Abstract
Background:
Hypertension development is predominantly influenced by inflammation, excessive fat deposition, and metabolic irregularities. Among these factors, liver fat accumulation is a critical metabolic disorder. However, the quantification of liver fat levels and its associated risk for hypertension incidence remain ambiguous. This project is designed to explore the association between liver fat levels and the risk of hypertension in a healthy population.
Methods:
This cross-sectional study involved 4955 participants from the Health Management Center at Henan Provincial People's Hospital who were surveyed between February 2020 and February 2023. Participants were categorized into four groups based on liver fat quartiles. Subgroup analyses, restricted cubic spline regression models, and logistic regression were utilized to assess the association between liver fat levels and hypertension risk. The relationships between liver fat levels and inflammatory markers were examined using multiple linear regression models. Additionally, a mediation analysis was conducted to explore the role of inflammatory factors in the relationship between liver fat and hypertension risk.
Results:
Participants with hypertension exhibited greater liver fat levels than did those without hypertension. An increased risk of hypertension was associated with elevated liver fat levels, even after adjusting for other covariates [Q4 vs. Q1 in model II: odds ratio (OR = 1.28), 95% confidence interval (CI) = 1.04–1.59, P = 0.022; P for trend = 0.039]. A nonlinear relationship was observed between liver fat level and hypertension risk, with a notable increase in hypertension risk occurring at liver fat levels greater than 8.65%. Additionally, a positive correlation was found between inflammatory markers and liver fat levels. A mediation effect of 4.76% was noted, linking hypertension risk and liver fat levels through neutrophils.
Conclusion:
Liver fat levels exceeding 8.65% significantly elevated the risk of hypertension. Inflammatory factors serve as crucial mediators of the relationship between liver fat and hypertension.
Keywords: Chinese, hypertension, inflammation, liver fat, physical examination
INTRODUCTION
Hypertension, a prevalent cardiovascular disease risk factor that is preventable, is characterized by persistent systemic arterial hypertension and is the leading cause of global mortality and disability, accounting for 14% of all deaths [1]. In the United States, hypertension affects approximately one-third of adults [2]. According to the latest survey data in China, approximately 24.7% of the adult population currently suffers from hypertension [3], and high SBP has been linked to more than 25 million disability-adjusted life years attributed to stroke [4]. This condition poses a significant burden on individuals, property safety, and healthcare resources. Several studies have consistently identified inflammation, abnormal fat accumulation, and metabolic abnormalities as the primary factors contributing to the development of hypertension [5], and recent increases in the prevalence of hypertension among the obese population in Shanghai have further supported these findings [6]. Therefore, investigating and mitigating abnormal fat accumulation to prevent the development of hypertension is crucial.
Normal hepatocellular fat accounts for 2–4% of the wet weight of the liver, and metabolic dysfunction-associated steatotic liver disease (MASLD) is diagnosed when triglycerides are present in more than 5% of hepatocytes, along with the presence of metabolic dysregulatory factors such as obesity, type 2 diabetes mellitus, hypertension, and dyslipidemia [7,8]. Abnormal hepatic fat accumulation may lead to cirrhosis, liver failure, and hepatocellular carcinoma [9]. Numerous studies have established a correlation between blood pressure and MASLD, with 49.5% of hypertensive patients having MASLD [10]. Epidemiological evidence also indicates that the incidence of hypertension within the MASLD population is greater than that in the general population [11]. Similar findings regarding the relationship between MASLD and hypertension were observed in another prospective cohort study, the Framingham Heart Study [12]. Despite the correlation between the two diseases and current data are insufficient to clarify the causal relationship between MASLD and hypertension, the exact impact of quantified liver fat levels on the risk of developing hypertension has not yet been determined. MASLD can induce adverse systemic responses, such as insulin resistance, activation of the renin–angiotensin system, activation of the sympathetic nervous system, and local and systemic inflammatory responses [13]. In turn, the inflammatory response can expedite the development and progression of hypertension [14].
Given the substantial role of inflammation in the development of hypertension, our study aimed to investigate the association between the risk of hypertension and liver fat by analyzing data from a population of medical examination participants.
MATERIALS AND METHODS
Individuals and the criteria for inclusion
The data for the study were obtained from the medical records of participants examined at the Health Management Center of Henan Provincial People's Hospital between February 2020 and February 2023, and consent was obtained from the participants. The research protocol received ethics approval from the Ethics Committee at Beijing Jishuitan Hospital, in conformity with the Declaration of Helsinki guidelines (No. 2015-12-02). This dataset, which is registered with clinicaltrials.gov (code: NCT03699228), is an integral part of the China Health Quantitative CT Big Data Research Project Group.
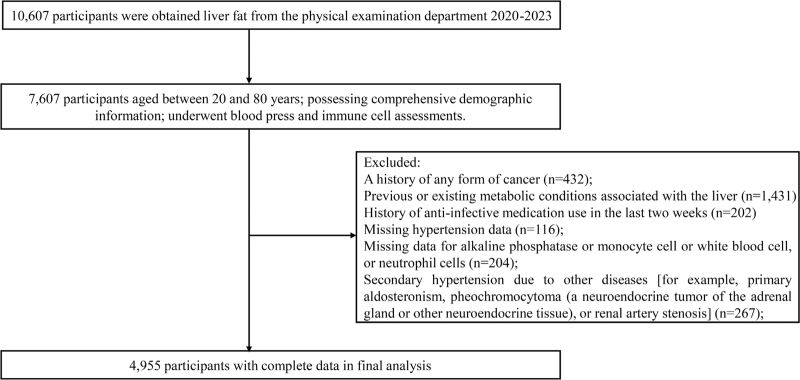
The screening criteria for participants included age between 20 and 80 years; complete general demographic and questionnaire information; and routine low-dose chest computed tomography (CT) scan, blood pressure, and blood immune cell tests. The exclusion criteria included a history of any form of cancer; previous or existing metabolic conditions associated with the liver (including chronic hepatitis, autoimmune hepatitis, hepatomegaly, alcoholic fatty liver disease, etc.); history of antiinfective medication use in the last 2 weeks; missing data for alkaline phosphatase, monocyte, white blood cell, or neutrophil cells; and secondary hypertension due to other diseases [e.g. primary aldosteronism, pheochromocytoma (a neuroendocrine tumor of the adrenal gland or other neuroendocrine tissue), or renal artery stenosis]. A total of 10 607 participants were recruited for liver fat quantification in this study. Of these, 7491 participants met the inclusion criteria, 2536 participants were excluded, and ultimately, 4955 participants were included in further analysis. Skilled research staff conducted on-site surveys to collect comprehensive data on all participants, including age, sex, ethnicity, medical history, and medication history. The process of subject screening is visually depicted in Fig. 1.
