Abstract
Background:
Impairment in semantic knowledge contributes to Alzheimer disease (AD)-related decline. However, the particulars of the impact AD has on specific domains of knowledge remain debatable.
Objective:
To investigate the impact of AD on specific semantic categories that are integral to daily functions—living things and man-made objects.
Method:
We administered a free-listing task (written version) to 19 individuals with AD and 15 cognitively normal older adults and assessed the task’s relationship with other cognitive and functional tests in clinical use. We compared the contents of the lists of salient concepts generated by the AD and control groups.
Results:
Group membership (AD or control), after controlling for age, sex, formal education, and an estimate of premorbid intellectual ability, predicted the groups’ performance on the free-listing task across two categories. Functional status was inversely related to performance on the free-listing task, holding demographic variables constant. Based on a comparison of the contents of the free lists that were generated by the two groups, it was possible to conclude that, in individuals with AD, conceptual knowledge central to the respective categories was well preserved, whereas the peripheral conceptual material showed evidence of degradation.
Conclusion:
The free-listing task, which is an easy-to-administer and cost-effective tool, could aid in the preliminary detection of semantic knowledge dysfunction, revealing concepts that are better preserved and, possibly, the characterization of AD. Cognitive assessment tools that can be applied across cultures are needed, and the free-listing task has the potential to address this gap.
Key Words: Alzheimer disease, man-made objects, living things, category fluency, free-listing, semantic knowledge, memory, cultural domain analysis
- AD
Alzheimer disease
- AMNART
American National Adult Reading Test
- CDA
cultural domain analysis
- COVID-19
coronavirus disease 2019
- HC
healthy control
- FAST
Functional Assessment Staging Scale
- MMSE
Mini-Mental State Examination
- MoCA
Montreal Cognitive Assessment
Semantic memory mediates the retention of conceptual and factual knowledge (Gazzaniga et al., 2018; Rogers and Friedman, 2008; Tulving, 1972), and semantic memory impairment is a prominent manifestation of Alzheimer disease (AD) (Arroyo-Anlló et al., 2012; Butters et al., 1987; Hodges et al., 1992; Martin and Fedio, 1983; Salmon et al., 1999; Weintraub et al., 2012; Zannino et al., 2015). In AD, semantic memory impairment is associated with the actual degradation of semantic knowledge rather than merely a retrieval deficit (Chan et al., 1995, 1997; Chertkow and Bub, 1990; Cross et al., 2008; Hodges et al., 1992; Luzzatti et al., 2020; Mårdh et al., 2013). This degradation manifests relatively early in the disease trajectory, with studies showing evidence of such degradation in individuals with mild cognitive impairment and those with preclinical AD (Adlam et al., 2006, 2010; Kim et al., 2019; Papp et al., 2016; Venneri et al., 2019; Vonk et al., 2020).
Studies have shown that early semantic impairment predicts future cognitive decline in individuals with mild cognitive impairment and those with preclinical AD (Alegret et al., 2018; Papp et al., 2016; Vonk et al., 2020). These individuals exhibit difficulties in naming things and have an impoverished vocabulary, even when their articulatory motor skills and syntactic awareness are preserved (Appell et al., 1982; Cummings et al., 1985; Flanagan et al., 2013; Laws, Adlington et al., 2007; Taler and Phillips, 2008). This impairment has also been shown to negatively impact individuals’ daily activities, such as using objects inappropriately, given their impoverished knowledge of the objects’ functions (Corbett et al., 2015; Falchook et al., 2012; Roll et al., 2019; Silveri and Ciccarelli, 2009).
The specific network responsible for semantic knowledge processing is predominantly left lateralized and involves the dorsomedial and ventromedial prefrontal cortices; the fusiform, inferior frontal, middle temporal, parahippocampal, and posterior cingulate gyri; and the posterior inferior parietal lobe (Binder et al., 2009). AD pathology (Braak and Braak, 1991; Masters et al., 2015) affects several of these regions—some early in the disease course—and propagates along the neural networks that are critical for semantic knowledge processing, thus exposing individuals to early semantic impairment.
The majority of the literature on semantic impairment in individuals with AD has concentrated on the general impact of the disease on semantic knowledge and the associated biomarkers and neuroanatomical correlates, whereas the impact of AD on specific domains of knowledge remains debatable. There are competing models that attempt to explain how AD (and related disorders) may distinctly impact specific domains of knowledge (Albanese, 2007; Caramazza and Shelton, 1998; Garrard et al., 1998; Gonnerman et al., 1997; Lesourd et al., 2021; Luzzatti et al., 2020; Mahon et al., 2009; McCarthy and Warrington, 2016; Perri et al., 2012; Silveri et al., 1991; Whatmough et al., 2003; Zannino et al., 2006). Regardless of the adopted model and the hypothesized pathway leading to specific impairments, studies have consistently shown that different domains of knowledge can be affected by AD.
In this study, we further the investigation of the impact of AD on the organization and preservation of semantic knowledge in the animate category of living kinds (i.e., living things or things that are alive, such as conspecifics, animals, etc.) and the inanimate category of artifacts (i.e., man-made objects or material things that are made by humans, such as tools, clothing, etc.) in individuals with AD in mild and moderate stages via the free-listing task, which was developed in cultural domain analysis (CDA). From here on, we will refer to living kinds and artifacts as living things and man-made objects, respectively.
The animate–inanimate distinction is a core principle that structures the cognitive process of conceptual categorization in humans (Caramazza and Shelton, 1998; Gelman, 1990; Mahon et al., 2009) and is critical for daily functioning. Therefore, the goals of our research are as follows:
Determine whether the free-listing task can distinguish between individuals with AD and cognitively normal older adults such that the free-listing task can be added to the armamentarium of tools for detecting AD and monitoring its progression.
Conduct a detailed analysis of the contents of the free lists generated by the two groups, exploring the demarcation between core and peripheral knowledge and the impact of this distinction on the preservation of information in individuals with AD.
Cultural Domain Analysis (CDA)
How people think and reason about things (e.g., objects, concepts, and people) in order to function appropriately has long interested ethnoscientists and cognitive anthropologists (D’Andrade, 1995). The field of CDA arose from the original idea that studying how people categorize things might provide insights into their understanding of the world and, from there, their culture-specific experiences of that world (D’Andrade, 1995; McGee and Warms, 2017; Sturtevant, 1964). CDA was developed to standardize the investigation of questions, focusing on the association between cognition, language, and culture (Goodenough, 1957; Sturtevant, 1964).
CDA researchers have developed methods and analytical tools that permit a systematic investigation of culture-specific categorical domains of knowledge (Bernard, 2018a, 2018b; Borgatti, 1994, 1998). More specifically, CDA provides tools for describing knowledge content, structure, and distribution. Examples of domains or categories that have been studied using CDA include the animal kingdom, plant world, color vocabulary, kinship terminology, illness classification, emotions, geographic and landscape terminology, ethnobotany, and technology (Berlin, 1973; Berlin and Kay, 1969; Berlin et al., 1974; Bernard et al., 2009; Borgatti, 1994; Bulmer, 1967; Conklin, 1955; Frake, 1961; Lounsbury, 1956; Weller and Romney, 1988). The ethnoscientists’ original objective of studying group-specific classification systems in order to uncover cultural differences in the interpretation and experience of the world was ancillary to the discovery of universal patterns that are systematically instantiated in human classifications.
Data collection in CDA starts with the demarcation of a domain of knowledge X by a sample of individuals who are asked to name or write the names of as many items as they can imagine that they think belong to domain X. The procedure is known as free-listing, which can be administered as an oral or written task.
Free elicitation prevents the imposition of a priori ideas by the experimenter that may not correspond to the native knowledge of the specific cultural group being studied (Borgatti, 1994; Weller and Romney, 1988). Terms that appear earlier or with a higher frequency on free lists indicate a high consensus among respondents (i.e., the concept is an important component of the category); these items are considered to be more salient in the domain under investigation (Bousfield and Barclay, 1950; Weller et al., 2018).
In cognitively normal adults, the written version of the free-listing task typically leads to the generation of larger, more exhaustive lists than oral elicitation, thereby permitting a better assessment of the core and peripheral knowledge of the categorical domain being investigated (Gravlee et al., 2013). To the best of our knowledge, no study has used the CDA free-listing task with individuals with AD or related dementias. Therefore, in this study, we chose to use the written version of the free-listing task in order to elicit long lists.
Given that memory impairment is a hallmark of AD, a written as opposed to an oral task also creates a more favorable test environment for individuals with AD. Instead of having to keep track of the generated words in an oral task, the individual is able to view the generated words, thereby reducing his or her cognitive burden, as the words generated are visible at all times. We used the written task to compare the performance of individuals with AD with that of cognitively normal older adults in order to assess whether the performance of the AD group diverges from that of the controls. A matched control group is necessary to determine the impact of the presence or absence of disease on the observed findings.
Hypotheses
In agreement with several researchers, we adopt the view that semantic impairment in AD is not simply a byproduct of general cognitive decline, but instead involves a specific degradation of semantic knowledge (Butters et al., 1987; Chan et al., 1997; Hodges et al., 1992; Salmon et al., 1999; Verma and Howard, 2012). We hypothesize that individuals with AD will perform worse than cognitively normal older adults on the quantitative free-listing task (as measured by the length of the list) for both the living things and man-made objects categories. We further hypothesize that the qualitative analysis of the specific contents of the free lists (i.e., the detailed descriptive analysis of the terms generated by participants) will reveal a greater preservation of the central concepts that occupy the core of a given semantic category. The following sections elaborate on our hypotheses.
Semantic Knowledge and Hierarchical Organization
Semantic knowledge acquisition follows a rather systematic path; cross-culturally, individuals rely on hierarchical cognitive structures to capture systematic relationships between objects and concepts (Berlin, 1973; Gelman, 2022; Medin and Atran, 2004; Rakison et al., 2022; Rosch, 1978; Waxman, 1999). These hierarchical structures are made up of three to six levels: superordinate (e.g., general forms such as animals or mammals for living things and mode of transportation or vehicles for man-made objects), intermediate or basic (e.g., cat, dog, airplane, and car), and subordinate (e.g., varietal such as a tabby cat, terrier dog, or short- vs long-haired tabby and a sedan or convertible car).
Central concepts (i.e., concepts that are an essential component of a given category) are easily acquired during development (Boyer and Barrett, 2016; Gelman, 2022; Hirschfeld and Gelman, 1994; Markman, 1989, 1990; Quinn, 2010; Waxman, 1999). Central concepts tend to belong to the intermediate (Gelman, 2022; Waxman, 1999; Waxman and Gelman, 1986) or more general superordinate (Quinn, 2010) levels rather than to the more specific subordinate levels and are widely distributed among individuals. The intermediate conceptual information for cat or car (typically acquired early in development) is buttressed from cognitive erosion because it is categorized under several broader conceptual classes such as living thing, animal, mammal, man-made object, or vehicle, which provides that conceptual information with some form of redundancy along with different routes of retrieval. Broader (compared to more specific) conceptual categories are closer to the general templates that drive domain-specific knowledge acquisition (Gelman, 1990; Keil, 1981; Sperber, 1994).
We hypothesize that when AD neurodegeneration sets in and semantic knowledge begins to degrade, more fine-grained knowledge (i.e., peripheral knowledge typically acquired later in development), such as tabby or calico or sedan or convertible, is affected first (Hodges et al., 1992) because that conceptual information, being significantly more specific, is less likely to be represented redundantly in the hierarchical cognitive structure.
Living Things
Category-specific semantic impairments—which involve the loss of the ability to identify, name, or reason about items from a specific semantic category while the ability to process information about other categories is preserved—have been observed in individuals with both focal and diffuse brain pathologies, including individuals with AD (Capitani et al., 2003; Caramazza and Shelton, 1998; Gainotti, 2000; Garrard et al., 1998; Gonnerman et al., 1997; Laws, Crawford et al., 2007; Mahon and Caramazza, 2011; Mahon et al., 2009; McCarthy and Warrington, 2016; Silveri et al., 1991; Warrington, 1975; Warrington and McCarthy, 1983; Warrington and Shallice, 1984). Evidence of category-specific semantic impairments leads to the inference that categories influence the organization of semantic knowledge in the brain.
For the living things category, our hypotheses are as follows:
Concepts such as agency and biological essence (or underlying causal nature) will be critical for the characterization of living things.
The subcategories that emerge under the broad category of living things will include animals, conspecifics, and plants.
Individuals with AD may include inanimate items in this category in the setting of a deteriorating semantic knowledge store.
The Concept of Agency. The concept of agency—the property of self-propelled things (i.e., in no need of applied force to set themselves in motion)— is essential in order for humans to organize their interactions with, and interpret, their environment. The distinction between agentive/nonagentive objects operates early in development, organizing the adaptive perception of, and response to, the world (Carey, 2009; Gelman, 1990; Zaitchik and Solomon, 2008).
People and animals, being prominent objects in the human environment, constitute prototypical biological agents that occupy an important place in human folkbiology—people’s common understanding and interpretation of and reasoning about the biological world (Medin and Atran, 1999; Zaitchik and Solomon, 2008). Because agency is the most salient characteristic of people and animals, the word alive is mapped onto and strongly associated with the concept of agency early in development. Zaitchik and Solomon (2008) showed that individuals with AD appear to overattribute life to objects that are capable of motion as higher level folkbiological concepts degrade (such as conceptual information regarding reproduction, birth, growth, and internal function).
Owing to the impoverishment of higher level folkbiological knowledge in individuals with AD, the concept of living things deteriorates faster than that of its fundamental and definitional components, that is, agency (Zaitchik and Solomon, 2008). In other words, the stripped-down notion of alive (i.e., the core conceptual element organizing knowledge about biological agents) would no longer suffice for properly classifying and reasoning about objects in the environment, thus forcing individuals to revert to the core concept of agency, that is, the self-propelling property of objects as a cue for living things. If this is the case, we hypothesize that individuals with AD may include inanimate objects that are capable of self-generated activity (e.g., fire, sun, and water) and/or inanimate objects that are capable of motion (e.g., vehicles) in the living things category of the free-listing task.
Folkbiology. The universally most salient level of living things categorizations appears to be at the generic species level or rank (e.g., oak, tiger, and piranha) rather than at the folk kingdom (e.g., plant or animal), life form (e.g., tree, flower, bush, bird, fish, or mammal), folk-specific (e.g., red oak, bald eagle, hammerhead shark, Bengal tiger, tabby, or calico), or varietal (e.g., white-collard hammerhead shark, short-haired tabby, or long-haired calico) level (Medin and Atran, 1999, 2004). Indeed, cross-cultural investigations have shown that members of the basic or intermediate level, those of generic species, are systematically itemized in elicitation conditions, whereas members of all other levels often remain underverbalized, and, for more inclusive (i.e., nesting) categories (e.g., folk kingdom and life form), remain regularly, underlexicalized, or fully unlexicalized (unlexicalized categories still exert their organizing effect on semantic knowledge about living things but as implicit categories) (Berlin, 1973; Gelman and Meyer, 2011; Waxman, 1999).
We hypothesize that the organizational principle will be preserved in cognitively normal older adults and eventuated in the frequency of items at different categorization levels in the lists they produce. Will this principle apply to the lists that are generated by the individuals with AD? We hypothesize that, given the centrality of the species level, the lists of the individuals with AD will still manifest the operation of that structural principle but in a weakened fashion. Given the degradation of detailed semantic knowledge, the AD group’s lists should display a reduction in the frequency of generic species items with an increase in more upstream terms (nesting categories such as folk kingdom and life form), requiring less detailed information but information that is essential for the acquisition of lower level detailed information about generic species, subspecies, and varietals.
Man-made Objects
Consistent with information reported in previous studies (Capitani et al., 2003; Gibson and Ingold, 1993; Lesourd et al., 2021; Magri et al., 2021; Warrington and McCarthy, 1987; Warrington and Shallice, 1984), we hypothesize that functional properties, size, and manipulability are essential for characterizing man-made objects.
Size and manipulability (i.e., whether manipulable by hand or not) have been proposed as properties that play a fundamental role in the brain’s specific handling of information regarding inanimate objects (Magri et al., 2021).
The capacity to recognize, represent, invent, and use tools has been critical in human evolutionary history (Bi et al., 2015; Buss, 2016; Gibson and Ingold, 1993). For most of that history, tools have generally been small, handheld/graspable, and manipulable by hand (Coolidge and Wynn, 2016; Magri et al., 2021). Given the centrality of our evolutionary history, it is probable that small objects will account for a fair number of man-made objects in the lists that are generated by cognitively normal older adults.
