening and smoking cessation counseling.
While CT screening guidelines have now existed for the past 10 years, actual screening rates have remained modest. A 2022 report from the American Lung Association showed that only 5.8% of those eligible in the United States were screened with a LDCT. Delaware had an above average rate of screening of 6.3%.8 To improve these numbers, efforts were made at both the state and local levels. At the state level, the Delaware Cancer Consortium created the Screening for Life Program in 1997. This program utilized nurse navigators to provide education and outreach. Partnering with community agencies and with federally qualified health centers were key to eliminating barriers and enhancing access. Through these efforts, a total of 48 community events were organized reaching over 2,054 individuals in 2023.
At a local level, the Christiana lung cancer screening program was started in 2016. There were 500 patients initially screened; however, this increased to 1,900 patients by 2022. Of the 1,900 patients, 22 lung cancers were detected (1.16%), 12 of which were stage I (12/22= 54.5%). This data is in line with the NLST data which showed an incidence of 0.65%, of which 50% were stage I.5 To raise additional awareness, the National Lung Cancer Screening Day is an annual event created to promote in-person screening. Also, a Lung Cancer Community Research Advisory Board (CRAB) was convened by the thoracic multidisciplinary team at the Helen F Graham Cancer Center & Research Institute at ChristianaCare to engage researchers, clinicians and community members in reducing inequities and improving access.
In the past few years, further efforts have been made to improve screening in underserved areas and to reduce disparities. Racial differences exist in lung cancer incidence, mortality and screening. African American males have the highest rate of age-adjusted lung cancer incidence in the US (73.5 per 100,000 vs 63.5 per 100,000 for white males) and have the highest lung cancer mortality (62.1 vs 51.7 for white males).9 Kunitomo group published a meta-analysis demonstrating lower screening rate in African Americans, but an equal participation rate once referred for a LDCT scan.10 Barriers to screening are multifactorial including education level, socioeconomic status, implicit bias, distrust of the medical community, and lack of insurance.11,12 In an effort to reduce disparities and to include more African Americans who developed lung cancer at a younger age and with less smoking exposure, the American Cancer Society lowered the NLST smoking and age requirements for screening.7
The Christiana Lung Health Program recognized racial disparities within its health system. Accreditation for screening at an inner-city hospital CT scanner was obtained, but it was not being utilized to full capacity. An intervention including provider education and additional support was established and results were monitored. The uptake of screening patients in low socioeconomic zip codes and demographics were evaluated (Table 1).
Table 1. Intervention Plan for improving LDCT Screening.
| Improve LDCT Screening Recruitment |
|---|
| ● Nurse navigator |
| ● Provider education |
| ● Smoking cessation education |
| ● Primary care letter to patients |
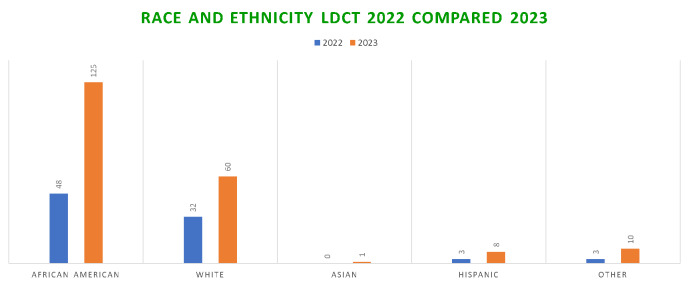
The Wilmington Adult Medical Clinic was the primary care site chosen for the intervention. Physicians received lung cancer screening and smoking cessation education. Philanthropic funds were utilized to fund a dedicated nurse navigator to facilitate enrollment in screening. Education began in May 2021 and nurse navigator involvement started in July 2021. In 2022, 170 total patients were screened, 48 of which were African-American. By 2023, 549 total patients were screened, 125 of which were African-American. (Table 2, Figure 1). This observational study demonstrates that providing dedicated support to train, educate and assist providers can improve screening of African Americans in an inner-city hospital.
Table 2. LDCT Screening in High-Risk Communities.
| Zip Code | 2018 | 2019 | 2020 | 2021 | 2022 | 2023 |
|---|---|---|---|---|---|---|
| 19801 | 24 | 29 | 40 | 60 | 21 | 91 |
| 19802 | 42 | 47 | 56 | 86 | 44 | 125 |
| 19805 | 17 | 21 | 42 | 44 | 47 | 138 |
| 19808 | 1 | 0 | 3 | 3 | 58 | 195 |
| Total | 84 | 97 | 141 | 193 | 170 | 549 |
Figure 1.
Race and Ethnicity LDCT 2022 vs 2023
In the future, as technology continues to evolve, other options may supplement or supplant LDCTs as a screening method. A positron emission tomography (PET) scan has a sensitivity of 68-95% in detecting stage I lung cancers; however, the high financial cost of this test currently limits its utility as a screening modality.13 Artificial intelligence could be applied in a number of ways including the development of personalized screening programs or more accurate detection on imaging tests.14 Significant research continues in developing a simple blood test that could either detect circulating tumor DNA fragments or a biomarker that would identify those with lung cancer.15 Initial progress has been made; however, continued efforts will be needed to implement widespread screening and decrease lung cancer related mortality in the state of Delaware.