FIGURE 1.
Flowchart of participant selection.
Hypertension status
Prior to the measurements taken by the research staff in the morning, the participants fasted for 12 h. Following a 5 min rest period, the research staff utilized an electronic sphygmomanometer (Omron Company, OMRON U30, Kyoto, Japan) to measure SBP and DBP, respectively, with the right arm placed in a semiflexed position at heart level. Two measurements were reported, and an analysis was conducted using the mean of the two readings.
Two consecutive blood pressure readings SBP at least 140 mmHg or DBP at least 90 mmHg, self-reported hypertension, antihypertensive medication use, or antihypertensive treatment, were considered to indicate hypertension [3]. Individuals who met the above diagnostic criteria were categorized as hypertensive, whereas those who did not meet this category were categorized as nonhypertensive.
Laboratory measurements
Prior to commencing the surveys, all the researchers underwent standardized training to ensure data impartiality and accuracy. A standardized questionnaire was utilized to obtain the necessary data from the participants. This questionnaire included disclosure of the participants’ medical history, such as previous or current experiences with various cancers, liver diseases, thyroid and endocrine diseases, and recent antibiotic medication usage within the past 2 weeks. Once the questionnaire was completed, the data were compiled, summarized, and verified.
Total cholesterol (TC), low-density lipoprotein cholesterol (LDL-C), triglycerides, high-density lipoprotein cholesterol (HDL-C), globulin, indirect bilirubin, alanine aminotransferase (ALT), aspartate transaminase (AST), glutamyl transpeptidase (GGT), total protein, white protein, fasting blood glucose (FBG), alkaline phosphatase, monocyte cells, white blood cells, and neutrophil cells were among the various laboratory parameters measured from fasting blood samples at 8 a.m. Blood glucose levels were measured using an Olympus AU 5400 automated biochemistry analyzer (Olympus Corporation, Japan, Shizuoka Prefecture). Standard laboratory procedures were employed to evaluate the remaining indicators.
Liver fat measurement
Liver fat was measured based on a low-dose chest CT scan, which can include the liver. This was also part of the participants’ routine health assessment. This approach avoided further radiation exposure. All participants had uniform CT data. The Quantitative CT (QCT) Pro 6.0 Supplementary Tissue Measurement application from Mindways software was used to measure the amount of liver fat in the chest CT images. The program utilized Hounsfield units and information from the calibration phantom to directly measure liver fat in regions of interest (ROIs) within the liver parenchyma. ROIs were positioned at the entry point of the right branch of the portal vein into the liver. Three ROIs were assigned to the periphery of the left lobe, right anterior lobe, and right posterior lobe, each measuring 300 mm2 (deviation: 10 mm2). In cases where the left lobe was not visible, measurements were taken at the level with the largest transverse area of the left lobe. The mean of the three ROIs was used for the final quantitative CT measurement of liver fat. Care was taken to avoid intrahepatic calcification, liver cysts, major blood vessels and bile ducts, rib-related artifacts, and gas in the gastrointestinal or lung tracts when selecting ROIs. All the radiologists who had received specialized training performed all the analyses using QCT software. The applicability of this strategy in Chinese individuals was confirmed by a previously published study [15].
To ensure uniform quality control throughout the study, regular calibrations and cross-comparisons between systems were conducted using the European Spinal Prosthesis-145 (ESP-145) as a reference.
Variables
Covariate data were collected as follows: demographic data, including age, sex, and ethnicity; physical examination parameters: BMI, SBP, and DBP; laboratory indicators: TC, LDL-C, triglycerides, HDL-C, globulin, ALT, AST, GGT, total protein, white protein, FBG, alkaline phosphatase, monocyte cells, white blood cells, and neutrophil cells.
The formula for calculating BMI was weight divided by height squared (kg/m2). Additionally, BMI was categorized based on thresholds for overweight (24 kg/m2) and obesity (28 kg/m2). In addition to immune cells, laboratory indices were grouped according to their clinical significance. Among them, lipids were grouped according to the criteria of the latest Chinese lipid management guidelines [16]. FBG levels were divided into two groups based on the threshold value for diabetes. The other indicators were divided into three groups based on tertiles.
Statistical analysis
Statistical analysis was performed using R software version 4.2. For each dataset, a normality test was used to identify continuous variables with a normally distributed mean ± standard deviation. Descriptive statistics are presented as the means or medians with quartiles for continuous variables and proportions for categorical variables and were used to describe participant characteristics. Variance estimation and chi-square tests were employed to address significant variations in the dataset. Univariate and multivariate logistic regression analyses were also conducted to investigate the association between liver fat levels and the risk of hypertension, considering confounding variables. The rudimentary model did not include any covariate adjustment. Model I was modified to account for age, sex, and ethnicity, while model II was adjusted for each covariate individually. The relationship between liver fat levels and the risk of hypertension was investigated using restricted cubic spline analysis. Subgroup analysis was also conducted to evaluate the correlation between liver fat levels and the risk of hypertension. Additionally, multivariable linear regression models were used to assess the associations between liver fat concentrations and potential inflammatory factors. Causal mediation analysis was employed to investigate the potential mediating role of inflammation in the relationship between liver fat levels and the risk of hypertension.