Function-related knowledge (i.e., which tool is used for its inventor’s intended function) is an essential element in the characterization of man-made objects (Capitani et al., 2003; Gibson and Ingold, 1993; Lesourd et al., 2021; Warrington and McCarthy, 1987; Warrington and Shallice, 1984). Left-lateralized white matter tracts between the frontal, temporal, and parietal cortices of the brain are critical for understanding the function and identity of tools (Bi et al., 2015). These brain regions are affected early in the AD continuum. Therefore, the lists generated by individuals with AD may include fewer terms belonging to the category of small manipulable objects compared to larger objects (i.e., too big to be manipulated by hand, such as furniture, building/structures, etc.). For man-made objects of other sizes, we are uncertain of what is expected, and very few studies on these categories have been conducted.
Plural and Singular Terms
For both biological (e.g., living things) and nonbiological (e.g., man-made objects) nouns, superordinate-level lexemes are more likely to be plural terms, whereas intermediate- or basic-level lexemes are more likely to be singular terms (Schalley and Zaefferer, 2007; Wisniewski and Murphy, 1989). If more fine-grained knowledge (typically at the lower levels of a hierarchical structure) deteriorates first in individuals with AD, as we hypothesize, more terms closer to the superordinate level and, thereby, more plural terms, should be generated by individuals with AD than by cognitively normal older adults.
Broader Impact
This is the first study to rely on a CDA free-listing task to analyze the impact of AD on distinct semantic categories of knowledge. We assess the relationship between this method of analyzing semantic knowledge and other cognitive and functional tests that are currently used in clinical and research settings.
Historically, most studies on semantic knowledge in individuals with AD have relied on predetermined sets of stimuli to assess the individual’s knowledge (e.g., inventory of pictures for naming fluency) without being careful to tease apart categorical knowledge preservation or impairment. For instance, questions such as how the semantic knowledge of living things would be split by individuals if they are not biased toward categorizing plants and animals as living things has not been fully answered. In the present study, we attempt to answer this question by asking the individuals with AD to generate the content of these categories.
Understanding the preservation and degradation of specific categories of semantic knowledge that are critical to human interactions with the environment may lead to a better understanding of how individuals with AD experience the world around them. This study has the potential to further our understanding of the impact of disease progression on an individual’s cognitive faculties and competencies, which, in turn, could lead to the development of targeted interventions with the potential to palliate some of the cognitive consequences of the disease.
We acknowledge that there are numerous cognitive assessments that are currently in use to investigate semantic knowledge (e.g., Pyramids and Palm Trees Test, category/semantic fluency, object naming such as the Boston Naming Test, and word-to-picture matching, among others) (Hodges, 2017; Howard and Patterson, 1992; Kaplan et al., 1983; Strauss et al., 2006) and overall cognitive impairment in individuals with AD and those with related dementias. Nevertheless, we aim to use the free-listing task to investigate semantic knowledge. Beyond its quantitative utility, this method has the potential to aid in determining which concepts are more resistant to degradation in AD (and, if so, why?). The findings of this study could also provide a new opportunity to investigate the age-old question of how semantic knowledge is organized.
METHOD
Participants
We recruited individuals with AD from the Cleveland Clinic Nevada Lou Ruvo Center for Brain Health. For the healthy control (HC) group, we recruited cognitively normal older adults, age ≥60 years, from the Greater Las Vegas, Nevada community. All individuals voluntarily participated in the study. Participants were recruited using ads/flyers. There were no incentives/payments provided for participation.
The study was conducted at the Cleveland Clinic Nevada Lou Ruvo Center for Brain Health. Constraints associated with coronavirus disease 2019 (COVID-19) led to a decision to reduce the original intended sample size of 15–30 participants for each of the following groups: mild to moderate AD and HC. The recruitment time was amended for a longer period for individual identification. The first individual was enrolled in mid-July of 2020, and the last individual in mid-March of 2021. The study required an in-person visit, and mandatory COVID-19 health and safety procedures were adhered to.
The inclusion criteria for both groups required that each individual had to be able to speak, read, and write English. For the AD group, individuals had to have an AD diagnosis, as defined by the National Institute on Aging and Alzheimer’s Association guidelines, at mild-to-moderate stage (as assessed by a neurologist at the Cognitive Disorders Clinic, Cleveland Clinic Nevada) (Jack et al., 2018; McKhann et al., 2011). For the HC group, individuals had to be healthy, cognitively normal, with no known neurologic disease (per participant’s self-report).
The exclusion criteria for both groups were a history of stroke, seizures, or major psychiatric disorders, including depression.
We obtained the following demographic information from all members of both groups via an in-person interview before conducting the cognitive assessments: age, sex, race, ethnicity, length of formal education, employment status, and whether the individual lived most of his or her life in an urban or rural environment.
When available, we obtained medical records of the AD group regarding their amyloid status via CSF analysis or PET (Jack et al., 2018). We also collected data on the AD group’s current use of antidementia agents. Because such drugs are part of the standard of care (Ulep et al., 2018), the majority of individuals with AD take such medications, and their use was not exclusionary.
Written informed consent was obtained from all individuals before enrollment. The study protocol was approved by the institutional review boards of the Cleveland Clinic Nevada and the University of Nevada, Las Vegas.
Assessments
Cognitive Tests
We administered the Montreal Cognitive Assessment (MoCA; Nasreddine et al., 2005) and the Mini-Mental State Examination (MMSE; Folstein et al., 1975) to characterize the global cognitive status and disease severity of all individuals in both groups. A third test, the Functional Assessment Staging (FAST; Sclan and Reisberg, 1992) scale, was used to refine the assessment of disease staging and severity in the AD group. The FAST scale is used by the Centers for Medicare and Medicaid Services (2021) to assess AD severity based on daily functions for determining hospice eligibility.
The individuals in both groups were required to reach the following scores on the MMSE and FAST scale for enrollment into the study: ≥12 and 3–5, respectively, for the AD group; and ≥26 and 1–2, respectively, for the HC group (Folstein et al., 1975; Galasko et al., 2005; Nasreddine et al., 2005; Perneczky et al., 2006; Roalf et al., 2013; Rossetti et al., 2011; Sclan and Reisberg, 1992).
It has been shown that current verbal fluency and National Adult Reading Test (Nelson and Willison, 1991) performances are highly correlated (Crawford et al., 1992). Thus, we administered the American National Adult Reading Test (AMNART; Grober and Sliwinski, 1991), a valid estimate of premorbid verbal intelligence (Strauss et al., 2006), to both groups. The AMNART is a North American version (Strauss et al., 2006) of the National Adult Reading Test that was developed in Britain (Nelson and Willison, 1991). We used the AMNART to assess whether a relationship exists between performance on the free-listing task and the estimated premorbid verbal intelligence before cognitive decline. A 1-minute semantic verbal fluency task (also known as a category fluency task), which is a measure of semantic processing that is currently used in research and clinical settings (Hodges, 2017; Papp et al., 2016; Vonk et al., 2020), for the category animal was also administered to assess its relationship with the free-listing task. The performance of all individuals on the MoCA’s 1-minute phonemic verbal fluency task for the letter F was recorded for comparison.
Free-Listing Task
We gave each individual one pen and two sheets of white copy paper, one for each category being assessed, and told them they had 5 minutes to write down as many items as possible for each category (i.e., living things and man-made objects).
We asked the individuals to list their answers to the following inquiry: “List all (category) you can think of,” or to variations of that statement, such as “Write down all (category) you can think of” or “Name all (category) you can think of.” As previously noted, the purpose of the free-listing task is to elicit a definition of the categories of interest (e.g., for this study, living things and man-made objects) from the participants. Thus, to prevent bias, participants were not provided with a definition of the categories or any examples of items belonging to the categories.
To elicit as many items as possible, we uttered probes as needed (within the 5-minute time frame) in a stepwise approach, starting from nonspecific prompting, followed by reading of the list produced, to semantic cuing. Nonspecific prompting included “What other kinds of (category) are there?” When reading the list, the examiner (M.G.U.) assessed the individual’s current list and repeated the nonspecific prompts. Semantic cuing included prompts such as, “You mentioned ______; what other kinds of (relevant category) are there like that?”
At the 2-minute mark, M.G.U. informed the individuals of the time remaining. Quantitative performance in the free-listing task was operationalized as the sum of the written words that were generated in the 5-minute time frame. We reviewed the lists for congruence between the written words and the given categories. Repeated words were counted once, and incongruent words (i.e., not belonging to the category) were not counted in the sum of the items generated.
Statistical Analysis
We used Spearman rank correlation coefficients to assess the relationships between the entire sample’s (N = 34) performance on the free-listing task, demographics (age and education), and cognitive/clinical variables (MoCA, MMSE, FAST, AMNART, and category and phonemic verbal fluency scores). The demographic characteristics and cognitive and clinical data of the two groups were compared using an independent samples t test for continuous variables and Fisher exact test for categorical variables. A two-sample Wilcoxon rank-sum (Mann–Whitney) test was used for continuous variables that did not meet the assumptions of the independent samples t test.
We used multivariable linear regression models to assess how well group membership predicts free-listing performance. Free-listing performance in either of the two categories was the dependent variable, and group membership was the predictor variable (control being the reference group), controlling for performance on the AMNART, age (in years), sex (male being the reference group), and education (in years). We constructed a second multivariable linear regression model using the same control variables as those used in the first regression model in order to assess how well functional status (measured using the FAST score) predicts free-listing performance.
Statistical significance was set at P < 0.05. The Shapiro–Wilk W test was used to assess the normality of the residuals. The variance inflation factor was calculated for each independent variable in the regression models to test for multicollinearity. Heteroscedasticity was assessed using the Breusch–Pagan/Cook–Weisberg test. Both the link and Ramsey regression specification error tests were performed to detect model specification errors. Omega-squared (ω2) and partial omega-squared ( ) values were computed to assess the effect sizes. All statistical analyses were performed using Stata, version 17.0.
Detailed Descriptive Analysis of the Contents of the Free Lists
Once the two groups had listed all of the items they thought belonged to the given categories of living things and man-made objects, we analyzed the contents of the lists for each category.
Each list was reviewed to identify repetitions and errors (i.e., items that did not belong to the category). Repetitions and errors were not counted; only the original was counted. A term appearing in its singular and plural forms in the same list (e.g., if cat and cats or dog and dogs were present in the list) was counted once, at its first appearance in the list.
We used Free List Analysis in R Environment to generate scree plots that enabled us to analyze the contents of the free lists. The scree plots facilitated the delineation of the core of the investigated categories (i.e., the terms listed by many participants by considering the rank where those terms appear in the list sequences), as well as the periphery (i.e., the terms occasionally listed by considering the rank of their appearance).
A cultural saliency index score, also called the Bʹ score (Robbins et al., 2017; Wencelius et al., 2017), was computed for each item. The Bʹ score considers a value between 0 and 1, with scores closer to 1 indicating a higher saliency and high consensus (i.e., high saliency indicates that the members of the group studied agree with the categorization of the term in the semantic domain investigated). Thus, concepts with high Bʹ scores belong to the core of their respective semantic categories and constitute a fundamental part of the domain-specific knowledge of the assessed group.
The Bʹ score is computed as follows (Robbins et al., 2017): , where Z is the number of lists; F x is the frequency of mention of item x across the lists; is the cultural salience of item x; and is the number of items preceding item x in list i, which is expressed as , where is the length of list i, and is the rank of item x in list i. We chose the Bʹ score as a measure of cognitive salience because it was specifically developed to adjust for the limitations of previous measures, such as the Smith index, where an item listed in the final position by several individuals and an item listed in the final position by one individual are given the same index value (Robbins et al., 2017).
Subcategories, Classification Ranks, and Plural Terms
Living Things. We recorded the frequency of animal and plant terms that were generated at distinct folk-biological classificatory ranks, adopting the cognitively universal ranking for folkbiology that was proposed by Medin and Atran (1999, 2004): folk kingdom (e.g., plant and animal), life form (e.g., tree, flower, bush, bird, fish, and mammal), generic species (e.g., oak, pine, lily, tulip, eagle, shark, tiger, and cat), folk-specific (e.g., red oak, bald eagle, hammerhead shark, Bengal tiger, tabby, and calico), and varietal (e.g., white-collared hammerhead shark, short-haired tabby, and long-haired calico).
Man-made Objects. We recorded the frequency of items of three different sizes: small (i.e., handheld, manipulable by hand), medium (i.e., too big to be manipulated by hand, such as furniture and appliances), and large (such as vehicles and buildings).
Singular and Plural Terms. We recorded the frequency of singular and plural terms in the lists generated by the two groups.
RESULTS
Participants
Twenty-one individuals with AD and 16 HCs responded to our invitation to participate in the study. One individual with AD who inquired about the study was excluded because of a history of a major psychiatric disorder. Another individual with AD and one HC changed their decision to participate and cancelled their appointments. Thus, we enrolled 19 individuals with AD and 15 HCs in our study.
The demographic characteristics of the two groups are presented in Table 1. The ages of the individuals in the AD and HC groups ranged between 63 and 89 years and 66 and 78 years, respectively (t 27 = –1.97, P = 0.06). There was no difference between the groups in terms of sex (P = 0.50), race (P = 0.08), ethnicity (P = 0.44), length of education (t 28 = 1.20, P = 0.24), or employment status (retired vs employed; P = 0.20). Most of the individuals (84.21% in the AD group and 80% in the HC group) lived primarily in an urban environment (P = 1.00).
TABLE 1.
Demographic Characteristics of the AD and HC Groups
| Demographic | Total Sample (N=34) |
AD Group (n=19) |
HC Group (n=15) |
P (AD vs HC) |
|---|---|---|---|---|
| Age, years (min, max) |
(63, 89) | (63, 89) | (66, 78) | 0.06 |
| M (SD) | 75.15 (6.29) | 76.84 (7.47) | 73 (3.57) | |
| Median | 74.5 | 77 | 74 | |
| Sex, frequency (%) | ||||
| Female | 20 (58.82) | 10 (52.63) | 10 (66.67) | 0.50 |
| Male | 14 (41.18) | 9 (47.37) | 5 (33.33) | |
| Race, frequency (%) | ||||
| White/Caucasian | 30 (88.24) | 18 (94.74) | 12 (80.00) | 0.08 |
| Black/African American | 1 (2.94) | 1 (5.26) | 0 | |
| Asian | 3 (8.82) | 0 | 3 (20.00) | |
| Ethnicity, frequency (%) | ||||
| Hispanic or Latino | 1 (2.94) | 0 | 1 (6.67) | 0.44 |
| Not Hispanic or Latino | 33 (97.06) | 19 (100.00) | 14 (93.33) | |
| Formal education, years (min, max) | (12, 22) | (12, 20) | (12, 22) | 0.24 |
| M (SD) | 16 (2.86) | 15.47 (2.70) | 16.67 (3.02) | |
| Median | 16 | 16 | 16 | |
| Employment status, frequency (%) | ||||
| Retired | 27 (79.41) | 17 (89.47) | 10 (66.67) | 0.20 |
| Employed | 7 (20.59) | 2 (10.53) | 5 (33.33) | |
| Rural vs urban environment, frequency (%) | 1.00 | |||
| Rural | 6 (17.65) | 3 (15.79) | 3 (20.00) | |
| Urban | 28 (82.35) | 16 (84.21) | 12 (80.00) | |
An independent samples t test was used to assess age and education. Fisher exact test was used to assess the other demographic variables.
AD = Alzheimer disease. HC = healthy control.
Table 2 presents the clinical data and assessment results of both groups. Of the 19 individuals with AD, five (26.3%) had a confirmed positive amyloid status based on CSF analysis or PET scan results. Only one of the HCs possessed data regarding their amyloid status (negative, based on a PET scan). Seventy-nine percent of the AD group were on antidementia medication; none of the HC group took antidementia medication.
TABLE 2.