Acknowledgement
The authors are grateful to the Katzin Family for their support of the Lung Cancer Screening Program at the Helen F Graham Cancer Center & Research Institute.
References
- 1.Brett, G. Z. (1968, July). The value of lung cancer detection by six-monthly chest radiographs. Thorax, 23(4), 414–420. 10.1136/thx.23.4.414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sharma, D., Newman, T. G., & Aronow, W. S. (2015, October 12). Lung cancer screening: History, current perspectives, and future directions. Archives of Medical Science, 11(5), 1033–1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Oken, M. M., Hocking, W. G., Kvale, P. A., Andriole, G. L., Buys, S. S., Church, T. R., . . .. Berg, C. D., & the PLCO Project Team. (2011, November 2). Screening by chest radiograph and lung cancer mortality: The Prostate, Lung, Colorectal, and Ovarian (PLCO) randomized trial. JAMA, 306(17), 1865–1873. 10.1001/jama.2011.1591 [DOI] [PubMed]
- 4.Henschke, C. I., Yankelevitz, D. F., Libby, D. M., Pasmantier, M. W., Smith, J. P., & Miettinen, O. S., & the International Early Lung Cancer Action Program Investigators . (2006, October 26). Survival of patients with stage I lung cancer detected on CT screening. The New England Journal of Medicine, 355(17), 1763–1771. 10.1056/NEJMoa060476 [DOI] [PubMed] [Google Scholar]
- 5.Aberle, D. R., Adams, A. M., Berg, C. D., Black, W. C., Clapp, J. D., Fagerstrom, R. M., . . .. Sicks, J. D., & the National Lung Screening Trial Research Team. (2011, August 4). Reduced lung-cancer mortality with low-dose computed tomographic screening. The New England Journal of Medicine, 365(5), 395–409. 10.1056/NEJMoa1102873 [DOI] [PMC free article] [PubMed]
- 6.De Koning, H., Aalst, C., Haaf, K., & Oudkerk, M. (2018). Effects of volume CT lung cancer screening: Mortality results of the NELSON randomised-controlled population based trial. J Thor Onc, 13(10), S185. 10.1016/j.jtho.2018.08.012 [DOI] [Google Scholar]
- 7.Wolf, A. M. D., Oeffinger, K. C., Shih, T. Y.-C., Walter, L. C., Church, T. R., Fontham, E. T. H., et al. Smith, R. A. (2024, January-February). Screening for lung cancer: 2023 guideline update from the American Cancer Society. CA: a Cancer Journal for Clinicians, 74(1), 50–81. 10.3322/caac.21811 [DOI] [PubMed] [Google Scholar]
- 8.American Lung Association. (2023). State of Lung Cancer: 2022 Report. https://www.lung.org/getmedia/647c433b-4cbc-4be6-9312-2fa9a449d489/solc-2022-print-report
- 9.Haddad, D. N., Sandler, K. L., Henderson, L. M., Rivera, M. P., & Aldrich, M. C. (2020, April). Disparities in lung cancer screening: A review. Annals of the American Thoracic Society, 17(4), 399–405. 10.1513/AnnalsATS.201907-556CME [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kunitomo, Y., Bade, B., Gunderson, C. G., Akgün, K. M., Brackett, A., Tanoue, L., & Bastian, L. A. (2022, November). Evidence of racial disparities in lung cancer screening process: A systematic review and meta-analysis. Journal of General Internal Medicine, 37(14), 3731–3738. 10.1007/s11606-022-07613-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Carter-Harris, L., Slaven, J. E., Jr., Monahan, P. O., Shedd-Steele, R., Hanna, N., & Rawl, S. M. (2018, February 3). Understanding lung cancer screening behavior: Racial, gender, and geographic differences among Indiana long-term smokers. Preventive Medicine Reports, 10, 49–54. 10.1016/j.pmedr.2018.01.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Steiling, K., Loui, T., Asokan, S., Nims, S., Moreira, P., Rebello, A., et al. Suzuki, K. (2020, May). Age, race, and income are associated with lower screening rates at a safety net hospital. The Annals of Thoracic Surgery, 109(5), 1544–1550. 10.1016/j.athoracsur.2019.11.052 [DOI] [PubMed] [Google Scholar]
- 13.Schöder, H., & Gönen, M. (2007, January). Screening for cancer with PET and PET/CT: Potential and limitations. Journal of Nuclear Medicine, 48(Suppl 1), 4S–18S. [PubMed] [Google Scholar]
- 14.Cellina, M., Cacioppa, L. M., Cè, M., Chiarpenello, V., Costa, M., Vincenzo, Z., et al. Floridi, C. (2023, August 30). Artificial intelligence in lung cancer screening: The future is now. Cancers (Basel), 15(17), 4344. 10.3390/cancers15174344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kossenkov, A. V., Qureshi, R., Dawany, N. B., Wickramasinghe, J., Liu, Q., Majumdar, R. S., et al. Showe, L. C. (2019, January 1). A gene expression classifier from whole blood distinguishes benign from malignant lung nodules detected by low-dose CT. Cancer Research, 79(1), 263–273. 10.1158/0008-5472.CAN-18-2032 [DOI] [PMC free article] [PubMed] [Google Scholar]