RESULTS
Baseline details about the participants
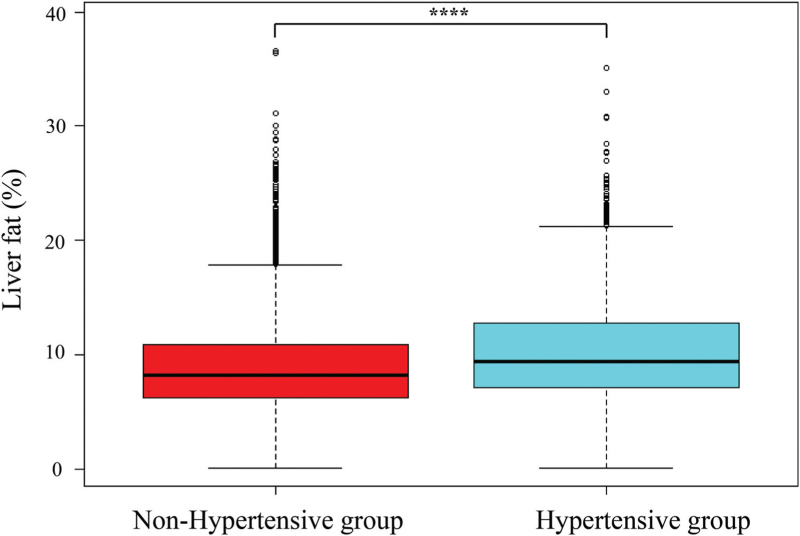
A total of 4955 participants from the health management department of Henan Provincial People's Hospital between February 2020 and February 2023 were included in this study. Liver fat was greater in hypertensive patients than in nonhypertensive patients, as depicted in Fig. 2. The prevalence of hypertension in the study population was 27.59%. Participants were divided into four groups based on liver fat quartiles: Q1 group (0.10% ≤ liver fat <6.31%, n = 1239), Q2 group (6.32% ≤ liver fat <8.60%, n = 1238), Q3 group (8.61% ≤ liver fat <11.45%, n = 1239), and Q4 group (11.46% ≤ liver fat <39.32%, n = 1239). As shown in Table 1, significant differences were observed in sex, BMI, TC, LDL-C, triglycerides, HDL-C, ALT, AST, GGT, total protein, white protein, FBG, alkaline phosphatase, monocyte cells, white blood cells, and neutrophil cells among the four groups (P <0.05). However, no significant differences were found in age, nationality, or globulin level (P > 0.05).
FIGURE 2.
Liver fat levels in the nonhypertensive and hypertensive groups. ∗∗∗∗P < 0.0001, compared with the nonhypertensive group.
TABLE 1.
Characteristics of participants according to quartiles of liver fat
| Variables | Q1 (0.10–6.31) | Q2 (6.32–8.60) | Q3 (8.61–11.45) | Q4 (11.46–39.32) | P value |
| Age [n (%)] | 0.438 | ||||
| <40 years | 201 (16.22) | 221 (17.85) | 199 (16.06) | 229 (18.48) | |
| 40–50 years | 380 (30.67) | 382 (30.86) | 385 (31.07) | 383 (30.91) | |
| 51–60 years | 437 (35.27) | 390 (31.50) | 409 (33.01) | 385 (31.07) | |
| >60 years | 221 (17.84) | 245 (19.79) | 246 (19.85) | 242 (19.53) | |
| Sex [n (%)] | <0.001 | ||||
| Female | 593 (47.86) | 496 (40.06) | 388 (31.32) | 316 (25.50) | |
| Male | 646 (52.14) | 742 (59.94) | 851 (68.68) | 923 (74.50) | |
| Nationality [n (%)] | 0.887 | ||||
| Nonhan | 14 (1.13) | 13 (1.05) | 12 (0.97) | 16 (1.29) | |
| Han | 1225 (98.87) | 1225 (98.95) | 1227 (99.03) | 1223 (98.71) | |
| BMI [n (%)] | <0.001 | ||||
| <24 kg/m2 | 710 (57.30) | 560 (45.23) | 386 (31.15) | 210 (16.95) | |
| 24–28 kg/m2 | 448 (36.16) | 569 (45.96) | 629 (50.77) | 608 (49.07) | |
| >28 kg/m2 | 81 (6.54) | 109 (8.80) | 224 (18.08) | 421 (33.98) | |
| TC [n (%)] | 0.011 | ||||
| <3.0 mmol/l | 5 (0.40) | 13 (1.05) | 19 (1.53) | 16 (1.29) | |
| 3.0–5.7 mmol/l | 981 (79.18) | 998 (80.61) | 993 (80.15) | 947 (76.43) | |
| >5.7 mmol/l | 253 (20.42) | 227 (18.34) | 227 (18.32) | 276 (22.28) | |
| LDL-C [n (%)] | <0.001 | ||||
| <2.1 mmol/l | 167 (13.48) | 178 (14.38) | 217 (17.51) | 150 (12.11) | |
| 2.1–3.1 mmol/l | 625 (50.44) | 596 (48.14) | 600 (48.43) | 568 (45.84) | |
| >3.1 mmol/l | 447 (36.08) | 464 (37.48) | 422 (34.06) | 521 (42.05) | |
| TG [n (%)] | <0.001 | ||||
| <0.5 mmol/l | 11 (0.89) | 12 (0.97) | 4 (0.32) | 1 (0.08) | |
| 0.5–1.7 mmol/l | 878 (70.86) | 815 (65.83) | 684 (55.21) | 494 (39.87) | |
| >1.7 mmol/l | 350 (28.25) | 411 (33.20) | 551 (44.47) | 744 (60.05) | |
| HDL-C [n (%)] | <0.001 | ||||
| <0.9 mmol/l | 49 (3.95) | 60 (4.85) | 99 (7.99) | 143 (11.54) | |
| 0.9–1.8 mmol/l | 1096 (88.46) | 1123 (90.71) | 1087 (87.73) | 1066 (86.04) | |
| >1.8 mmol/l | 94 (7.59) | 55 (4.44) | 53 (4.28) | 30 (2.42) | |
| Globulin [n (%)] | 0.428 | ||||
| <20 g/l | 61 (4.92) | 58 (4.68) | 78 (6.30) | 57 (4.60) | |
| 20–30 g/l | 1059 (85.47) | 1048 (84.65) | 1043 (84.18) | 1052 (84.91) | |
| >30 g/l | 119 (9.60) | 132 (10.66) | 118 (9.52) | 130 (10.49) | |
| ALT [n (%)] | <0.001 | ||||