Clinical Data and Assessment Results
| Test/Clinical Information | Total Sample (N=34) |
AD Group (n=19) |
HC Group (n=15) |
P (AD vs HC) |
|---|---|---|---|---|
| Amyloid, frequency (%) | ||||
| PET scan (+) | 4 (11.8) | 4 (21.0) | 0 | NA |
| CSF (+) | 1 (2.9) | 1 (5.3) | 0 | |
| PET scan (–) | 1 (2.9) | 0 | 1 (6.7) | |
| None | 28 (82.4) | 14 (73.7) | 14 (93.3) | |
| Medications, frequency (%) | ||||
| Donepezil (Aricept) | 6 (17.6) | 6 (31.6) | 0 | NA |
| Galantamine (Razadyne) | 1 (3.0) | 1 (5.0) | 0 | |
| Rivastigmine (Exelon) | 2 (5.9) | 2 (10.5) | 0 | |
| Donepezil (Aricept) and memantine (Namenda) | 3 (8.8) | 3 (15.8) | 0 | |
| Rivastigmine (Exelon) and memantine (Namenda) | 3 (8.8) | 3 (15.8) | 0 | |
| None | 19 (55.9) | 4 (21.1) | 15 (100) | |
| MoCA score | 0.0000*** | |||
| M (SD) | 21.53 (7.53) | 16.90 (7.05) | 27.4 (1.96) | |
| Median | 24.5 | 18 | 28 | |
| MMSE score | 0.0000*** | |||
| M (SD) | 24.74 (5.63) | 21.42 (1.28) | 28.93 (.23) | |
| Median | 27.5 | 23 | 29 | |
| FAST score | ||||
| M (SD) | 2.76 (1.44) | 3.89 (.77) | 1.33 (.49) | 0.0000*** |
| Median | 3 | 4 | 1 | |
| AMNART score | ||||
| M (SD) | 114.57 (7.76) | 110.91 (6.81) | 119.20 (6.39) | 0.0010* |
| Median | 114.51 | 112.17 | 118.01 | |
| Verbal fluency, semantic† (category animals) | 0.0000*** | |||
| M (SD) | 14.71 (8.18) | 9.63 (6.32) | 21.13 (5.20) | |
| Median | 15 | 8 | 21 | |
| Verbal fluency, phonemic†
(Letter F) | ||||
| M (SD) | 11.24 (4.20) | 9.63 (4.02) | 13.27 (3.58) | 0.0098* |
| Median | 12 | 10 | 14 | |
An independent samples t test was used to compare performance on the FAST, AMNART, and verbal fluency (semantic and phonemic) assessments. The Wilcoxon rank-sum (Mann–Whitney) test was used to compare performance on the MoCA and MMSE.
Significant at P < 0.05.
Significant at P < 0.001.
Measured as the total number of words produced in 1 minute.
AD = Alzheimer disease. AMNART = American National Adult Reading Test. FAST = Functional Assessment Staging scale. HC = healthy control. MMSE = Mini-Mental State Examination. MoCA = Montreal Cognitive Assessment. NA = not assessed.
Assessments
Cognitive Tests
The AD group performed significantly worse than the HC group on the MoCA (z = 4.28, P < 0.001), MMSE (z = 4.17, P < 0.001), AMNART (t 32 = 3.62, P < 0.05), both verbal fluency tasks (category task: t 32 = 5.69, P < 0.001; phonemic task: t 32 = 2.75, P<0.05), and FAST scale (t 32 = –11.58, P < 0.001).
Free-listing Task
The mean number of items in each list generated by the AD and HC groups was 15 and 38, respectively, for living things, and 17 and 43, respectively, for man-made objects. There was no significant difference between the HC group’s performance on the free-listing of living things and man-made objects (t 14 = –1.08, P = 0.30). There was also no significant difference between the AD group’s performance on the free-listing of living things and man-man objects (t 18 = –1.88, P = 0.08). The AD group performed significantly worse than the HC group in the free-listing task for both categories (living things: t 32 = 6.24, P < 0.001; man-made objects: t 32 = 5.48, P < 0.001). Additional details are presented in Table 3.
TABLE 3.
Free-listing Data
| Category | AD Group (n=19) |
HC Group (n=15) |
P (AD vs HC) |
|---|---|---|---|
| Living things† | 0.0000*** | ||
| M (SD) | 15.05 (10.09) | 38.47 (11.78) | |
| Median | 12 | 39 | |
| Man-made objects† | 0.0000*** | ||
| M (SD) | 17.42 (11.60) | 42.87 (15.48) | |
| Median | 18 | 45 | |
| Living things vs man-made objects (comparing performance of each individual on free-listing task based on the two different categories) |
P = 0.076 Paired t test |
P = 0.299 Paired t test |
(As reported in the cells to the left) |
An independent samples t test was used to compare performance on the free-listing task for both categories (living things and man-made objects).
Significant at P<0.001.
Measured as the total number of words produced in 5 minute.
AD = Alzheimer disease. HC = healthy control.
The computation of Spearman rank correlation (see Table 4 for ρ and P values) revealed significant findings. The performance of the total sample (N=34) in the free-listing of living things was correlated with the following variables in descending order: MoCA, MMSE, category verbal fluency task, FAST, phonemic verbal fluency task, and AMNART scores. Similarly, the performance of the total sample in the free-listing of man-made objects was correlated with the following variables in descending order: FAST, MoCA, MMSE, semantic verbal fluency, phonemic verbal fluency, and AMNART scores.
TABLE 4.
Correlation Data
| Living Things Category | Man-made Objects Category | |||
|---|---|---|---|---|
| Demographic, Cognitive/Clinical Variable | ρ | P | ρ | P |
| Age | –0.34 | 0.05 | –0.32 | 0.06 |
| Formal education | 0.15 | 0.41 | 0.06 | 0.76 |
| MoCA score | 0.82 | 0.0000*** | 0.77 | 0.0000*** |
| MMSE score | 0.81 | 0.0000*** | 0.73 | 0.0000*** |
| FAST score | –0.77 | 0.0000*** | –0.80 | 0.0000*** |
| AMNART score | 0.47 | 0.0051* | 0.47 | 0.0055* |
| Verbal fluency, semantic (Category animals) | 0.80 | 0.0000*** | 0.72 | 0.0000*** |
| Verbal fluency, phonemic (Letter F) | 0.69 | 0.0000*** | 0.63 | 0.0001*** |
Spearman rank correlation was used for analysis. N = 34. Degrees of freedom = 32.
Significant at P < 0.05.
Significant at P < 0.001.
AMNART = American National Adult Reading Test. FAST = Functional Assessment Staging scale. MMSE = Mini-Mental State Examination. MoCA = Montreal Cognitive Assessment.
The two multivariable regression models (Table 5) with group membership (AD or HC) as the predictor and performance on the free-listing task in the category of living things or man-made objects as the outcome variable indicated that group membership predicted free-listing performance in the two categories (living things: β = –0.64, P = 0.000; man-made objects: β = –0.59, P=0.001) after controlling for age, education, sex, and AMNART score. Both models demonstrated a sizable amount of variance (living things: R2 = 0.57, F 5, 28 = 7.46, P = 0.0001; man-made objects: R2 = 0.58, F 5, 28 = 7.59, P = 0.0001).
TABLE 5.
Multivariable Regression Models With Group Membership as the Primary Predictor of Interest and Performance on the Free-listing Task as the Outcome Variable
| AD Group | Age | Education | Female | AMNART Score | Constant | Observations R2 |
Adjusted R2 |
F | |
|---|---|---|---|---|---|---|---|---|---|
| Living things | |||||||||
| Coefficient | –20.17*** | –0.2990 | –0.1749 | –0.2619 | 0.2831 | 29.64 | |||
| β | –0.6383*** | –0.1181 | –0.0314 | –0.0082 | 0.1378 | ||||
| (SE) | (5.024) | (0.3343) | (0.7939) | (4.118) | (0.3472) | (43.55) | |||
| t | –4.01 | –0.89 | –0.22 | –0.06 | 0.82 | 0.68 | |||
| P | 0.000*** | 0.379 | 0.827 | 0.950 | 0.422 | 0.502 | |||
| 34 0.5711 |
0.4945 |
7.46*** |
|||||||
| Man-made objects | |||||||||
| Coefficient | –21.53** | –0.4604 | –1.066 | –6.077 | 0.5153 | 36.86 | |||
| β | –0.5890** | –0.1572 | –0.1654 | –0.1648 | 0.2168 | ||||
| (SE) | (5.784) | (0.3849) | (0.9139) | (4.741) | (0.3997) | (50.14) | |||
| t | –3.72 | –1.20 | –1.17 | –1.28 | 1.29 | 0.74 | |||
| P | 0.001** | 0.242 | 0.254 | 0.210 | 0.208 | 0.468 | |||
| 34 0.5754 |
0.4995 |
7.59*** |
|||||||
Significant at P < 0.01.
Significant at P < 0.001.
AD = Alzheimer disease. AMNART = American National Adult Reading Test.
Effect sizes were based on ω2 and values. For living things, 48.70% of the variance in the outcome variable was explained by the model (ω2=0.4870), and group membership alone explained 33.49% ( =0.3349) of the variance in the outcome variable after removing the variance accounted for by other terms. For man-made objects, 49.21% of the variance in the outcome variable was explained by the model (ω2=0.4921), and group membership alone explained 30% ( =0.3000) of the variance in the outcome variable after removing the variance accounted for by other terms. The computed observed powers for the multivariable regression models were 0.9993 and 0.9995 for living things and man-made objects, respectively.
The two multivariable regression models (Table 6) with functional status (i.e., FAST scores) as the predictor and performance on the free-listing task in the category of living things or man-made objects as the outcome variable indicated that functional status predicted free-listing performance in the two categories (living things: β = –0.68, P < 0.001; man-made objects: β = –0.70, P < 0.001) after controlling for age, education, sex, and AMNART score. Both models demonstrated a sizable amount of variance (living things: R2 = 0.61, F 5, 28 = 8.87, P<0.001; man-made objects: R2 = 0.67, F 5, 28 = 11.27, P<0.001).
TABLE 6.
Multivariable Regression Models With Functional Status as the Primary Predictor of Interest and Performance on the Free-listing Task as the Outcome Variable
| FAST | Age | Education | Female | AMNART Score | Constant | Observations R2 |
Adjusted R2 |
F | |
|---|---|---|---|---|---|---|---|---|---|
| Living things | |||||||||
| Coefficient | –7.572*** | –0.1299 | 0.1470 | 2.535 | 0.2590 | 22.57 | |||
| β | –0.6832*** | –0.0513 | 0.0264 | 0.0795 | 0.1261 | ||||
| (SE) | (1.657) | (0.3277) | (0.7606) | (3.826) | (0.3257) | (40.44) | |||
| t | –4.57 | –0.40 | 0.19 | 0.66 | 0.80 | 0.56 | |||
| P | 0.000*** | 0.695 | 0.848 | 0.513 | 0.433 | 0.581 | |||
| 34 0.6130 |
0.5438 | 8.87*** | |||||||
| Man-made objects | |||||||||
| Coefficient | –8.973*** | –0.2133 | –0.6651 | –3.175 | 0.4002 | 36.15 | |||
| β | –0.6998*** | –0.0729 | –0.1033 | −0.0861 | 0.1684 | ||||
| (SE) | (1.775) | (0.3511) | (0.8150) | (4.099) | (0.3490) | (43.33) | |||
| t | –5.05 | –0.61 | –0.82 | −0.77 | 1.15 | 0.83 | |||
| P | 0.000*** | 0.548 | 0.421 | 0.445 | 0.261 | 0.411 | |||
| 34 0.6680 |
0.6088 | 11.27*** | |||||||
Significant at P<0.001.
AD = Alzheimer disease. AMNART = American National Adult Reading Test. FAST = Functional Assessment Staging Scale.
For living things, 53.64% of the variance in the outcome variable was explained by the model (ω2=0.5364), and functional status alone explained 39.86% ( =0.3986) of the variance in the outcome variable after removing the variance accounted for by other terms. For man-made objects, 60.16% of the variance in the outcome variable was explained by the model (ω2 = 0.6016), and functional status alone explained 44.99% ( =0.4499) of the variance in the outcome variable after removing the variance accounted for by other terms. The computed observed powers for the multivariable regression models were 0.9999 and 1.000 for living things and man-made objects, respectively.
Detailed Descriptive Analysis of the Contents of the Free Lists
Comparison Between the AD and HC Groups
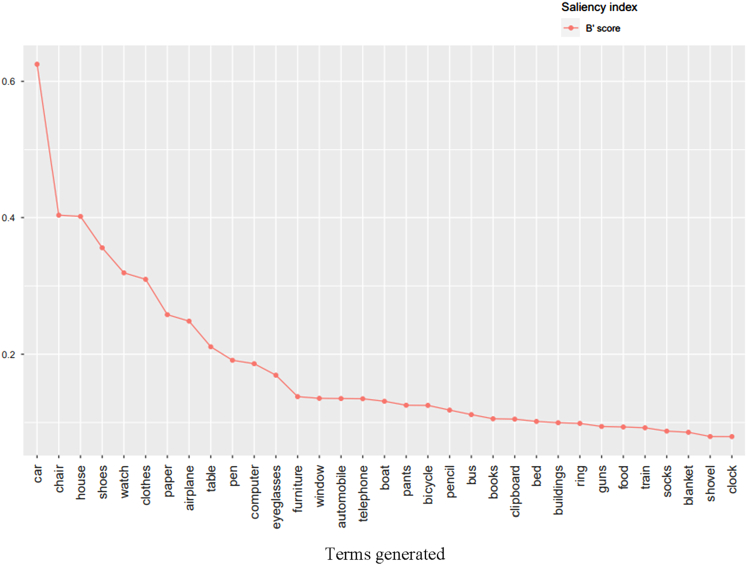
The AD group generated significantly fewer terms than the HC group on the free lists for the living things category, with 328 terms (169 distinct terms [i.e., no redundancies or errors]); the HC group generated 600 terms (260 distinct terms; Figure 1). Dog and cat were ranked first and second by both groups on the list of most salient terms for both groups.
FIGURE 1.
Number of terms generated by the two groups on the free-listing task by semantic category. AD = Alzheimer disease. HC = healthy control.
In the man-made objects category, the AD group generated 359 terms (201 distinct terms); the HC group generated 664 terms (378 distinct terms). Car, chair, and clothes were ranked first, second, and sixth on the list of most salient terms for both groups.
Content Structure—Highlights
Living Things—Supra/Subcategories and Privileged Levels. All of the terms generated by the AD and HC groups for the living things category are presented in Tables 7 and 8, respectively. The most salient terms generated by the AD and HC groups are shown in the scree plots in Figures 2 and 3, respectively. The AD and HC groups generated 26.23% and 10.27% of terms at the superordinate levels (folk kingdom and life form), respectively.
TABLE 7.
Cultural Saliency (Bʹ Score) of all Terms Generated by the AD Group for the Living Things Category (i.e., List Without Redundancies)
| 1. dog (0.6768) | 54. rhinoceros (0.0386) | 107. hearts (0.0111) | 160. bacteria (0.0007) |
| 2. cat (0.6703) | 55. sea urchin (0.0336) | 108. pig (0.0111) | 161. building a beautiful day for swimming (0) |
| 3. fish (0.3285) | 56. going to see a movie with my children (0.027) | 109. elderly people (0.0108) | 162. germs (0) |
| 4. tree (0.3235) | 57. horses are so much fun that I want to have one (0.027) | 110. heaters (0.0103) | 163. meat (0) |
| 5. people (0.3104) | 58. me (0.0262) | 111. elm (0.0097) | 164. neighbors (0) |
| 6. grass (0.3089) | 59. flea (0.0257) | 112. crow (0.0095) | 165. robin (0) |
| 7. horse (0.2687) | 60. canary (0.0256) | 113. homes (0.0095) | 166. spiders (0) |
| 8. bird (0.2676) | 61. deer (0.0253) | 114. Jack isn’t going to go into Henderson (0.009) | 167. stones (0) |
| 9. man (0.2483) | 62. gold fish (0.0249) | 115. leopards (0.009) | 168. We have a deer in our backyard (0) |
| 10. flower (0.248) | 63. tulips (0.0245) | 116. oak (0.009) | 169. worms (0) |
| 11. woman (0.2422) | 64. antelope (0.0244) | 117. orangutan (0.009) | |
| 12. plant (0.2017) | 65. elk (0.0235) | 118. pear (0.009) | |
| 13. bush (0.1875) | 66. butterflies (0.0232) | 119. running on a ride to the movie with my children (0.009) | |
| 14. rose (0.1748) | 67. bear (0.0227) | 120. harps (0.0087) | |
| 15. cow (0.1729) | 68. teaching our children to swim (0.0225) | 121. maple (0.0083) | |
| 16. rat (0.1697) | 69. tigers (0.0225) | 122. heels (0.0079) | |
| 17. whale (0.1294) | 70. ground hog (0.0222) | 123. reptiles (0.0077) | |
| 18. ants (0.1188) | 71. beaver (0.0218) | 124. pine (0.0076) | |
| 19. baby (0.1132) | 72. bass (0.0208) | 125. ponds (0.0072) | |
| 20. mice (0.1122) | 73. blue gill (0.0201) | 126. clams (0.007) | |
| 21. bugs (0.1116) | 74. Dall sheep (0.0201) | 127. parks (0.0064) | |
| 22. snakes (0.11) | 75. camels (0.0195) | 128. octopus (0.0061) | |
| 23. daughter (0.1088) | 76. salmon (0.0194) | 129. lettuce (0.006) | |
| 24. rabbit (0.108) | 77. rhubarb (0.0193) | 130. clothes (0.0058) | |
| 25. lion (0.1063) | 78. chihuahua (0.0191) | 131. puddles (0.0056) | |
| 26. animals (0.1055) | 79. moons (0.0191) | 132. persons (0.0055) | |
| 27. son (0.1051) | 80. wheat (0.019) | 133. Africans (0.0049) | |
| 28. child (0.1038) | 81. perch (0.0187) | 134. pokes (0.0048) | |
| 29. fly (0.0955) | 82. mine (0.0183) | 135. buffalo (0.0045) | |
| 30. hippo (0.0901) | 83. apes (0.018) | 136. daisy (0.0045) | |
| 31. zebra (0.0783) | 84. boys (0.018) | 137. making cookies (0.0045) | |
| 32. mouse (0.0749) | 85. bunny (0.018) | 138. Caucasians (0.0042) | |
| 33. mom (0.0704) | 86. drawing a picture of a elephant (0.018) | 139. donkey (0.0041) | |
| 34. dad (0.0675) | 87. marlin (0.018) | 140. carrots (0.004) | |
| 35. humans (0.0619) | 88. my mom had to work on the people’s (0.018) | 141. pots (0.004) | |
| 36. goat (0.0604) | 89. rice (0.018) | 142. caterpillars (0.0039) | |
| 37. elephant (0.0575) | 90. yours (0.0175) | 143. Latino (0.0035) | |
| 38. dolphin (0.0551) | 91. girls (0.0167) | 144. piles (0.0032) | |
| 39. squirrel (0.0534) | 92. his (0.0167) | 145. bees (0.003) | |
| 40. shark (0.0522) | 93. crocodile (0.0166) | 146. opossum (0.003) | |
| 41. frogs (0.0513) | 94. hers (0.0159) | 147. water melon (0.003) | |
| 42. fruit (0.0506) | 95. porpoise (0.0159) | 148. doctors (0.0028) | |
| 43. weeds (0.0495) | 96. cactus (0.0154) | 149. teacher (0.0027) | |
| 44. bat (0.0488) | 97. narwal (0.0152) | 150. ferrets (0.0025) | |
| 45. corn (0.0485) | 98. auto (0.0151) | 151. pokers (0.0024) | |
| 46. tomatoes (0.0475) | 99. brother (0.0146) | 152. nurses (0.0021) | |
| 47. apple (0.0445) | 100. star fish (0.0139) | 153. gardening (0.0019) | |
| 48. vegetables (0.0434) | 101. cars (0.0135) | 154. algae (0.0017) | |
| 49. orange (0.0425) | 102. going to write a picture for my children (0.0135) | 155. stops (0.0016) | |
| 50. insects (0.0418) | 103. pets (0.0135) | 156. COVID-19 virus (0.0014) | |
| 51. lizards (0.0417) | 104. feet (0.0127) | 157. seagull (0.0014) | |
| 52. buses (0.0413) | 105. sister (0.0125) | 158. tadpoles (0.0011) | |
| 53. giraffe (0.0409) | 106. chicken (0.0118) | 159. steps (0.0008) |
AD = Alzheimer disease.
TABLE 8.
Cultural Saliency (Bʹ Score) of all Terms Generated by the HC Group for the Living Things Category (i.e., List Without Redundancies)
| 1. dog (0.8967) | 54. fly (0.1281) | 107. cheetah (0.0452) | 160. egg (0.0197) | 213. sealions (0.0095) |
| 2. cat (0.8857) | 55. chipmunks (0.1279) | 108. gazelle (0.0424) | 161. mussels (0.0196) | 214. tortoise (0.0095) |
| 3. lion (0.638) | 56. chicken (0.1272) | 109. panther (0.0407) | 162. mesquite (0.0195) | 215. orange tree (0.0091) |
| 4. cow (0.6028) | 57. bug (0.127) | 110. lemur (0.0386) | 163. buffalo (0.0194) | 216. vine (0.0083) |
| 5. bird (0.5935) | 58. cactus (0.1219) | 111. grasshopper (0.0345) | 164. tulip (0.0194) | 217. praying mantis (0.0082) |
| 6. pig (0.5434) | 59. rooster (0.1218) | 112. husband (0.0345) | 165. slug (0.0191) | 218. MRSA (0.0081) |
| 7. fish (0.5228) | 60. elk (0.1208) | 113. son (0.0339) | 166. starfish (0.0189) | 219. flea (0.0076) |
| 8. snake (0.4556) | 61. gorilla (0.1192) | 114. daughter (0.0332) | 167. fern (0.0188) | 220. dove (0.0074) |
| 9. tiger (0.4527) | 62. amoeba (0.117) | 115. sister (0.0326) | 168. sperm (0.0185) | 221. insects (0.0074) |
| 10. tree (0.4462) | 63. sheep (0.116) | 116. brother (0.032) | 169. cougar (0.0181) | 222. Chinese cabbage (0.0073) |
| 11. horse (0.4447) | 64. frog (0.1066) | 117. crops (0.032) | 170. slime (0.0181) | 223. mole (0.0073) |
| 12. goat (0.3715) | 65. panda (0.1052) | 118. mourning dove (0.0318) | 171. yucca (0.0177) | 224. octopus (0.0073) |
| 13. camel (0.3711) | 66. parrot (0.1044) | 119. mother (0.0313) | 172. peony (0.0176) | 225. impala (0.0071) |
| 14. people (0.3524) | 67. alligator (0.0992) | 120. weeds (0.0309) | 173. ocean (0.0172) | 226. COVID (0.0068) |
| 15. flower (0.3195) | 68. bee (0.0979) | 121. father (0.0307) | 174. parakeets (0.0172) | 227. beans (0.0064) |
| 16. bear (0.3071) | 69. cockroach (0.0926) | 122. grandfather (0.0301) | 175. orcas (0.0169) | 228. mango (0.0054) |
| 17. monkey (0.3046) | 70. crab (0.088) | 123. grandmother (0.0295) | 176. salmon (0.0169) | 229. wildebeest (0.0053) |
| 18. baby (0.2845) | 71. humans (0.0853) | 124. grackles (0.0292) | 177. jojoba (0.0168) | 230. Bactrian camels (0.0047) |
| 19. elephant (0.2754) | 72. hawk (0.085) | 125. cousin (0.0288) | 178. trout (0.163) | 231. ram (0.0045) |
| 20. butterfly (0.2557) | 73. animal (0.0837) | 126. aunt (0.0282) | 179. daffodil (0.0162) | 232. brown bear (0.0044) |
| 21. child (0.2397) | 74. mockingbird (0.0787) | 127. uncle (0.0276) | 180. penguin (0.0162) | 233. grizzly (0.0038) |
| 22. plant (0.2377) | 75. coyote (0.0779) | 128. friend (0.027) | 181. forest (0.016) | 234. squid (0.0036) |
| 23. rat (0.232) | 76. hen (0.0762) | 129. toad (0.0269) | 182. mallow (0.0159) | 235. mules (0.0034) |
| 24. rabbit (0.2266) | 77. guinea pig (0.0749) | 130. caterpillar (0.0265) | 183. silverfish (0.0157) | 236. jellyfish (0.0033) |
| 25. zebra (0.2216) | 78. hamster (0.0749) | 131. boss (0.0263) | 184. blue whale (0.0156) | 237. wren (0.0032) |
| 26. turtle (0.2144) | 79. daisy (0.0747) | 132. halibut (0.0257) | 185. pine trees (0.0154) | 238. rattle snake (0.0031) |
| 27. whale (0.2126) | 80. pony (0.074) | 133. neighbor (0.0257) | 186. puffins (0.0149) | 230. wife (0.0031) |
| 28. deer (0.2096) | 81. seals (0.0714) | 134. moth (0.0248) | 187. redwood trees (0.0147) | 240. lawyers (0.0029) |
| 29. giraffe (0.2087) | 82. mosquito (0.0704) | 135. nurse (0.0245) | 188. rice (0.0145) | 241. anteater (0.0025) |
| 30. worm (0.2085) | 83. fox (0.0701) | 136. iguana (0.0242) | 189. lobster (0.0144) | 242. planets (0.0025) |
| 31. ant (0.2046) | 84. leopard (0.0693) | 137. scorpion (0.0241) | 190. cobra (0.0138) | 243. arachnids (0.002) |
| 32. spider (0.2027) | 85. otter (0.0682) | 138. patient (0.0238) | 191. bluegills (0.0136) | 244. sloth (0.0019) |
| 33. shark (0.2009) | 86. adult (0.068) | 139. coconut trees (0.0236) | 192. watermelon (0.0136) | 245. engineers (0.0014) |
| 34. eagle (0.1892) | 87. orangutan (0.0669) | 140. memory (0.0234) | 193. sparrow (0.0135) | 246. parasite (0.0014) |
| 35. rhino (0.1864) | 88. antelope (0.0664) | 141. bull (0.023) | 194. asp (0.0132) | 247. canary (0.0013) |
| 36. hummingbird (0.1817) | 89. bush (0.065) | 142. sage (0.023) | 195. fir trees (0.0132) | 248. solar system (0.0012) |
| 37. mouse (0.1817) | 90. bat (0.0647) | 143. gecko (0.0227) | 196. gold fish (0.0129) | 249. puppy (0.0008) |
| 38. moose (0.1809) | 91. doctor (0.0639) | 144. palm trees (0.0227) | 197. lark (0.0128) | 250. algae (0.0007) |
| 39. man (0.1724) | 92. ash tree (0.0635) | 145. orchid (0.0226) | 198. hibiscus (0.0127) | 251. warthog (0.0007) |
| 40. woman (0.1693) | 93. hippopotamus (0.0635) | 146. bison (0.0223) | 199. anaconda (0.0125) | 252. baboon (0) |
| 41. squirrel (0.1691) | 94. crocodile (0.0634) | 147. blood (0.0222) | 200. succulents (0.0125) | 253. boar (0) |
| 42. wolf (0.1679) | 95. fungus (0.0625) | 148. brittle brush (0.0221) | 201. bamboo (0.0118) | 254. Clydesdale (0) |
| 43. bacteria (0.1616) | 96. jaguar (0.0601) | 149. mink (0.0219) | 202. aloe (0.0117) | 255. common spider (0) |
| 44. virus (0.1606) | 97. duck (0.0598) | 150. shrubs (0.0218) | 203. agave (0.011) | 256. grebe (0) |
| 45. dolphin (0.1591) | 98. eel (0.0581) | 151. clams (0.0216) | 204. boy (0.011) | 257. miners (0) |
| 46. owl (0.1425) | 99. shrimps (0.0575) | 152. weasel (0.0214) | 205. pilinut tree (0.0109) | 258. snails (0) |
| 47. pigeon (0.1402) | 100. coral (0.0565) | 153. heather (0.0212) | 206. guppies (0.0108) | 259. termites (0) |
| 48. lizard (0.1363) | 101. lamb (0.0558) | 154. oysters (0.021) | 207. marine mammals (0.0108) | 260. water (0) |
| 49. donkey (0.1362) | 102. llama (0.0555) | 155. fetus (0.0209) | 208. girl (0.0103) | |
| 50. beetle (0.1337) | 103. tuna (0.0521) | 156. racoon (0.0207) | 209. mountain lions (0.0102) | |
| 51. grass (0.1331) | 104. turkey (0.0521) | 157. palo verde tree (0.0203) | 210. goose (0.01) | |
| 52. rose (0.1314) | 105. black widow (0.0509) | 158. iris (0.0201) | 211. peanuts (0.01) | |
| 53. chimpanzee (0.1302) | 106. black recluse (0.0496) | 159. water buffalo (0.0201) | 212. falcon (0.0097) |
HC = healthy control.
FIGURE 2.
Scree plot of the most salient terms (31) generated by the AD group for the living things category. AD = Alzheimer disease.
FIGURE 3.
Scree plot of the most salient terms (69) generated by the HC group for the living things category. HC = healthy control.
The subcategories to which the terms generated belong to the living things category were animals, plants, conspecifics, and microorganisms (Figure 4). In the AD group, of the 328 terms generated, 175 (53.35%) belonged to animals, 69 (21.04%) belonged to plants, 57 (17.38%) belonged to conspecifics, and 2 (0.61%) belonged to microorganisms. In the HC group, of the 600 terms generated, 449 (74.83%) belonged to animals (wren, hummingbird, elk, deer, brown recluse, etc.), 77 (12.83%) belonged to plants (daisy, rose, daffodil, cactus, yucca, etc.), 52 (8.67%) belonged to conspecifics (people, man, woman, child, grandmother, etc.), and 9 (1.5%) belonged to microorganisms (e.g., amoeba, fungus, bacteria, etc.). The HC group made fewer errors (2.17% of all terms generated) than the AD group (7.62% of all terms generated). Examples of errors made by the participants in the living things category are reviewed under the Discussion section.
FIGURE 4.
Detected subcategories for living things. Animals = percentage of terms belonging to the category animals. Plants = percentage of terms belonging to the category plants, including fruits and vegetables. Conspecifics = percentage of terms belonging to the category humans/people. Microorganisms = percentage of terms belonging to the category germs. Errors = terms incongruent with semantic category. AD = Alzheimer disease. HC = healthy control.
In the animal and plant subcategories, the frequency of words in the folk-biological ranks of folk kingdom, life form, generic species, and folk specific was 10 (4.10%), 54 (22.13%), 180 (73.77%), and 0 (0%), respectively, in the AD group; in the HC group, the corresponding values were 7 (1.33%), 47 (8.94%), 463 (88.02%), and 9 (1.71%), respectively (Figure 5).
FIGURE 5.
Folk-biological classification: Terms generated by rank. Folk kingdom = terms such as plants, animals, etc. Life form = terms such as tree, flower, fish, mammal, etc. Generic species = terms such as pine, tulip, shark, cat, etc. Folk-specific = terms such as red oak, Bengal tiger, tabby, etc. AD = Alzheimer disease. HC = healthy control.
Living Things—Conspecifics. Terms belonging to the class of conspecifics amounted to 17.38% and 8.67% of the terms generated by the AD and HC groups, respectively. The AD group generated terms pertaining to conspecifics in general (e.g., human people, and humans), kin relationship (e.g., brother, sister, and daughter), race classification (e.g., Africans, Caucasians, and Latino), gender (e.g., boy, girl, man, and woman), general relationships (e.g., neighbor), and occupation (e.g., teacher, doctors, and nurses). The HC group also generated terms pertaining to conspecifics in general (e.g., humans and people) as well as terms related to occupation (e.g., doctor, nurse, patient, lawyers, engineers, and miners). The HC group also generated terms pertaining to kin relationship, albeit with more variations (e.g., husband, grandfather, grandmother, cousin, aunt, and uncle).
Man-made Objects. All of the terms generated by the AD and HC groups for the man-made objects category are presented in Tables 9 and 10, respectively. The most salient terms generated by the AD and HC groups are shown in the scree plots in Figures 6 and 7, respectively. In general, the subcategories to which the terms generated belong to the man-made objects category were apparel, tools, household items/appliances/furniture, transportation-related items and modes of transportation, office-related items, buildings/structures, and musical instruments, among others (Figure 8). The AD and HC groups generated the following terms for man-made objects of different sizes and manipulability (Figure 9): 50.14% and 52.56% for small (e.g., handheld, manipulable by hand), 22.56% and 28.16% for medium (e.g., furniture appliances, etc.), and 19.78% and 16.57% for large (e.g., vehicles, buildings, etc.) items, respectively.
TABLE 9.