| ≤40 U/l | 1185 (95.64) | 1166 (94.18) | 1111 (89.67) | 928 (74.90) | |
| >40 U/l | 54 (4.36) | 72 (5.82) | 128 (10.33) | 311 (25.10) | |
| AST [n (%)] | <0.001 | ||||
| <= 40 U/l | 1217 (98.22) | 1214 (98.06) | 1205 (97.26) | 1124 (90.72) | |
| >40 U/l | 22 (1.78) | 24 (1.94) | 34 (2.74) | 115 (9.28) | |
| GGT [n (%)] | <0.001 | ||||
| ≤40 U/l | 1088 (87.81) | 1061 (85.70) | 953 (76.92) | 797 (64.33) | |
| >40 U/l | 151 (12.19) | 177 (14.30) | 286 (23.08) | 442 (35.67) | |
| TP [n (%)] | 0.002 | ||||
| Low (g/l) | 434 (35.03) | 422 (34.09) | 414 (33.41) | 340 (27.44) | |
| Middle (g/l) | 405 (32.69) | 389 (31.42) | 411 (33.17) | 450 (36.32) | |
| High (g/l) | 400 (32.28) | 427 (34.49) | 414 (33.41) | 449 (36.24) | |
| White protein [n (%)] | <0.001 | ||||
| Low (g/l) | 435 (35.11) | 418 (33.76) | 417 (33.66) | 347 (28.01) | |
| Middle (g/l) | 424 (34.22) | 411 (33.20) | 394 (31.80) | 408 (32.93) | |
| High (g/l) | 380 (30.67) | 409 (33.04) | 428 (34.54) | 484 (39.06) | |
| FBG [n (%)] | <0.001 | ||||
| ≤7 mmol/l | 1186 (95.72) | 1171 (94.59) | 1149 (92.74) | 1096 (88.46) | |
| >7 mmol/l | 53 (4.28) | 67 (5.41) | 90 (7.26) | 143 (11.54) | |
| Alkaline phosphatase (U/l) | 67.80 ± 20.89 | 69.16 ± 19.40 | 70.82 ± 22.69 | 73.84 ± 20.74 | <0.001 |
| Monocyte cells (1000 cells/μl) | 0.35 ± 0.15 | 0.35 ± 0.13 | 0.36 ± 0.13 | 0.39 ± 0.13 | <0.001 |
| White blood cells (1000 cells/μl) | 5.55 ± 2.04 | 5.65 ± 1.43 | 5.80 ± 1.42 | 6.08 ± 1.48 | <0.001 |
| Neutrophil cells (1000 cells/μl) | 3.15 ± 1.05 | 3.27 ± 1.05 | 3.39 ± 1.03 | 3.56 ± 1.11 | <0.001 |
ALT, alanine aminotransferase; AST, aspartate transaminase; BMI, body mass index; FBG, fasting blood glucose; GGT, glutamyl transpeptidase; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; TC, total cholesterol; TG, triglycerides; TP, total protein.
Relationship between liver fat and hypertension
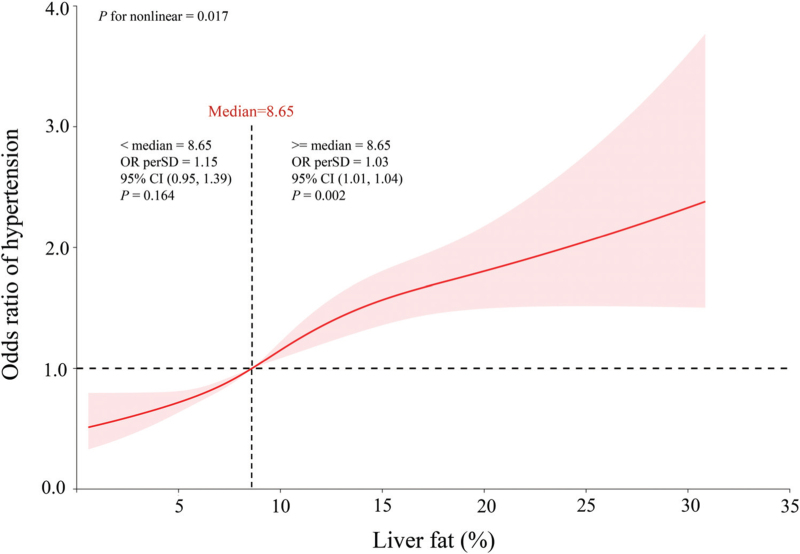
As shown in Supplemental Table 1, the results of the univariate logistic regression analysis demonstrated a correlation between liver fat levels and an increased risk of hypertension [odds ratio (OR) 1.07, 95% confidence interval (CI) 1.05–1.08; P < 0.001]. The independent relationship between liver fat levels and hypertension was further confirmed using multivariate logistic regression analysis. Table 2 shows that in both model I (OR 1.04, 95% CI 1.02–1.05, P < 0.001) and model II (OR 1.02, 95% CI 1.01–1.04, P = 0.004), continuous liver fat levels remained independently associated with the risk of hypertension after accounting for confounding factors. To convert continuous variables into categorical variables, quartiles of liver fat levels were utilized. According to all three models, participants in the highest quartile (Q4) had a significantly greater risk of hypertension than did those in the lowest quartile (Q1) (Q4 in the crude model: OR 2.22, 95% CI 1.86–2.66, P < 0.001, P for trend < 0.001; Q4 in model I: OR 1.50, 95% CI 1.22–1.83, P = 0.001, P for trend <0.001; Q4 in model II: OR 1.28, 95% CI 1.04–1.59, P = 0.022, P for trend = 0.039). This relationship is depicted in Fig. 3, which shows that even after adjustment for all confounding factors, there was a nonlinear association (P for nonlinearity = 0.017) between liver fat levels and the risk of hypertension. The OR per SD = 1.15 (95% CI 0.95–1.39, P = 0.164) for liver fat less than 8.65%. Notably, when liver fat greater than 8.65%, the risk of hypertension significantly increases (OR per SD = 1.03, 95% CI 1.01–1.04, P = 0.002).
TABLE 2.