Cultural Saliency (Bʹ Score) of all Terms Generated by the AD Group for the Man-made Objects Category (i.e., List Without Redundancies)
| 1. car (0.6215) | 52. tools (0.0512) | 103. boots (0.016) | 154. paintings (0.0065) |
| 2. chair (0.4035) | 53. refrigerator (0.0505) | 104. sweaters (0.016) | 155. fencing (0.0064) |
| 3. house (0.4017) | 54. bench (0.047) | 105. cups (0.0159) | 156. gauze (0.0062) |
| 4. shoes (0.3558) | 55. pictures (0.0459) | 106. exam table (0.0159) | 157. boxes (0.0061) |
| 5. watch (0.3191) | 56. baseball (0.045) | 107. teachers (0.0157) | 158. bracelets (0.0061) |
| 6. clothes (0.3096) | 57. paint (0.044) | 108. warm gloves (0.0154) | 159. wars (0.0061) |
| 7. paper (0.2579) | 58. fireplace (0.0409) | 109. stool (0.0152) | 160. happiness (0.006) |
| 8. airplane (0.2484) | 59. jewelry (0.0405) | 110. coats (0.015) | 161. walking shorts (0.0058) |
| 9. table (0.2109) | 60. motorcycles (0.0404) | 111. sons (0.015) | 162. band-aids (0.0055) |
| 10. pen (0.1911) | 61. sofa (0.0378) | 112. students (0.0148) | 163. peace (0.0052) |
| 11. computer (0.1861) | 62. faucet (0.0283) | 113. plumbing parts (0.0147) | 164. hoses (0.0051) |
| 12. eyeglasses (0.1693) | 63. making dinner (0.027) | 114. welding (0.0147) | 165. syringes (0.0049) |
| 13. furniture (0.1379) | 64. some men has to take a bow (0.027) | 115. keyboard (0.0139) | 166. trucks (0.0049) |
| 14. window (0.1354) | 65. cooked food (0.0252) | 116. a/c’s (0.0135) | 167. lawn furniture (0.0048) |
| 15. automobile (0.1351) | 66. underwear (0.0249) | 117. babies (0.0135) | 168. ace wraps (0.0042) |
| 16. telephone (0.1348) | 67. scooters (0.0246) | 118. clarinet (0.0135) | 169. freezer (0.0039) |
| 17. boat (0.1311) | 68. homes (0.0243) | 119. paper towels (0.0135) | 170. playing with our dogs (0.0039) |
| 18. pants (0.1253) | 69. garage (0.0233) | 120. ear drops (0.0129) | 171. car parts (0.0037) |
| 19. bicycle (0.1251) | 70. hose (0.0233) | 121. forks (0.0127) | 172. cigarettes (0.0037) |
| 20. pencil (0.1182) | 71. ladders (0.0233) | 122. paper plates (0.0125) | 173. tires (0.0037) |
| 21. bus (0.1116) | 72. china (0.0232) | 123. banks (0.0123) | 174. gloves (0.0032) |
| 22. books (0.1055) | 73. going to shop at the store by our home (0.0232) | 124. furnaces (0.0123) | 175. fun (0.003) |
| 23. clipboard (0.105) | 74. jacket (0.0229) | 125. knitting (0.0123) | 176. highways (0.0027) |
| 24. bed (0.1016) | 75. dishes (0.0222) | 126. ink (0.012) | 177. lawn mower (0.0026) |
| 25. buildings (0.0997) | 76. glassware (0.0222) | 127. parent (0.012) | 178. a/c parts (0.0025) |
| 26. ring (0.0987) | 77. hand tools (0.0221) | 128. asphalt (0.0118) | 179. lanterns (0.0025) |
| 27. guns (0.0942) | 78. dishwasher (0.0219) | 129. hearing aids (0.0116) | 180. weaving (0.0025) |
| 28. food (0.0935) | 79. ships (0.0218) | 130. singing children’s songs (0.0116) | 181. ink pens (0.0019) |
| 29. train (0.0923) | 80. wallet (0.0215) | 131. teeth from dentist (0.0112) | 182. chins (0.0017) |
| 30. socks (0.0874) | 81. desk (0.021) | 132. discs (0.011) | 183. sinks (0.0016) |
| 31. blanket (0.0857) | 82. noise (0.021) | 133. machinery (0.0106) | 184. rockets (0.0014) |
| 32. shovel (0.0795) | 83. two-wheelers (0.0209) | 134. computer modem (0.0104) | 185. fountain pens (0.001) |
| 33. clock (0.0794) | 84. toothbrush (0.0198) | 135. chalk (0.0103) | 186. skirts (0.001) |
| 34. saw (0.0779) | 85. stove (0.0193) | 136. medical equipment (0.0097) | 187. type writers (0.001) |
| 35. toys (0.0773) | 86. taking the children to see a beautiful movie (0.0193) | 137. silverware (0.0093) | 188. cans (0.0009) |
| 36. shirt (0.0756) | 87. sewers (0.0189) | 138. ability (0.009) | 189. spaceships (0.0009) |
| 37. hammer (0.0708) | 88. trailer (0.0189) | 139. get up and move the car (0.009) | 190. x-ray machines (0.0007) |
| 38. bridge (0.0703) | 89. streets (0.0183) | 140. handbags (0.0087) | 191. children love to color with crayons (0) |
| 39. nail (0.0683) | 90. fathers (0.018) | 141. shells (0.0087) | 192. copy machines (0) |
| 40. knives (0.0682) | 91. ice maker (0.018) | 142. shoe laces (0.0086) | 193. fountains (0) |
| 41. television (0.0681) | 92. lab coat (0.018) | 143. vaccines (0.0083) | 194. men like to go on to the races (0) |
| 42. towel (0.0622) | 93. men get on the horse and go (0.018) | 144. flowers (0.008) | 195. money (0) |
| 43. carpet (0.0602) | 94. door (0.0177) | 145. door knobs (0.0077) | 196. oil paintings (0) |
| 44. cabinet (0.0599) | 95. spoons (0.0175) | 146. making pictures for my children to enjoy (0.0077) | 197. opportunities (0) |
| 45. trashcan (0.0582) | 96. sidewalks (0.0174) | 147. rubber gloves (0.0077) | 198. pillow (0) |
| 46. pots (0.0567) | 97. hats (0.0172) | 148. aspirin (0.0076) | 199. rake (0) |
| 47. belt (0.0559) | 98. pottery (0.0172) | 149. purse (0.0074) | 200. septic systems (0) |
| 48. child (0.0557) | 99. football (0.017) | 150. sauce pan (0.0072) | 201. spade (0) |
| 49. pans (0.0548) | 100. fertilizer (0.0167) | 151. ammo (0.007) | |
| 50. lights (0.0544) | 101. fabrics (0.0164) | 152. medication (0.0069) | |
| 51. floor (0.0542) | 102. car seat (0.0162) | 153. winter coats (0.0068) |
AD = Alzheimer disease.
TABLE 10.
Cultural Saliency (Bʹ Score) of all Terms Generated by the HC Group for the Man-made Objects Category (i.e., List Without Redundancies)
| 1. car (0.8762) | 55. motorcycle (0.1125) | 109. patio (0.0554) | 163. sinks (0.0251) | 217. joy (0.0161) | 271. clothespin (0.0104) | 325. freezer (0.0045) |
| 2. chair (0.6587) | 56. desk (0.1107) | 110. frames (0.0553) | 164. slacks (0.025) | 218. copiers (0.016) | 272. canned food (0.0103) | 326. slide (0.0045) |
| 3. table (0.5874) | 57. gun (0.1081) | 111. coat (0.0545) | 165. corals (0.0249) | 219. wrapping papers (0.016) | 273. paintings (0.0103) | 327. cannon (0.0044) |
| 4. computer (0.5643) | 58. washing machine (0.104) | 112. radar (0.0541) | 166. apartment (0.0247) | 220. forge (0.0159) | 274. pottery (0.0103) | 328. tower (0.0044) |
| 5. shoes (0.558) | 59. bottle (0.1011) | 113. ammo (0.0524) | 167. bleachers (0.0243) | 221. flower pots (0.0157) | 275. pizzas (0.0102) | 329. wok (0.0044) |
| 6. clothes (0.4602) | 60. jewelry (0.0981) | 114. plastic (0.0506) | 168. sheetrock (0.0242) | 222. greeting cards (0.0154) | 276. screw driver (0.0102) | 330. maternal dresses (0.0043) |
| 7. house (0.4494) | 61. spoon (0.0964) | 115. compass (0.0475) | 169. babies (0.0241) | 223. scissors (0.0153) | 277. hair pins (0.0099) | 331. shopping mall (0.0042) |
| 8. eyeglasses (0.4419) | 62. steel (0.0962) | 116. telescope (0.0429) | 170. grass (0.0241) | 224. answering machine (0.015) | 278. club (0.0097) | 332. lens (0.0039) |
| 9. television (0.4052) | 63. dam (0.0934) | 117. motor (0.0428) | 171. tennis courts (0.0236) | 225. saucer (0.015) | 279. trashes (0.0097) | 333. bazookas (0.0038) |
| 10. bed (0.3994) | 64. gloves (0.0889) | 118. chest of drawers (0.0412) | 172. drums (0.0234) | 226. tie (0.015) | 280. tacos (0.0096) | 334. counters (0.0037) |
| 11. book (0.3336) | 65. dishes (0.0875) | 119. saw (0.0404) | 173. appliances (0.0233) | 227. choices (0.0149) | 281. ladies’ hats (0.0093) | 335. steno machine (0.0037) |
| 12. pencil (0.2739) | 66. blanket (0.0872) | 120. mixer (0.0382) | 174. hearing aids (0.0228) | 228. clamps (0.0149) | 282. light fixture (0.0093) | 336. trumpet (0.0037) |
| 13. airplane (0.2606) | 67. rake (0.0872) | 121. lake (0.0345) | 175. golf courses (0.0223) | 229. hairbrush (0.0148) | 283. microscopes (0.0093) | 337. parties (0.0034) |
| 14. telephone (0.2578) | 68. hat (0.0852) | 122. man-made lake (0.0345) | 176. false teeth (0.0222) | 230. toothpicks (0.0148) | 284. stories (0.0092) | 338. playground equipment (0.0034) |
| 15. refrigerator (0.2546) | 69. fork (0.0849) | 123. fireplace (0.0336) | 177. bureau (0.219) | 231. cleaning solutions (0.0142) | 285. soap (0.009) | 339. tanks (0.0033) |
| 16. stove (0.2536) | 70. tile (0.0837) | 124. cake (0.0333) | 178. slippers (0.0217) | 232. scope (0.0142) | 286. landscapes (0.0089) | 340. filters (0.0031) |
| 17. pen (0.2457) | 71. piano (0.0831) | 125. store (0.033) | 179. stadiums (0.0217) | 233. tieback (0.0141) | 287. bat (0.0088) | 341. drawers (0.003) |
| 18. glass (0.2359) | 72. roofs (0.0827) | 126. curtains (0.0328) | 180. telemetry (0.0216) | 234. nails (0.014) | 288. men belts (0.0088) | 342. signs (0.0028) |
| 19. building (0.2295) | 73. skirt (0.0808) | 127. electronics (0.0326) | 181. pills (0.0215) | 235. space craft (0.014) | 289. headsets (0.0086) | 343. AK47 (0.0027) |
| 20. bicycle (0.2213) | 74. microwave (0.0801) | 128. lumber (0.0326) | 182. china cabinets (0.0213) | 236. patio furniture (0.0139) | 290. meters (0.0086) | 344. tether (0.0027) |
| 21. truck (0.2114) | 75. printer (0.0797) | 129. bus (0.0318) | 183. dole (0.0212) | 237. fur (0.0138) | 291. underwear (0.0082) | 345. fertilizer (0.0026) |
| 22. lamp (0.2079) | 76. box (0.0781) | 130. stone (0.031) | 184. footballs (0.0211) | 238. love (0.0138) | 292. bucket (0.008) | 346. jeans (0.0026) |
| 23. pants (0.2044) | 77. tractor (0.0763) | 131. swimming pool (0.031) | 185. machine (0.0209) | 239. purses (0.0138) | 293. hotels (0.008) | 347. typewriters (0.0025) |
| 24. pillow (0.2018) | 78. ruler (0.0742) | 132. implements (0.0307) | 186. tree (0.0207) | 240. rockets (0.0137) | 294. parks (0.0078) | 348. bags (0.0024) |
| 25. paper (0.1959) | 79. cloth (0.073) | 133. ink (0.0306) | 187. pavement (0.0205) | 241. sponges (0.0137) | 295. shirts (0.0078) | 349. décor (0.0023) |
| 26. watch (0.1895) | 80. wrench (0.0728) | 134. barns (0.03) | 188. awnings (0.0204) | 242. BBQ (0.0135) | 296. ceramic pot (0.0077) | 350. grinders (0.0022) |
| 27. tools (0.1748) | 81. camera (0.0717) | 135. trailer (0.03) | 189. basketball courts (0.0204) | 243. stuffed animals (0.0135) | 297. girdle (0.0077) | 351. guitar (0.0022) |
| 28. couch (0.172) | 82. basket (0.0716) | 136. garden (0.0299) | 190. rifles (0.0202) | 244. chips (0.0134) | 298. spread (0.0076) | 352. military hardware (0.0022) |
| 29. train (0.1682) | 83. hammer (0.0711) | 137. blouse (0.0294) | 191. golf clubs (0.0198) | 245. ship (0.0133) | 299. swing (0.0075) | 353. sprinklers (0.002) |
| 30. picture (0.1672) | 84. paint (0.071) | 138. scale (0.0292) | 192. ballot (0.0195) | 246. try-squares (0.0133) | 300. x-ray machines (0.0075) | 354. shopping carts (0.0019) |
| 31. window (0.1662) | 85. towels (0.0699) | 139. scooter (0.0292) | 193. money (0.0195) | 247. waste baskets (0.0133) | 301. cleaners (0.0072) | 355. briefcase (0.0018) |
| 32. wheels (0.1581) | 86. tennis racket (0.0692) | 140. air conditioner (0.029) | 194. pistol (0.0195) | 248. Legos (0.0129) | 302. newspaper (0.0071) | 356. elevators (0.0018) |
| 33. lights (0.1531) | 87. pool (0.069) | 141. food (0.0287) | 195. earrings (0.0193) | 249. bread (0.0128) | 303. router (0.0071) | 357. jet liners (0.0016) |
| 34. flooring (0.1474) | 88. tires (0.0668) | 142. sweater (0.0286) | 196. brushes (0.0189) | 250. flower vases (0.0127) | 304. emotions (0.0069) | 358. suitcases (0.0016) |
| 35. bricks (0.1453) | 89. cellphone (0.0664) | 143. toaster (0.0285) | 197. parking lots (0.0188) | 251. friendship (0.0126) | 305. fabric (0.0069) | 359. ukulele (0.0015) |
| 36. furniture (0.1418) | 90. bridges (0.0663) | 144. axe (0.0283) | 198. ladder (0.0187) | 252. masks (0.0125) | 306. school (0.0069) | 360. pliers (0.0013) |
| 37. dishwasher (0.1405) | 91. bathtub (0.0659) | 145. walls (0.0282) | 199. necklace (0.0185) | 253. saxophone (0.0123) | 307. linings (0.0068) | 361. braces for teeth (0.0012) |
| 38. silverware (0.1397) | 92. photograph (0.0658) | 146. oven (0.0279) | 200. shower heads (0.0185) | 254. doughnuts (0.0121) | 308. needles (0.0067) | 362. firewalls (0.0012) |
| 39. fence (0.1385) | 93. toys (0.0658) | 147. face mask (0.0278) | 201. electric frying pans (0.0184) | 255. scuba tanks (0.0121) | 309. toilets (0.0067) | 363. seats (0.0011) |
| 40. shovel (0.1372) | 94. boat (0.065) | 148. fan (0.0277) | 202. support (0.0184) | 256. goggles (0.0118) | 310. polish (0.0065) | 364. war (0.0011) |
| 41. sheets (0.1371) | 95. wine (0.0647) | 149. drawing (0.0276) | 203. mats (0.0182) | 257. sidewalk (0.0118) | 311. aluminum (0.0064) | 365. clock (0.0009) |
| 42. carpet (0.1362) | 96. ring (0.0646) | 150. marble (0.0276) | 204. helicopter (0.018) | 258. board games (0.0117) | 312. drills (0.0063) | 366. shelves (0.0009) |
| 43. radio (0.1339) | 97. roads (0.064) | 151. lawn mower (0.0271) | 205. table saw (0.018) | 259. toothpaste (0.0117) | 313. yarn (0.0061) | 367. lampshade (0.0007) |
| 44. rug (0.1234) | 98. cabinets (0.0638) | 152. stroller (0.027) | 206. crockpot (0.0178) | 260. kindness (0.0115) | 314. shower (0.006) | 368. highways (0.0006) |
| 45. socks (0.1234) | 99. pillowcase (0.0638) | 153. canals (0.0268) | 207. halter (0.0177) | 261. scones (0.0115) | 315. staples (0.006) | 369. plate (0.0006) |
| 46. doll (0.1231) | 100. dryer (0.0615) | 154. utensils (0.0267) | 208. knobs (0.0176) | 262. shoe laces (0.0115) | 316. top (0.006) | 370. air masks for planes (0.0005) |
| 47. trashcan (0.1228) | 101. medicines (0.061) | 155. monitor (0.0266) | 209. band saw (0.0172) | 263. surf boards (0.0112) | 317. hanger (0.0059) | 371. batteries (0) |
| 48. sofa (0.1217) | 102. benches (0.0596) | 156. music (0.0264) | 210. hair dryer (0.0172) | 264. hockey sticks (0.0111) | 318. levels (0.0055) | 372. braces for limbs (0) |
| 49. violin (0.1188) | 103. belts (0.0595) | 157. ditches (0.0262) | 211. hatred (0.0172) | 265. statue (0.0111) | 319. titanium (0.0051) | 373. church (0) |
| 50. pots (0.1177) | 104. toothbrush (0.0591) | 158. bulbs (0.026) | 212. leather (0.0172) | 266. garage door (0.0109) | 320. high rise building (0.005) | 374. fire extinguisher (0) |
| 51. door (0.117) | 105. magazine (0.0583) | 159. food packaging (0.0259) | 213. saran wrap (0.0166) | 267. ice cream (0.0109) | 321. sewing machine (0.005) | 375. peace (0) |
| 52. faucet (0.1163) | 106. cup (0.0581) | 160. outlets (0.0259) | 214. mitre saw (0.0165) | 268. tablecloths (0.0109) | 322. rollers (0.0049) | 376. screws (0) |
| 53. pans (0.1154) | 107. golf ball (0.0574) | 161. orchards (0.0255) | 215. blinds (0.0163) | 269. bell (0.0106) | 323. draws (0.0046) | 377. snow plow (0) |
| 54. knife (0.1147) | 108. hose (0.0567) | 162. songs (0.0253) | 216. brakes (0.0161) | 270. bandages (0.0104) | 324. ideas (0.0046) | 378. wood (0) |
HC = healthy control.