Multivariate logistic regression analysis of liver fat for risk of hypertension
| Crude model | Model I | Model II | ||||
| OR (95% CI) | P value | OR (95% CI) | P value | OR (95% CI) | P value | |
| Liver fat | 1.07 (1.05–1.08) | <0.001 | 1.04 (1.02–1.05) | <0.001 | 1.02 (1.01–1.04) | 0.004 |
| Q1 | Reference | Reference | Reference | |||
| Q2 | 1.26 (1.04–1.52) | 0.016 | ||||
| Q3 | 1.46 (1.21–1.76) | <0.001 | 1.11 (0.90–1.36) | 0.323 | 1.04 (0.85–1.29) | 0.679 |
| Q4 | 2.22 (1.86–2.66) | <0.001 | 1.50 (1.22–1.83) | 0.001 | 1.28 (1.04–1.59) | 0.022 |
| P for trend | 1.30 (1.22–1.37) | <0.001 | 1.13 (1.06–1.21) | <0.001 | 1.07 (1.00–1.15) | 0.039 |
Crude model: no adjustment for model variables. Model I was adjusted for age, sex, and nationality. Model II was adjusted for all covariates. CI, confidence interval; OR, odds ratio.
FIGURE 3.
Odds ratio of hypertension according to liver fat levels in the overall population. A nonlinear association was found (P for nonlinearity = 0.017) between liver fat levels and risk of hypertension in a restricted cubic spline regression model. The solid line and shadow represented the odds ratio of hypertension and 95% confidence interval, respectively. Dashed vertical line indicated the threshold (liver fat = 8.65%) with the lowest risk of hypertension. All covariates were adjusted in this model.
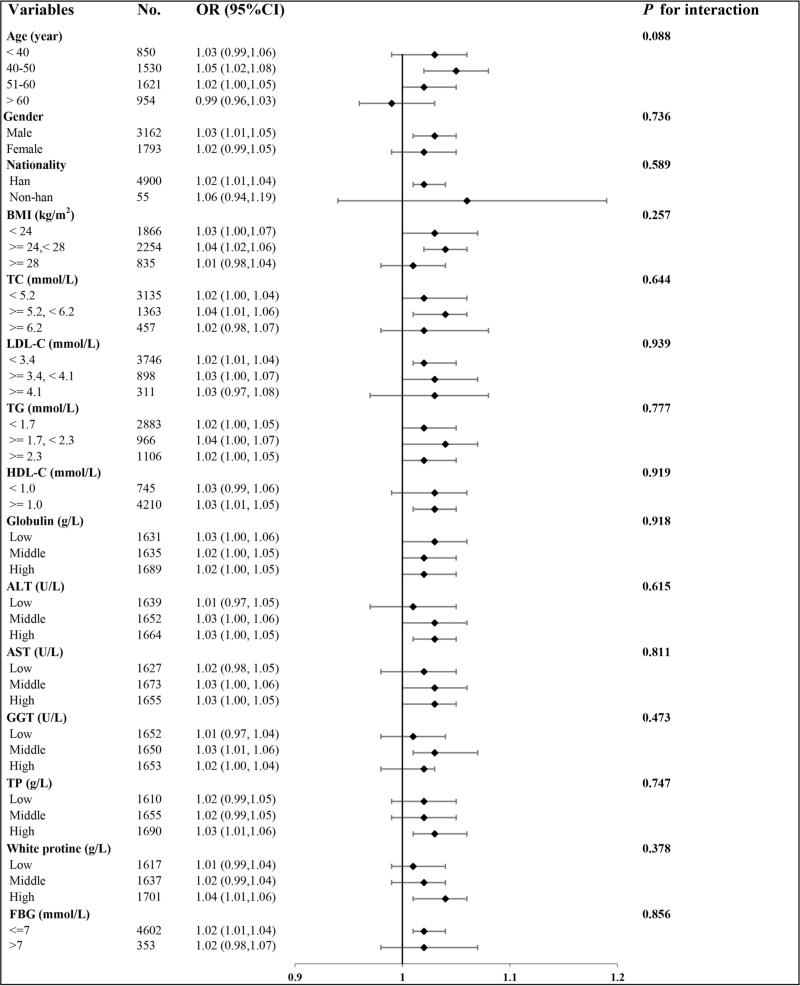
Subgroup analysis
Consistency was observed in the subgroup analyses, as illustrated in Fig. 4. Interactions were not significant when stratified by age, sex, ethnicity, BMI, TC, LDL-C, triglycerides, HDL-C, globulin, ALT, AST, GGT, total protein, white protein, or FBG. These findings validate the significant association between liver fat levels and the likelihood of hypertension across various subcategories (P for interaction >0.05).
FIGURE 4.
The relationship between liver fat and risk of hypertension according to different subgroups. ALT, alanine aminotransferase; AST, aspartate transaminase; CI, confidence interval; FBG, fasting blood glucose; GGT, glutamyl transpeptidase HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; OR, odds ratio; TC, total cholesterol; TG, triglycerides; TP, total protein.
Associations between liver fat and inflammatory factor levels
A multivariate linear regression model was used to investigate the associations between inflammatory factors and liver fat. As presented in Table 3, liver fat levels were significantly positively associated with alkaline phosphatase activity (β = 0.01; 95% CI −0.12 to 0.13; P < 0.001) and neutrophil counts (β = 0.04; 95% CI 0.02–0.06; P < 0.001) but not with monocyte (β = 0.01; 95% CI 0.12–0.13; P = 0.910) or white blood cell counts (β = −0.01; 95% CI −0.02 to 0.01; P = 0.492). These findings suggest a link between inflammation and liver fat.
TABLE 3.
Multivariate linear regression of liver fat levels with inflammatory factors
| n | β | 95%CI | P-value | |
| Alkaline phosphatase (U/l) | 4955 | 0.01 | (−0.12 to 0.13) | <0.001∗ |
| Monocyte cells, (1000 cells/μl) | 4955 | 0.01 | (0.12 to 0.13) | 0.910 |
| White blood cells (1000 cells/μl) | 4955 | −0.01 | (−0.02 to 0.01) | 0.492 |
| Neutrophils (1000 cells/μ) | 4955 | 0.04 | (0.02 to 0.06) | <0.001∗ |
All covariates were adjusted in this model. CI, confidence interval.
P < 0.05.