FIGURE 6.
Scree plot of the most salient terms (33) generated by the AD group for the man-made objects category. AD = Alzheimer disease.
FIGURE 7.
Scree plot of the most salient terms (62) generated by the HC group for the man-made objects category. HC = healthy control.
FIGURE 8.
Detected subcategories for man-made objects. AD = Alzheimer disease. HC = healthy control.
FIGURE 9.
Man-made objects: Terms generated by object size, and errors. Errors = terms incongruent with semantic category. Object size = small (e.g., handheld, manipulable by hands), medium (e.g., furniture, appliances, etc.), and large (e.g., vehicles, buildings, etc.). AD = Alzheimer disease. HC = healthy control.
The rate of errors amounted to 7.52% of all terms in the lists generated by the AD group, which is a significantly higher rate of errors than that found in the lists generated by the HC group (2.71%). Examples of errors made by the AD group in the man-made objects category included child, flowers, wars, peace, chin, students, teachers, noise, parent, ability, sons, and fun, among others. Examples of errors made by the HC group in the man-made objects category included orchards, corals, and some emotion-related terms such as support, hatred, emotions, and love, among others.
Plural Versus Singular Terms. The plural terms in the living things category accounted for 44.21% (145 items of 328) of the terms in the lists generated by the AD group and only 22.33% (134 terms of 600) of the terms in the lists generated by the HC group. For the man-made objects category, the plural terms accounted for 54.60% (196 terms of 359) of the terms in the lists generated by the AD group and 44.58% (296 terms of 664) of the terms in the lists generated by the HC group.
DISCUSSION
We used the CDA free-listing task to investigate the impact of AD on the integrity of two distinct semantic categories—living things and man-made objects—by comparing the performance of individuals with AD with that of a matched sample of cognitively normal older adults. We had hypothesized that the AD group would perform worse than the HC group on the quantitative free-listing task for the categories of both living things and man-made objects. The lengths of the lists generated by the AD group in the free-listing task differed significantly from those generated by the HC group for both living things and man-made objects. Specifically, the AD group performed worse than the HC group in both categories, supporting our hypothesis. For the qualitative free-listing (i.e., detailed descriptive analysis of the contents of the free-lists), we had hypothesized that central, as opposed to peripheral, knowledge would be better preserved. Based on a comparison between the contents of the free lists that were generated by the two groups, we concluded that, in the AD group, conceptual knowledge that is central to the respective categories was well preserved, whereas the peripheral conceptual material showed evidence of degradation, again supporting our hypothesis.
Quantitative Free-listing: A Diagnostic Tool for Detecting AD?
Our multivariable regression models showed that group membership, after controlling for age, education, sex, and AMNART score, predicted quantitative free-listing performance (i.e., number of words generated in 5 minutes) in both categories. Age, education, sex, and AMNART score were not significant predictors of free-listing performance in either category, thereby suggesting that the demographic variables did not significantly influence the performance of the two groups, whereas disease status (presence or absence of AD) did. The findings also indicated a significant inverse relationship between functional status (i.e., FAST score) and performance on the free-listing task when demographic variables (age, education, and sex) and AMNART score were held constant. Several studies have suggested that the loss of semantic knowledge negatively impacts everyday functioning (Corbett et al., 2015; Kirchberg et al., 2012; Roll et al., 2019; Snowden, 2015).
The results showed a correlation between performance on the free-listing task and performance on a category verbal fluency task. The free-listing task may be reminiscent of category verbal fluency, although they are not identical. The goals of the tasks, and thereby their methods of administration, are different. The free-listing task differs from the category fluency task in that free-listing focuses on maximizing list elicitation to reveal the central content of semantic categories as exhaustively as possible (Bernard, 2018a) in order to aid anthropologists in examining the age-old question of how culture, language, and thought intersect. Therefore, probes such as nonspecific prompting, reading of the list produced, and semantic cuing were allowed (as needed) in the free-listing task. However, such probes are not used in oral category fluency tasks (or a written verbal fluency task; Strauss et al., 2006).
In the setting that the AD group still performed worse than the HC group in both semantic categories, even when the individuals were provided with probes to aid in eliciting longer lists in order to potentially ease the burden on domain-general processes (e.g., executive function, attention, etc.), might it be that free-listing would be able to detect individuals with AD from HCs earlier in the disease process? Perhaps future studies can compare the performance of individuals on the free-listing task and the verbal category fluency task in order to distinguish individuals with AD from HCs and to prove whether the free-listing task can detect individuals with AD earlier in the disease trajectory. With time constraints as a given in busy clinical settings, additional studies could also record the length of the free lists that were generated at other time intervals (e.g., 2 minutes, 3 minutes, etc.) and compare the sensitivity and specificity of the task in differentiating between individuals with AD and HCs at each time point.
The performance of the two groups in the free-listing task also showed strong correlations (P < 0.001) with performance on the other cognitive tests (MoCA, MMSE, and FAST), suggesting that the free-listing task could be a reliable tool for detecting and staging AD. Additional studies are required to establish performance on the free-listing task for each FAST stage. With semantic dysfunction occurring early in AD, as global cognitive function deteriorates, it is expected that individuals with AD will have more difficulties in accessing, retrieving, and preserving semantic knowledge, which may explain the positive correlation between the free-listing task and MoCA and MMSE scores. To clarify this, future research investigating the differences in performance in the free-listing task between individuals at different stages of the cognitive continuum—cognitively normal, mild cognitive impairment, and dementia—is needed.
Conceptual Saliency: Core and Peripheral Semantic Knowledge in AD?
The AD group generated fewer distinct terms than the HC group in both categories. A comparison of the contents of the free lists of living things and man-made objects that were generated by the AD and HC groups highlighted differences and similarities. Though ranked differently, the most salient concepts appeared in prominent positions in the lists generated by the AD and HC groups as expected, given that the individuals in both groups shared one culture.
Living Things
The terms generated by both the AD and HC groups that were elaborated on in the lists under the broad category of living things covered the subcategories of animals, plants (including fruits and vegetables), conspecifics, and microorganisms. The prominent mention of the microorganisms subcategory may be connected to the increased salience of that class of items, given that the study was conducted during the COVID-19 pandemic. Threats related to pathogens have been well-controlled in affluent Western societies until the current pandemic, which likely activated the evolved inferential system about “contamination risks” (Liénard and Boyer, 2006) in the context of daily cultural inputs (e.g., news regarding the pandemic, interactions with others, etc.). In 2019, the three leading causes of death in the United States were heart disease, cancer, and accidents (Ahmad and Anderson, 2021). In 2020, COVID-19 replaced accidents as the third leading cause of death in the United States.
For both the AD and HC groups, the most prominent living things subcategory was animals, amounting to 53.35% of the terms in the living things category in the lists generated by the AD group and 74.83% in those generated by the HC group. Twice (17.38%) as many terms belonging to the class of conspecifics were found in the AD group’s lists than in the HC group’s lists (8.67%). The AD group was more prone to make errors than the HC group (3.5 times higher), with 7.62% of their terms produced being errors, as opposed to 2.17% for the HCs.
The errors made by the AD group in the lists of living things included inanimate items that are capable of motion (e.g., cars, buses, hearts, feet, and heaters). The HC group behaved similarly, including inanimate items that are capable of self-generated activity (e.g., ocean, blood, planets, and water) in their lists. Although the lists of both groups included inanimate items, those generated by the HC group only included inanimate natural kinds (e.g., planets, solar system, water, etc.). The lists generated by the AD group included both natural kinds and man-made objects (e.g., autos, buses, harps, hearts, heaters, heels, stones, etc.).
As higher level folk-biological concepts degraded in the AD group, the group reverted to relying on core principles (e.g., the association between agency and alive) when reasoning about living things; this finding is similar to that reported by Zaitchik and Solomon (2008).
The level at which most inductive inferences are produced is considered the basic/generic or privileged level of a hierarchical structure (Gelman, 2022; Medin and Atran, 2004; Rosch, 1978; Waxman, 1999). Both the AD group (73.77%) and the HC group (88.02%) generated the most items at the generic species level in the living things category. Thus, this level appeared to be the most salient level for both groups. These results are consistent with those reported in previous studies (Berlin, 1973; Berlin et al., 1974; Medin and Atran, 2004). This structural aspect of biological cognition appears to be preserved in the mild stages of AD.
Although both groups showed a privileged level at the generic species level, the frequency of terms generated for each level differed between the groups. For example, the AD group generated more terms than the HC group at the superordinate levels (i.e., folk kingdom and life form). The percentage of terms generated by the AD group at the superordinate level were 22.13% (life form) and 4.10% (folk kingdom), and those generated by the HC group were 8.94% (life form) and 1.33% (folk kingdom). For lower levels (i.e., subordinate level folk-specific), the percentage of terms generated by the AD group (0%) was significantly lower than that generated by the HC group (1.71%).
This finding supports previous findings that have been reported in the literature (Arroyo-Anlló et al., 2012; Hodges et al., 1992; Salmon et al., 1999; Simoes Loureiro and Lefebvre, 2016; Verma and Howard, 2012) as well as our hypothesis that more fine-grained or peripheral knowledge is affected first, and there is greater preservation of more general core knowledge further into the disease. These results are also consistent with those reported by Medin and Atran (2004), who proposed that, as knowledge in a particular domain is impoverished, the privileged level moves up the hierarchy, devolving from the basic level to more superordinate levels. In other words, the privilege level of a hierarchical structure depends on experience and knowledge. In the present study, we interpreted the observed devolution as a consequence of the neurodegenerative process in AD, indicating semantic knowledge degradation.
Hodges et al. (1992) supported the hypothesis of a greater preservation of superordinate concepts and the loss of subordinate conceptual information occurring early in the disease trajectory. The researchers relied on a sorting task to assess individuals’ semantic knowledge about the items in the test. Hodges and colleagues (1992) found that knowledge at the most general level (living vs artifact categories) is preserved in individuals with AD. However, as sorting requires more specific knowledge (i.e., grouping the items into less inclusive categories, such as birds vs land animals vs sea creatures), impairment became evident. The greatest impairment was observed at the subordinate level, which required more in-depth information (e.g., fierceness, locality, and size). The finding of better preservation of more general knowledge as opposed to detailed knowledge in individuals with AD has been corroborated by several studies (Arroyo-Anlló et al., 2012; Perri et al., 2012; Salmon et al., 1999; Simoes Loureiro and Lefebvre, 2016; Verma and Howard, 2012).
The percentage of terms belonging to the conspecifics category (under the broader living things category) generated by the AD group was twice as large as that generated by the HC group. The social categories mentioned by both groups belonged to the general categories of conspecifics (e.g., humans and people), kin relationship, gender, general relationships, and occupation. The HC group listed more specific terms for conspecifics. For instance, kin relationship terms (e.g., grandfather, grandmother, cousin, aunt, and uncle) were listed by the HC group but not by the AD group. The subcategory race or race classification was found in the lists generated by the AD group but not in those generated by the HC group. The terms mentioned were Africans, Caucasians, and Latino. The terms related to race might have been noted in the lists generated by the AD group but not in those generated by the HC group because it has been found to be a central aspect of social categorization (i.e., classifying conspecifics into categories), preceding other characteristics such as occupation (Hirschfeld, 1995, 1996). The central principles of domain knowledge are expected to be better preserved than peripheral principles, which are often acquired later.
The representation of social categories has been observed in young children as early as 3–12 months of age (Kelly et al., 2005; Liberman et al., 2017). At the core of social categorization is the notion of an essence, or an underlying causal nature (extended from folkbiology), that is responsible for the physical features (e.g., appearance), growth and development, behavior, and inheritance of the members, which guides how people classify conspecifics into distinct types (Hirschfeld, 1996). A series of sorting experiments (Hirschfeld, 1995) exploring which variables (body build, race, or occupation) children believed to be more significant in a person’s identity showed that children as young as 3 years were more likely to select race over body build or occupation. The tendency to classify conspecifics into categories, such as essentialized groups (i.e., races), is found across cultures; however, the specific characteristics that distinguish or define distinct races (e.g., physical attributes, religion, etc.) are culture specific and are shaped by the cultural context (Cosmides et al., 2003; Gedeon et al., 2021; Gelman, 2022; Hailey and Olson, 2013; Hirschfeld, 1995; Kelly et al., 2005; Pauker et al., 2016).
Man-made Objects
The terms generated by both the AD and HC groups that were elaborated on in the lists under the broad category of man-made objects covered specific subcategories, such as apparel, tools, household items/appliances/furniture, transportation-related items and modes of transportation, office-related items, buildings/structures, medical equipment, and a few musical instruments. The AD group listed fewer terms for all subcategories than the HC group (Figure 8), except for apparel, modes of transportation/transportation-related items, and medical equipment. It is possible that the AD group generated more terms pertaining to medical equipment and apparel because these objects were readily available to them during the task. Because the study was conducted in a clinical setting, medical supplies were clearly visible in the examination rooms. Perhaps, in the context of a degrading semantic structure, it is plausible that individuals with AD would rely more on readily available objects as a strategy to generate more terms for the task at hand.
Most of the man-made object terms (a little >50%) generated by both groups were of a small size (i.e., handheld, manipulable by hand), such as a pen, pencil, eyeglasses, paper, among others. For both groups, medium-sized object terms, such as table, chair, and television, were the second most frequently mentioned items. The HC group (28.16%) generated slightly more terms in this category than the AD group (22.56%). Finally, the AD group (19.78%) generated more terms than the HC group (16.57%) in the large-sized item category (e.g., cars, airplanes, houses, etc.). Object properties, specifically size and manipulability, are fundamental aspects that are considered for the conceptual organization and processing of, and reasoning about, inanimate objects.
The distinct properties of smallness/manipulability, and large amplitude, stationary, relevant for navigation objects, include different processing pathways involving specific brain regions, such as the lateral occipitotemporal regions for small manipulable objects and the ventromedial occipitotemporal regions for large and stable inanimate objects (He et al., 2013; Magri et al., 2021; Mahon et al., 2009). The activation of distinct brain regions based on object size and type (animate vs inanimate) showed similar findings in studies comparing sighted and congenitally blind individuals, indicating that visual sensory experience is not necessary for this specialization to develop, implying a fundamental role for categories in the organization of semantic knowledge (He et al., 2013; Mahon et al., 2009). In an eye-tracking study evaluating tool use, Federico and Brandimonte (2020) showed that semantic processing precedes sensorimotor processing.