Analysis of the mediating effects of inflammatory factors
To understand the potential mediating role of inflammatory factors in the association between liver fat and the risk of hypertension, mediation analysis was conducted. Table 4 shows that the correlation between liver fat and the risk of hypertension was significantly mediated by neutrophils. The mediation percentage was calculated to be 4.76% (P = 0.012). Conversely, other inflammatory cells did not significantly change.
TABLE 4.
The mediating effects of inflammatory factors on the association between liver fat and risk of hypertension
| Inflammatory factors | Indirect effects | Direct effects | Total effects | ||
| OR (95% CI) | OR (95% CI) | OR (95% CI) | Mediated proportion (%) | P value | |
| Alkaline phosphatase | 2.49e-04 (−3.49e-04 8.84e-04) | 2.00e-02 (0.62e-02, 3.35e-02)∗ | 2.03e-02 (0.64e-02, 3.37e-02)∗ | 1.22 | 0.496 |
| Monocyte cells | 0.01e-02 (−0.04e-02, 0.08e-02) | 2.00e-02 (0.62e-02, 3.34e-02)∗ | 2.02e-02 (0.63e-02, 3.37e-02)∗ | 0.71 | 0.494 |
| White blood cells | 0.71e-04 (−0.25e-03, 0.53e-03) | 2.00e-02 (0.62e-02, 3.34e-02)∗ | 2.00e-02 (0.63e-02, 3.36e-02)∗ | 0.36 | 0.690 |
| Neutrophils | 0.10e-02 (0.02e-02, 0.23e-02)∗ | 2.00e-02 (0.62e-02, 3.34e-02)∗ | 2.10e-02 (0.73e-02, 3.45e-02)∗ | 4.76 | 0.012∗ |
All covariates were adjusted in this model. CI, confidence interval.
P < 0.05.
DISCUSSION
The WHO has identified hypertension as a key global prevention target because it is the primary cause of premature death and affects approximately 1.6 billion people worldwide [17]. As a result, it is crucial to identify risk factors early to prevent the development of hypertension. MASLD, a multisystemic disease characterized by excessive hepatic lipid accumulation, can have a significant impact on extrahepatic organs and lead to increased morbidity and mortality [5]. To explore the relationship between liver fat levels and the risk of hypertension, a cross-sectional study was conducted using data from 4955 patients who underwent physical examinations at a health management center. The study showed that liver fat levels were significantly greater in patients with hypertension than in those without hypertension. Additionally, a positive correlation between the risk of hypertension and liver fat levels was observed across all the subgroups. Furthermore, liver fat levels above 8.56% significantly increased the risk of hypertension. Neutrophils were found to play a role in the impact of liver fat on hypertension risk, and liver fat levels were also positively correlated with inflammatory factor levels. Therefore, this study demonstrated that liver fat promotes inflammation, ultimately leading to a greater risk of hypertension.
The complex interactions between pathophysiological and environmental factors that impact various systems, along with genetic predispositions, constitute the cause of hypertension. Visceral obesity and metabolic diseases are well known risk factors for hypertension [18]. The MASLD score serves as a reliable indicator of visceral obesity and strongly correlates with hypertension [19]. Therefore, identifying metabolic changes arising from adipose tissue is crucial for preventing hypertension. Recently, MASLD has been recognized as the abnormal accumulation of fat in the liver, but its health risks extend beyond hepatic implications. Numerous studies have reported that MASLD contributes to cardiovascular disease, chronic kidney disease, polycystic ovarian syndrome, and malignancies [20]. Furthermore, substantial evidence indicates a link between the presence and severity of MASLD syndrome and the risk of hypertension. Ultrasound-detected hepatic steatosis has been identified as an independent risk factor for hypertension in the Korean population [21]. This association persisted even after considering several confounding factors, such as variations in BMI (hazard ratio = 1.36, 95% CI 1.10–1.67; P = 0.004) [22]. A fatty liver index (FLI) exceeding 60, a screening tool for steatosis, is considered an unfavorable predictor of the development of hypertension. This correlation remained significant even after controlling for sex differences and conventional risk factors [23]. A study conducted by Siafi et al.[24] demonstrated that in hypertensive patients not receiving antihypertensive therapy, the FLI independently predicted the occurrence of cardiovascular events. Using MRI, Lorbeer et al.[10] measured liver fat content and discovered that higher levels of liver fat were associated with an increased risk of hypertension (hazard ratio = 2.16, P = 0.025). Additionally, a study conducted in Shanghai, China, identified liver fat as a sensitive early warning indicator for hypertension and its complications [25]. However, the aforementioned studies lacked direct measurements of liver fat levels. In the present study, we identified a nonlinear correlation between the risk of hypertension and liver fat level, with liver fat exceeding 8.65% substantially increasing the risk of hypertension. These findings suggest that the pathophysiology of hypertension may involve liver fat. To the best of our knowledge, this study is the first to directly measure liver fat levels using QCT technology and quantify its relationship with hypertension risk. QCT is a simple, well tolerated, efficient, and cost-effective technique for measuring liver fat levels. Clinically, it is particularly suitable for liver fat level assessments in participants of large-scale health screenings, thereby helping them evaluate and reduce their risk of hypertension. Moreover, elucidating the quantitative relationship between liver fat levels and hypertension will aid clinicians in better understanding and managing the cause of hypertension, as well as in exploring its pathogenesis.
Multiple cellular processes are implicated in the pathogenesis of hypertension. Numerous studies have suggested that inflammation plays a pivotal role in the cause of hypertension [26]. Hypertensive patients exhibit heightened levels of systemic inflammation, which is closely associated with arterial stiffness and the development of atherosclerosis [27]. Experimental animal studies have demonstrated that inhibiting the inflammatory response can attenuate hypertension [28]. The activation of Ras and the sympathetic nervous system, which are key pathophysiological mechanisms of hypertension, can be triggered by MASLD, resulting in various systemic adverse effects, including insulin resistance and local as well as systemic inflammatory responses. These findings are corroborated by a growing body of literature [13]. Hepatic steatosis leads to a substantial accumulation of diverse immune cells in the liver, which subsequently triggers the release of cytokines into the bloodstream and induces systemic inflammatory responses [29]. Haukeland et al.[30] discovered that the development of hypertension in individuals with MASLD is largely mediated by the inflammatory response, which is complexly linked to a systemic inflammatory reaction. Moreover, local inflammatory responses in the kidneys, adipose tissues, and blood vessels within the context of MASLD can directly expedite the onset and progression of hypertension [14]. Furthermore, neutrophil activation promotes endothelial dysfunction and accelerates the progression of hypertension [31]. Nevertheless, the underlying mechanisms that connect liver fat accumulation with hypertension are not yet fully understood. The results of the present study align with previous research by demonstrating a positive correlation between liver fat levels and inflammatory factors that mediate the effects of liver fat on hypertension. Therefore, inflammation could serve as a potential mechanism, underlying the association between hypertension and liver fat.