The brain regions (frontal, temporal, and parietal cortices) that are critical for the names and identity of objects, such as tools (Bi et al., 2015), typically of small size, are affected by AD pathology early in the disease. Individuals with AD may produce more terms pertaining to transportation/modes of transportation and relatively larger objects in order to maximize knowledge that is better preserved in order to compensate for weaknesses in another domain. Large inanimate objects (e.g., different types of transportation) rely on different brain networks for processing (He et al., 2013; Magri et al., 2021; Mahon et al., 2009).
The errors made by the AD group in the lists of man-made objects included terms related to social interactions (e.g., wars, peace, and fun), human activity (e.g., noise), reproduction (e.g., child and sons), and social categorization (e.g., teacher and parent). These items could arguably fall under the class of “things” that humans make, though they are not artifacts, which was the specific category of interest in this study. Therefore, we counted them as errors. The errors made by the HC group in the lists of man-made objects included terms related to emotions (e.g., hatred, love, emotions, etc.).
Plural Versus Singular Terms
For both living things and man-made objects, the AD group generated more plural terms than the HC group, supporting our hypothesis. We had hypothesized that if more detailed knowledge (typically those occupying the lower levels of a hierarchical structure) deteriorates first in individuals with AD, then more terms closer to the superordinate level and, thereby, more plural terms should be generated by the AD group compared to the HC group. Indeed, superordinate-level lexemes (e.g., animals, trees, appliances, and vehicles) that identify conceptual collections of items are typically more strongly associated with plural lexical forms, and the AD group generated more terms at the superordinate levels than the HC group. Subordinate lexemes (cat, tabby, chair, table, etc.) are more likely to appear in singular forms as itemizations of a specific genus rather than as representations of conceptual collectives (Rosch, 1978; Schalley and Zaefferer, 2007; Wisniewski and Murphy, 1989). This study is the first to investigate whether individuals with AD generate more plural terms than singular terms in a fluency task. The results are preliminary findings, and additional studies are required to support this observation.
Limitations and Future Vision
This preliminary study has several limitations. The primary limitation is the small sample size, which limited its generalizability. Generally, large sample sizes are required to detect subtle effects or differences that are not detectable in small samples. Despite the small sample size of our study, divergences (and similarities) in the performance and output of individuals with AD were detected, suggesting robust findings.
One of the foundational assumptions of CDA is that domain knowledge is shared among individuals in a given population. In other words, the agreement between individuals regarding what belongs to a domain of interest is generally high, making possible studies with smaller sample sizes valid (Weller, 2007; Weller and Romney, 1988). Nevertheless, future studies with larger sample sizes are required to confirm our findings and to further evaluate the quantitative and qualitative utility of the free-listing task, which could aid in diagnosing, staging, and revealing concepts that remain salient as the disease progresses. Additional research is also needed to investigate the task’s test–retest and inter-rater reliability; sensitivity in detecting individuals affected by AD; and specificity in excluding healthy, cognitively normal, age-matched adults.
Another limitation of the study is that identifying the role that impairment of domain-specific mechanisms and domain-general processes (e.g., attention, executive function, etc.) plays in the differences in performance that were observed between the AD and HC groups extends beyond the scope of our research. This divergence is likely a consequence of the complex interplay between these components. However, further studies are required to confirm this hypothesis.
Given that certain elements of knowledge in two distinct semantic categories were better preserved than others, as noted in our descriptive analysis of the contents of the free lists, impairment in domain-general process explanations alone would not be able to fully account for why specific aspects of semantic knowledge were more intact. Effective performance on similar tasks, such as verbal semantic/category fluency, has been shown to depend on an intact semantic knowledge store and not just on a successful search to retrieve semantic information (Adlam et al., 2010; Chertkow and Bub, 1990; Hodges, 2017; Hodges et al., 1992).
Additional studies could also compare the performance of individuals who have been diagnosed with other neurodegenerative disorders that affect semantic knowledge (e.g., AD vs semantic dementia) in order to assess whether the task can aid in differentiating dementia syndromes. Dementia is a global problem affecting ~35.6 million individuals worldwide, with 58% living in middle- to low-income countries (World Health Organization, 2021). Yet, current cognitive assessment tools are generally Western-centric (Alzheimer’s Association, 2022).
Novel reliable tools that can be used in underserved and diverse populations are needed to aid in distinguishing cognitively normal individuals from those with cognitive impairments. The free-listing task can contribute to addressing this gap. Anthropologists have successfully used this low-tech method (both oral and written) since the 1960s in small- and large-scale societies with diverse cultural backgrounds. Given its simplicity, cost-effectiveness, and noninvasive nature, this method has the potential to aid in the detection of semantic degradation in neurodegenerative diseases and in the diagnosis and staging of such diseases in less affluent societies where brain imaging technologies, memory centers, and neuropsychological testing resources are sparse and dementia prevalence is predicted to increase the most (Alzheimer’s Association, 2022; Alzheimer’s Disease International, 2022).
CONCLUSION
In this preliminary study, we demonstrated the potential utility of the quantitative and qualitative free-listing of living things and man-made objects in AD research. Our findings suggest that the free-listing task could aid in the detection of semantic knowledge dysfunction in individuals with AD. The median MoCA, MMSE, and FAST scores of 18, 23, and 4, respectively, indicated that the AD group was made up of individuals with relatively mild AD, that is, the early stages of disease progression. Two conclusions can be drawn:
Knowledge degradation in two distinct semantic categories—living things and man-made objects—is detectable early in the disease trajectory of AD.
The assessment of knowledge degradation via the free-listing task could aid in the preliminary characterization of AD.
Our findings also indicate that the fundamental aspects of the structure of knowledge for semantic categories of living things and man-made objects are preserved to some extent in individuals with mild-to-moderate AD. The categories at broader levels (e.g., superordinate and intermediate/basic), which drive knowledge acquisition early in development, were better preserved than the more specific (e.g., subordinate) levels. At the core of domain-specific knowledge structures are general templates (Gelman and Meyer, 2011; Keil, 1981; Sperber, 1994) and broader categories on the basis of which more specific levels are built in later stages. Knowledge that requires more detailed information was less intact, as this finding was made obvious by the greater use of more inclusive terms in the list that was generated by the AD group.
Degradation of more detailed knowledge can also be detected in the strategies that were used by the AD group. Specifically, the AD group seemed to rely more on core principles when defining semantic categories compared with the HC group. For instance, the overreliance of the AD group on the concept of agency for defining living things may reflect a loss of more fine-grained knowledge. In addition, perhaps because detailed knowledge (subordinate concepts, often learned later) of features that distinguish members of different categories or classify them as belonging to shared categories deteriorates in individuals with AD, individuals are left to rely on more elemental features of animate objects. Although the overall knowledge structure is maintained in AD in the mild-to-moderate stages, it is more confined to the core structure and to concepts that enable the acquisition of more specific knowledge later in development.
Although semantic knowledge structures are relatively similar across cultures, the saliency of concepts is culture specific. Thus, future studies should recruit individuals from diverse cultural backgrounds and individuals with confirmed biomarkers (Jack et al., 2018) and neuroimaging data throughout the AD continuum (preclinical AD, mild cognitive impairment due to AD, and AD dementia). Cross-cultural comparisons would provide valuable insights into the conceptual material that is not only culture specific but would also detect the conceptual information that remains highly resilient to deterioration in AD, potentially indicating a more universal core knowledge that is critical to human beings. Additional studies could further our insights into several topics that have long interested researchers across disciplines, such as the organization of semantic knowledge; the question of how the brain achieves the complex processes of acquiring typical knowledge based on tokens; and the intersection between cognition, culture, and language.
ACKNOWLEDGMENTS
First, this research endeavor is dedicated to the memory of Pierre Liénard, who passed away during the preparation of this manuscript. Professor Liénard had a brilliant mind and was an exceptional human being. He will be remembered for his mentorship, kindness, strength, and scientific expertise. Second, the authors would like to thank all of the participants and their care partners for their contributions in making this study possible. The authors also wish to thank Daniel C. Benyshek, Jeffrey L. Cummings, and William Jankowiak, University of Nevada, Las Vegas; Jennifer Kawi, University of Texas Health Science Center at Houston; and Gabriel C. Léger, University of California, San Diego, for their comments on the previous version of the manuscript. Gratitude is owed to Brittany Lapin, Cleveland Clinic, and David F. Damore, University of Nevada, Las Vegas for providing general guidance on the statistical methods. A special thanks is also owed to Nancy M. Albert, Cleveland Clinic; and Aaron R. Ritter, Hoag Memory and Cognitive Disorders Program, for their assistance in making this study possible.
Footnotes
The authors declare no conflicts of interest.
A previous version of this manuscript was posted to PsyArXiv as doi:10.31234/osf.io/wa56t.
Contributor Information
Maileen G. Ulep, Email: maileen.ulep-reed@unlv.edu.
Pierre Liénard, Email: pierre.lienard@unlv.edu.
REFERENCES
- Adlam ALR, Bozeat S, Arnold R, et al. 2006. Semantic knowledge in mild cognitive impairment and mild Alzheimer’s disease. Cortex. 42:675–684. doi: 10.1016/s0010-9452(08)70404-0 [DOI] [PubMed] [Google Scholar]
- Adlam ALR, Patterson K, Bozeat S, et al. 2010. The Cambridge Semantic Memory Test Battery: detection of semantic deficits in semantic dementia and Alzheimer’s disease. Neurocase. 16:193–207. doi: 10.1080/13554790903405693 [DOI] [PubMed] [Google Scholar]
- Ahmad FB, Anderson RN. 2021. The leading causes of death in the US for 2020. JAMA. 325:1829–1830. doi: 10.1001/jama.2021.5469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albanese E. 2007. The “hidden” semantic category dissociation in mild-moderate Alzheimer’s disease patients. Neuropsychologia. 45:639–643. doi: 10.1016/j.neuropsychologia.2006.07.018 [DOI] [PubMed] [Google Scholar]
- Alegret M, Peretó M, Pérez A, et al. 2018. The role of verb fluency in the detection of early cognitive impairment in Alzheimer’s disease. J Alzheimers Dis. 62:611–619. doi: 10.3233/JAD-170826 [DOI] [PubMed] [Google Scholar]
- Alzheimer’s Association . 2022. Alzheimer’s Disease Facts and Figures. Accessed May 16, 2022. https://www.alz.org/alzheimers-dementia/facts-figures
- Alzheimer’s Disease International . 2022. Dementia Statistics. Accessed May 16, 2022. https://www.alzint.org/about/dementia-facts-figures/dementia-statistics/
- Appell J, Kertesz A, Fisman M. 1982. A study of language functioning in Alzheimer patients. Brain Lang. 17:73–91. doi: 10.1016/0093-934x(82)90006-2 [DOI] [PubMed] [Google Scholar]
- Arroyo-Anlló EM, Lorber M, Rigaleau F, et al. 2012. Verbal fluency in Alzheimer’s disease and aphasia. Dementia. 11:5–18. doi: 10.1177/1471301211416609 [DOI] [Google Scholar]
- Berlin B. 1973. Folk systematics in relation to biological classification and nomenclature. Annu Rev Ecol Evol Syst. 4:259–271. [Google Scholar]
- Berlin B, Kay P. 1969. Basic Color Terms: Their Universality and Evolution. University of California Press. [Google Scholar]
- Berlin B, Breedlove DE, Raven PH. 1974. Principles of Tzeltal Plant Classification: An Introduction to the Botanical Ethnography of a Mayan-Speaking, People of Highland, Chiapas. New York and London: Academic Press. [Google Scholar]
- Bernard HR. 2018a. Research Methods in Anthropology: Qualitative and Quantitative Approaches, 6th ed. Lanham, Maryland: Rowman & Littlefield. [Google Scholar]
- Bernard HR. 2018b. Research Methods in Cognitive Anthropology: Cultural Domain Analysis [PowerPoint slides]. Accessed May 16, 2022. https://ufl.instructure.com/courses/351891/files/38150668/download?wrap=1
- Bernard HR, Ryan GW, Borgatti SP. 2009. Green cognition and behavior: a cultural domain analysis. In: Greiner C, Kokot W, eds. Networks, Resources and Economic Action: Ethnographic Case Studies in Honor of Hartmut Lang. Berlin, Germany: Dietrich Reimer Verlag; 189–215. [Google Scholar]
- Bi Y, Han Z, Zhong S, et al. 2015. The white matter structural network underlying human tool use and tool understanding. J Neurosci. 35:6822–6835. doi: 10.1523/JNEUROSCI.3709-14.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binder JR, Desai RH, Graves WW, et al. 2009. Where is the semantic system? A critical review and meta-analysis of 120 functional neuroimaging studies. Cereb Cortex. 19:2767–2796. doi: 10.1093/cercor/bhp055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borgatti SP. 1994. Cultural domain analysis. J Quantitative Anthropol. 4:261–278. [Google Scholar]
- Borgatti SP. 1998. Elicitation techniques for cultural domain analysis. In: Shensul J, LeCompte M, eds. The Ethnographer’s Toolkit. Walnut Creek, California: Altamira; 1–26. [Google Scholar]
- Bousfield WA, Barclay WD. 1950. The relationship between order and frequency of occurrence of restricted associative responses. J Exp Psychol. 40:643–647. doi: 10.1037/h0059019 [DOI] [PubMed] [Google Scholar]
- Boyer P, Barrett HC. 2016. Intuitive ontologies and domain specificity Buss DM, ed. The Handbook of Evolutionary Psychology, 2nd ed. Hoboken, New Jersey: John Wiley & Sons; 161–179. [Google Scholar]
- Braak H, Braak E. 1991. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol. 82:239–259. doi: 10.1007/BF00308809 [DOI] [PubMed] [Google Scholar]
- Bulmer RNH. 1967. Why is the cassowary not a bird? A problem of zoological taxonomy among the Karam of the New Guinea Highlands. Man. 2:5–25. doi: 10.2307/2798651 [DOI] [Google Scholar]
- Buss DM. 2016. The Handbook of Evolutionary Psychology, 2nd ed. Hoboken, New Jersey: John Wiley & Sons. [Google Scholar]
- Butters N, Granholm E, Salmon DP, et al. 1987. Episodic and semantic memory: a comparison of amnesic and demented patients. J Clin Exp Neuropsychol. 9:479–497. doi: 10.1080/01688638708410764 [DOI] [PubMed] [Google Scholar]
- Capitani E, Laiacona M, Mahon B, et al. 2003. What are the facts of semantic category-specific deficits? A critical review of the clinical evidence. Cogn Neuropsychol. 20:213–261. doi: 10.1080/02643290244000266 [DOI] [PubMed] [Google Scholar]