This study has several notable strengths. First, the participants were drawn from a health checkup population that accurately reflects the general community population, enhancing the study's external validity. Second, the study was diligently adjusted for potential confounders and utilized subgroup analyses, yielding results that were robust and consistent across various subgroups. Third, the study employed QCT technology, which is a straightforward and efficient method that does not prolong the scanning time or increase radiation exposure, to measure liver fat levels. This feature facilitated the conduction of large-scale reproducible studies. The limitations of the present study must be acknowledged. First, the accuracy of liver fat quantification based on CT scanning may be somewhat different from that of MRI-measured liver fat content, but CT scanning has the advantage of being inexpensive, with no increase in scanning time and no increase in radiation dose and allows for large-scale population screening for liver fat. Second, there may have been confounding variables that were not considered in the study, such as a history of alcohol consumption, but we excluded participants with alcoholic fatty liver disease when we asked for a history. Finally, because of its cross-sectional design, this study cannot establish a causal relationship between liver fat levels and the risk of hypertension. Consequently, further research is necessary to explore and understand the connection between the risk of hypertension and liver fat levels.
In conclusion, the present study revealed a noteworthy link between elevated liver fat levels (above 8.65%) and an increased risk of hypertension. Notably, a nonlinear correlation was revealed between liver fat levels and hypertension in individuals undergoing physical examination. Furthermore, inflammation has emerged as a conceivable mechanism mediating the association between liver fat and hypertension progression. To establish the validity of these findings, comprehensive prospective studies are warranted. (Supplemental Table 2; Supplemental Fig. 1; Supplemental Fig. 2).
ACKNOWLEDGEMENTS
Source of funding: this study was supported by the National Natural Science Foundation of China (82071884); National Key R&D Program of China (2022YFC2010001); Medical Science and Technology Research Project of Henan Province (SBGJ202302011, LHGJ20210054); Central Plains Science and Technology Innovation leading talent Program (244200510016). Henan Provincial Science and Technology Tackling Program Project Funding (242102311018, 242102311121, 242102310299).
Author contributions: Y.S. and Y.L. contributed the central idea, Y.S. and X.Q. analyzed most of the data. Y.S. wrote the initial draft of the article. X.W. helped in revising the manuscript. X.L., Y.Z., Y.D., A.L., J.Z., and F.S. contributed to the data collection, and Xue. Lv., J.Z., Z.L., Z.Z., M.Z., Y.H., F.L., and H.L. contributed to the opinion refinement, supplementary analysis, and finalization of this article. The author(s) read and approved the final manuscript.
Data availability statement: contact the first author for all data relating to this study on reasonable request.
Publication history – posted history: this manuscript has not been published elsewhere.
Conflicts of interest
There are no conflicts of interest.
Supplementary Material
Footnotes
Abbreviations: ALT, alanine aminotransferase; AST, aspartate transaminase; CI, confidence interval; FBG, fasting blood glucose; GGT, glutamyl transpeptidase; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; MASLD, metabolic dysfunction-associated steatotic liver disease; OR, odds ratio; QCT, quantitative CT; ROI, regions of interest
Supplemental digital content is available for this article.
REFERENCES
- 1.Yano Y, Kim HC, Lee H, Azahar N, Ahmed S, Kitaoka K, et al. Isolated diastolic hypertension and risk of cardiovascular disease: controversies in hypertension - pro side of the argument. Hypertension 2022; 79:1563–1570. [DOI] [PubMed] [Google Scholar]
- 2.Wang F, Sun Y, Luo R, Lu X, Yang B, Yang T. COX-2-independent activation of renal (pro)renin receptor contributes to DOCA-salt hypertension in rats. Am J Physiol Renal Physiol 2020; 319:F647–F653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhang M, Shi Y, Zhou B, Huang Z, Zhao Z, Li C, et al. Prevalence, awareness, treatment, and control of hypertension in China, 2004-18: findings from six rounds of a national survey. BMJ 2023; 380:e071952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ma Q, Li R, Wang L, Yin P, Wang Y, Yan C, et al. Temporal trend and attributable risk factors of stroke burden in China, 1990-2019: an analysis for the Global Burden of Disease Study 2019. Lancet Public Health 2021; 6:e897–e906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wu W, Zhang Z, Qi Y, Zhang H, Zhao Y, Li J. Association between dietary inflammation index and hypertension in participants with different degrees of liver steatosis. Ann Med 2023; 55:2195203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bastien M, Poirier P, Lemieux I, Després JP. Overview of epidemiology and contribution of obesity to cardiovascular disease. Prog Cardiovasc Dis 2014; 56:369–381. [DOI] [PubMed] [Google Scholar]
- 7.Brunt EM, Janney CG, Di Bisceglie AM, Neuschwander-Tetri BA, Bacon BR. Nonalcoholic steatohepatitis: a proposal for grading and staging the histological lesions. Am J Gastroenterol 1999; 94:2467–2474. [DOI] [PubMed] [Google Scholar]
- 8. MSD Manual Professional Edition. Metabolic dysfunction-associated liver disease (MASLD). MSD Manual Professional Edition, 2023, Retrieved from https://www.msdmanuals.com/professional/hepatic-and-biliary-disorders/approach-to-the-patient-with-liver-disease/metabolic-dysfunction%E2%80%93associated-liver-disease-masld. [Accessed 19 June 2024] [Google Scholar]
- 9.Starekova J, Hernando D, Pickhardt PJ, Reeder SB. Quantification of liver fat content with CT and MRI: state of the art. Radiology 2021; 301:250–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lorbeer R, Bayerl C, Auweter S, Rospleszcz S, Lieb W, Meisinger C, et al. Association between MRI-derived hepatic fat fraction and blood pressure in participants without history of cardiovascular disease. J Hypertens 2017; 35:737–744. [DOI] [PubMed] [Google Scholar]
- 11.Ciardullo S, Monti T, Grassi G, Mancia G, Perseghin G. Blood pressure, glycemic status and advanced liver fibrosis assessed by transient elastography in the general United States population. J Hypertens 2021; 39:1621–1627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ma J, Hwang SJ, Pedley A, Massaro JM, Hoffmann U, Chung RT, et al. Bi-directional analysis between fatty liver and cardiovascular disease risk factors. J Hepatol 2017; 66:390–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Oikonomou D, Georgiopoulos G, Katsi V, Kourek C, Tsioufis C, Alexopoulou A, et al. Nonalcoholic fatty liver disease and hypertension: coprevalent or correlated? Eur J Gastroenterol Hepatol 2018; 30:979–985. [DOI] [PubMed] [Google Scholar]
- 14.Sinn DH, Kang D, Jang HR, Gu S, Cho SJ, Paik SW, et al. Development of chronic kidney disease in patients with nonalcoholic fatty liver disease: A cohort study. J Hepatol 2017; 67:1274–1280. [DOI] [PubMed] [Google Scholar]
- 15.Guo Z, Blake GM, Li K, Liang W, Zhang W, Zhang Y, et al. Liver fat content measurement with quantitative CT validated against MRI proton density fat fraction: a prospective study of 400 healthy volunteers. Radiology 2020; 294:89–97. [DOI] [PubMed] [Google Scholar]
- 16.Joint Committee on the Chinese Guidelines for Lipid Management. Chinese guidelines for lipid management (2023). Zhonghua Xin Xue Guan Bing Za Zhi 2023; 51:221–255. [in Chinese]. [DOI] [PubMed] [Google Scholar]
- 17.Carey RM, Moran AE, Whelton PK. Treatment of hypertension: a review. JAMA 2022; 328:1849–1861. [DOI] [PubMed] [Google Scholar]
- 18.Kuang M, Lu S, Xie Q, Peng N, He S, Yu C, et al. Abdominal obesity phenotypes are associated with the risk of developing nonalcoholic fatty liver disease: insights from the general population. BMC Gastroenterol 2022; 22:311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chang Y, Jung HS, Cho J, Zhang Y, Yun KE, Lazo M, et al. Metabolically healthy obesity and the development of nonalcoholic fatty liver disease. Am J Gastroenterol 2016; 111:1133–1140. [DOI] [PubMed] [Google Scholar]
- 20.Kim H, Lee CJ, Ahn SH, Lee KS, Lee BK, Baik SJ, et al. MAFLD predicts the risk of cardiovascular disease better than NAFLD in asymptomatic subjects with health check-ups. Dig Dis Sci 2022; 67:4919–4928. [DOI] [PubMed] [Google Scholar]
- 21.Ryoo JH, Suh YJ, Shin HC, Cho YK, Choi JM, Park SK, et al. Clinical association between nonalcoholic fatty liver disease and the development of hypertension. J Gastroenterol Hepatol 2014; 29:1926–1931. [DOI] [PubMed] [Google Scholar]
- 22.Sung KC, Wild SH, Byrne CD. Development of new fatty liver, or resolution of existing fatty liver, over five years of follow-up, and risk of incident hypertension. J Hepatol 2014; 60:1040–1045. [DOI] [PubMed] [Google Scholar]
- 23.Higashiura Y, Furuhashi M, Tanaka M, Takahashi S, Mori K, Miyamori D, et al. Elevated fatty liver index is independently associated with new onset of hypertension during a 10-year period in both male and female subjects. J Am Heart Assoc 2021; 10:e021430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Siafi E, Andrikou I, Thomopoulos C, Konstantinidis D, Kakouri N, Tatakis F, et al. Fatty liver index and cardiovascular outcomes in never-treated hypertensive patients: a prospective cohort. Hypertens Res 2023; 46:119–127. [DOI] [PubMed] [Google Scholar]
- 25.Zhu XP, Han GC, Chen Q, Zhang ZY, Wang LS, Zhang B. Fatty liver is a sensitive early warning for hypertension and its complication in the Chinese population. Clin Exp Hypertens 2022; 44:306–312. [DOI] [PubMed] [Google Scholar]
- 26.Wu M, Si J, Liu Y, Kang L, Xu B. Association between composite dietary antioxidant index and hypertension: insights from NHANES. Clin Exp Hypertens 2023; 45:2233712. [DOI] [PubMed] [Google Scholar]
- 27.Wu Q, Yang H, Zheng Q, Chen Q, Li X, Guo J. κ-opioid receptors improve vascular endothelial dysfunction in salt-sensitive hypertension via PI3K/Akt/eNOS signaling pathway. Oxid Med Cell Longev 2023; 2023:5352959. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 28.Niu LG, Sun N, Liu KL, Su Q, Qi J, Fu LY, et al. Genistein alleviates oxidative stress and inflammation in the hypothalamic paraventricular nucleus by activating the Sirt1/Nrf2 pathway in high salt-induced hypertension. Cardiovasc Toxicol 2022; 22 (10–11):898–909. [DOI] [PubMed] [Google Scholar]
- 29.Nunes KP, de Oliveira AA, Mowry FE, Biancardi VC. Targeting toll-like receptor 4 signalling pathways: can therapeutics pay the toll for hypertension? Br J Pharmacol 2019; 176:1864–1879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Haukeland JW, Damås JK, Konopski Z, Løberg EM, Haaland T, Goverud I, et al. Systemic inflammation in nonalcoholic fatty liver disease is characterized by elevated levels of CCL2. J Hepatol 2006; 44:1167–1174. [DOI] [PubMed] [Google Scholar]
- 31.Meeuwsen JAL, de Vries J, Zoet GA, Franx A, Fauser BCJM, Maas AHEM, et al. Circulating neutrophils do not predict subclinical coronary artery disease in women with former preeclampsia. Cells 2020; 9:E468. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.