- Caramazza A, Shelton JR. 1998. Domain-specific knowledge systems in the brain: the animate-inanimate distinction. J Cogn Neurosci. 10:1–34. doi: 10.1162/089892998563752 [DOI] [PubMed] [Google Scholar]
- Carey S. 2009. The Origin of Concepts. New York, New York: Oxford University Press. [Google Scholar]
- Centers for Medicare and Medicaid Services . 2021. Hospice Alzheimer’s Disease & Related Disorders. Accessed May 16, 2022. https://www.cms.gov/medicare-coverage-database/view/lcd.aspx?LCDId=34567
- Chan AS, Butters N, Salmon DP. 1997. The deterioration of semantic networks in patients with Alzheimer’s disease: a cross-sectional study. Neuropsychologia. 35:241–248. doi: 10.1016/s0028-3932(96)00067-x [DOI] [PubMed] [Google Scholar]
- Chan AS, Salmon DP, Butters N, et al. 1995. Semantic network abnormality predicts rate of cognitive decline in patients with probable Alzheimer's disease. J Int Neuropsychol Soc. 1:297–303. doi: 10.1017/s1355617700000291 [DOI] [PubMed] [Google Scholar]
- Chertkow H, Bub D. 1990. Semantic memory loss in dementia of Alzheimer's type: What do various measures measure? Brain. 113:397–417. doi: 10.1093/brain/113.2.397 [DOI] [PubMed] [Google Scholar]
- Conklin HC. 1955. Hanunóo color categories. Southwest J Anthropol. 11:339–344. doi: 10.1086/soutjanth.11.4.3628909 [DOI] [Google Scholar]
- Coolidge FL, Wynn T. 2016. An introduction to cognitive archaeology. Curr Dir Psychol Sci. 25:386–392. doi: 10.1177/0963721416657085 [DOI] [Google Scholar]
- Corbett F, Jefferies E, Burns A, et al. 2015. Deregulated semantic cognition contributes to object‐use deficits in Alzheimer's disease: a comparison with semantic aphasia and semantic dementia. J Neuropsychol. 9:219–241. doi: 10.1111/jnp.12047 [DOI] [PubMed] [Google Scholar]
- Cosmides L, Tooby J, Kurzban R. 2003. Perceptions of race. Trends Cogn Sci. 7:173–179. doi: 10.1016/s1364-6613(03)00057-3 [DOI] [PubMed] [Google Scholar]
- Crawford JR, Moore JW, Cameron IM. 1992. Verbal fluency: a NART‐based equation for the estimation of premorbid performance. Br J Clin Psychol. 31:327–329. doi: 10.1111/j.2044-8260.1992.tb00999.x [DOI] [PubMed] [Google Scholar]
- Cross K, Smith EE, Grossman M. 2008. Knowledge of natural kinds in semantic dementia and Alzheimer’s disease. Brain Lang. 105:32–40. doi: 10.1016/j.bandl.2008.01.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummings JL, Benson F, Hill MA, et al. 1985. Aphasia in dementia of the Alzheimer type. Neurology. 35:394–397. doi: 10.1212/wnl.35.3.394 [DOI] [PubMed] [Google Scholar]
- D’Andrade RG. 1995. The Development of Cognitive Anthropology. Cambridge, United Kingdom: Cambridge University Press. [Google Scholar]
- Falchook AD, Mosquera DM, Finney GR, et al. 2012. The relationship between semantic knowledge and conceptual apraxia in Alzheimer disease. Cogn Behav Neurol. 25:167–174. doi: 10.1097/WNN.0b013e318274ff6a [DOI] [PubMed] [Google Scholar]
- Federico G, Brandimonte MA. 2020. Looking to recognise: the pre-eminence of semantic over sensorimotor processing in human tool use. Sci Rep. 10:6157. doi: 10.1038/s41598-020-63045-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flanagan KJ, Copland DA, Chenery HJ, et al. 2013. Alzheimer's disease is associated with distinctive semantic feature loss. Neuropsychologia. 51:2016–2025. doi: 10.1016/j.neuropsychologia.2013.06.008 [DOI] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. 1975. “Mini-mental state”: a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 12:189–198. doi: 10.1016/0022-3956(75)90026-6 [DOI] [PubMed] [Google Scholar]
- Frake CO. 1961. The diagnosis of disease among the Subanun of Mindanao. Am Anthropol. 63:113–132. doi: 10.1525/aa.1961.63.1.02a00070 [DOI] [PubMed] [Google Scholar]
- Gainotti G. 2000. What the locus of brain lesion tells us about the nature of the cognitive defect underlying category-specific disorders: a review. Cortex. 36:539–559. doi: 10.1016/s0010-9452(08)70537-9 [DOI] [PubMed] [Google Scholar]
- Galasko D, Schmitt FA, Thomas RG, et al. 2005. Detailed assessment of activities of daily living in moderate to severe Alzheimer’s disease. J Int Neuropsychol Soc. 11:446–453. doi: 10.1017/s1355617705050502 [DOI] [PubMed] [Google Scholar]
- Garrard P, Patterson K, Watson PC, et al. 1998. Category specific semantic loss in dementia of Alzheimer's type. Functional-anatomical correlations from cross-sectional analyses. Brain. 121:633–646. doi: 10.1093/brain/121.4.633 [DOI] [PubMed] [Google Scholar]
- Gazzaniga MS, Ivry RB, Mangun GR. 2018. Cognitive Neuroscience: The Biology of the Mind, 5th ed. New York, New York: W.W. Norton. [Google Scholar]
- Gedeon C, Badea C, Esseily R. 2021. Racial categorization and intergroup relations in children: the role of social status and numerical group size. Front Psychol. 12:719121. doi: 10.3389/fpsyg.2021.719121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelman R. 1990. First principles organize attention to and learning about relevant data: number and the animate‐inanimate distinction as examples. Cogn Sci. 14:79–106. doi: 10.1016/0364-0213(90)90027-T [DOI] [Google Scholar]
- Gelman SA. 2022. Development of qualitative thinking: language and categorization Houdé O, Borst G, eds. The Cambridge Handbook of Cognitive Development. Cambridge, United Kingdom: Cambridge University Press; 341–360. [Google Scholar]
- Gelman SA, Meyer M. 2011. Child categorization. Wiley Interdiscip Rev Cogn Sci. 2:95–105. doi: 10.1002/wcs.96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson KR, Ingold T. 1993. Tools, Language and Cognition in Human Evolution. Cambridge, United Kingdom: Cambridge University Press. [Google Scholar]
- Gonnerman LM, Andersen ES, Devlin JT, et al. 1997. Double dissociation of semantic categories in Alzheimer’s disease. Brain Lang. 57:254–279. doi: 10.1006/brln.1997.1752 [DOI] [PubMed] [Google Scholar]
- Goodenough WH. 1957. Cultural anthropology and linguistics Garvin, P., ed. Report of the Seventh Annual Round Table Meeting on Linguistics and Language Study. Washington, DC: Georgetown University Press; 167–173. [Google Scholar]
- Gravlee CC, Bernard HR, Maxwell CR, et al. 2013. Mode effects in free-list elicitation: comparing oral, written, and web-based data collection. Soc Sci Comput Rev. 31:119–132. doi: 10.1177/0894439312455312 [DOI] [Google Scholar]
- Grober E, Sliwinski M. 1991. Development and validation of a model for estimating premorbid verbal intelligence in the elderly. J Clin Exp Neuropsychol. 13:933–949. doi: 10.1080/01688639108405109 [DOI] [PubMed] [Google Scholar]
- Hailey SE, Olson KR. 2013. A social psychologist's guide to the development of racial attitudes. Soc Personal Psychol Compass. 7:457–469. doi: 10.1111/spc3.12038 [DOI] [Google Scholar]
- He C, Peelen MV, Han Z, et al. 2013. Selectivity for large nonmanipulable objects in scene-selective visual cortex does not require visual experience. Neuroimage. 79:1–9. doi: 10.1016/j.neuroimage.2013.04.051 [DOI] [PubMed] [Google Scholar]
- Hirschfeld LA. 1995. Do children have a theory of race? Cognition. 54:209–252. doi: 10.1016/0010-0277(95)91425-r [DOI] [PubMed] [Google Scholar]
- Hirschfeld LA. 1996. Race in the Making: Cognition, Culture, and the Child's Construction of Human Kinds. Cambridge, Massachusetts: MIT Press. [Google Scholar]
- Hirschfeld LA, Gelman SA. 1994. Mapping the Mind: Domain Specificity in Cognition and Culture. Cambridge, United Kingdom: Cambridge University Press; 10.1017/CBO9780511752902 [DOI] [Google Scholar]
- Hodges JR. 2017. Cognitive Assessment for Clinicians, 3rd ed. Oxford, United Kingdom: Oxford University Press; 10.1093/med/9780198749189001.0001 [DOI] [Google Scholar]
- Hodges JR, Salmon DP, Butters N. 1992. Semantic memory impairment in Alzheimer’s disease: failure of access or degraded knowledge. Neuropsychologia. 30:301–314. doi: 10.1016/0028-3932(92)90104-t [DOI] [PubMed] [Google Scholar]
- Howard D, Patterson K. 1992. The Pyramids and Palm Trees Test: A Test of Semantic Access From Pictures and Words. Bury St Edmunds, UK: Thames Valley Test. [Google Scholar]
- Jack CR, Jr, Bennett DA, Blennow K, et al. 2018. NIA-AA research framework: toward a biological definition of Alzheimer’s disease. Alzheimers Dement. 14:535–562. doi: 10.1016/j.jalz.2018.02.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan E, Goodglass H, Weintraub S. 1983. The Boston Naming Test, 2nd ed. Philadelphia, Pennsylvania: Lea & Febiger. [Google Scholar]
- Keil FC. 1981. Constraints on knowledge and cognitive development. Psychol Rev. 88:197–227. doi: 10.1037/0033-295X.88.3.197 [DOI] [Google Scholar]
- Kelly DJ, Quinn PC, Slater AM, et al. 2005. Three‐month‐olds, but not newborns, prefer own‐race faces. Dev Sci. 8:F31–F36. doi: 10.1111/j.1467-7687.2005.0434a.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JE, Park SH, Hong YJ, et al. 2019. Qualitative comparison of semantic memory impairment in patients with amnestic mild cognitive impairment based on β-amyloid status. J Clin Neurol. 15:27–37. doi: 10.3988/jcn.2019.15.1.27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirchberg BC, Cohen JR, Adelsky MB, et al. 2012. Semantic distance abnormalities in mild cognitive impairment: their nature and relationship to function. Am J Psychiatry. 169:1275–1283. doi: 10.1176/appi.ajp.2012.12030383 [DOI] [PubMed] [Google Scholar]
- Laws KR, Adlington RL, Gale TM, et al. 2007. A meta-analytic review of category naming in Alzheimer’s disease. Neuropsychologia. 45:2674–2682. doi: 10.1016/j.neuropsychologia.2007.04.003 [DOI] [PubMed] [Google Scholar]
- Laws KR, Crawford JR, Gnoato F, et al. 2007. A predominance of category deficits for living things in Alzheimer’s disease and Lewy body dementia. J Int Neuropsychol Soc. 13:401–409. doi: 10.1017/S1355617707070610 [DOI] [PubMed] [Google Scholar]
- Lesourd M, Servant M, Baumard J, et al. 2021. Semantic and action tool knowledge in the brain: identifying common and distinct networks. Neuropsychologia. 159:107918. doi: 10.1016/j.neuropsychologia.2021.107918 [DOI] [PubMed] [Google Scholar]
- Liberman Z, Woodward AL, Kinzler KD. 2017. The origins of social categorization. Trends Cogn Sci. 21:556–568. doi: 10.1016/j.tics.2017.04.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liénard P, Boyer P. 2006. Whence collective rituals? A cultural selection model of ritualized behavior. Am Anthropol. 108:814–827. doi: 10.1525/aa.2006.108.4.814 [DOI] [Google Scholar]
- Lounsbury FG. 1956. A semantic analysis of the Pawnee kinship usage. Language. 32:158–194. doi: 10.2307/410664 [DOI] [Google Scholar]
- Luzzatti C, Mauri I, Castiglioni S, et al. 2020. Evaluating semantic knowledge through a semantic association task in individuals with dementia. Am J Alzheimers Dis Other Demen. 35:1533317520917294. doi: 10.1177/1533317520917294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magri C, Konkle T, Caramazza A. 2021. The contribution of object size, manipulability, and stability on neural responses to inanimate objects. Neuroimage. 237:118098. doi: 10.1016/j.neuroimage.2021.118098 [DOI] [PubMed] [Google Scholar]
- Mahon BZ, Caramazza A. 2011. What drives the organization of object knowledge in the brain? Trends Cogn Sci. 15:97–103. doi: 10.1016/j.tics.2011.01.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahon BZ, Anzellotti S, Schwarzbach J, et al. 2009. Category-specific organization in the human brain does not require visual experience. Neuron. 63:397–405. doi: 10.1016/j.neuron.2009.07.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mårdh S, Nägga K, Samuelsson S. 2013. A longitudinal study of semantic memory impairment in patients with Alzheimer’s disease. Cortex. 49:528–533. doi: 10.1016/j.cortex.2012.02.004 [DOI] [PubMed] [Google Scholar]
- Markman EM. 1989. Categorization and Naming in Children: Problems of Induction. Cambridge, Massachusetts: MIT Press. [Google Scholar]
- Markman EM. 1990. Constraints children place on word meanings. Cogn Sci. 14:57–77. doi: 10.1016/0364-0213(90)90026-S [DOI] [Google Scholar]
- Martin A, Fedio P. 1983. Word production and comprehension in Alzheimer's disease: the breakdown of semantic knowledge. Brain Lang. 19:124–141. doi: 10.1016/0093-934x(83)90059-7 [DOI] [PubMed] [Google Scholar]
- Masters CL, Bateman R, Blennow K, et al. 2015. Alzheimer’s disease. Nat Rev Dis Primers. 1:15056. doi: 10.1038/nrdp.2015.56 [DOI] [PubMed] [Google Scholar]
- McCarthy RA, Warrington EK. 2016. Past, present, and prospects: reflections 40 years on from the selective impairment of semantic memory (Warrington, 1975. Q J Exp Psychol (Hove). 69:1941–1968. doi: 10.1080/17470218.2014.980280 [DOI] [PubMed] [Google Scholar]
- McGee RJ, Warms RL. 2017. Structure, language, and cognition McGee RJ, Warms RL, eds. Anthropological Theory: An Introduction History, 6th ed. Lanham, Maryland: Rowman & Littlefield; 339–343. [Google Scholar]
- McKhann GM, Knopman DS, Chertkow H, et al. 2011. The diagnosis of dementia due to Alzheimer’s disease: recommendations from the National Institute on Aging–Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement. 7:263–269. doi: 10.1016/j.jalz.2011.03.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medin DL, Atran S. 1999. Folkbiology. Cambridge, Massachusetts: MIT Press. [Google Scholar]
- Medin DL, Atran S. 2004. The native mind: biological categorization and reasoning in development and across cultures. Psychol Rev. 111:960–983. doi: 10.1037/0033-295X.111.4.960 [DOI] [PubMed] [Google Scholar]
- Nasreddine ZS, Phillips NA, Bédirian V, et al. 2005. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc. 53:695–699. doi: 10.1111/j.1532-5415.2005.53221.x [DOI] [PubMed] [Google Scholar]
- Nelson HE, Willison J. 1991. National Adult Reading Test (NART). Windsor, UK: NFER-Nelson. [Google Scholar]
- Papp KV, Mormino EC, Amariglio RE, et al. 2016. Biomarker validation of a decline in semantic processing in preclinical Alzheimer’s disease. Neuropsychology. 30:624–630. doi: 10.1037/neu0000246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pauker K, Williams A, Steele JR. 2016. Children's racial categorization in context. Child Dev Perspect. 10:33–38. doi: 10.1111/cdep.12155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perneczky R, Wagenpfeil S, Komossa K, et al. 2006. Mapping scores onto stages: mini-mental state examination and clinical dementia rating. Am J Geriatr Psychiatry. 14:139–144. doi: 10.1097/01.JGP.0000192478.82189.a8 [DOI] [PubMed] [Google Scholar]
- Perri R, Zannino G, Caltagirone C, et al. 2012. Alzheimer’s disease and semantic deficits: a feature-listing study. Neuropsychology. 26:652–663. doi: 10.1037/a0029302 [DOI] [PubMed] [Google Scholar]
- Quinn PC. 2010. Born to categorize. In: Goswani U, ed. The Wiley-Blackwell Handbook of Childhood Cognitive Development, 2nd ed. Hoboken, New Jersey: Wiley-Blackwell; 129–152. doi: 10.1002/9781444325485.ch5 [DOI] [Google Scholar]
- Rakison DH, Benton DT, Dinh PN. 2022. Infant categorization. In: Houdé O, Borst G, eds. The Cambridge Handbook of Cognitive Development. Cambridge, United Kingdom: Cambridge University Press; 195–215. [Google Scholar]
- Roalf DR, Moberg PJ, Xie SX, et al. 2013. Comparative accuracies of two common screening instruments for classification of Alzheimer's disease, mild cognitive impairment, and healthy aging. Alzheimers Dement. 9:529–537. doi: 10.1016/j.jalz.2012.10.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins MC, Nolan JM, Chen D. 2017. An improved measure of cognitive salience in free listing tasks: a Marshallese example. Field Methods. 29:395–403. doi: 10.1177/1525822X17726726 [DOI] [Google Scholar]
- Rogers SL, Friedman RB. 2008. The underlying mechanisms of semantic memory loss in Alzheimer’s disease and semantic dementia. Neuropsychologia. 46:12–21. doi: 10.1016/j.neuropsychologia.2007.08.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roll EE, Giovannetti T, Libon DJ, et al. 2019. Everyday task knowledge and everyday function in dementia. J Neuropsychol. 13:96–120. doi: 10.1111/jnp.12135 [DOI] [PubMed] [Google Scholar]
- Rosch E. 1978. Principles of categorizations. In: Rosch E, Lloyd BB, eds. Cognition and Categorization. Mahwah, New Jersey: Lawrence Erlbaum; 27–48. [Google Scholar]
- Rossetti HC, Lacritz LH, Cullum CM, et al. 2011. Normative data for the Montreal Cognitive Assessment (MoCA) in a population-based sample. Neurology. 77:1272–1275. doi: 10.1212/WNL.0b013e318230208a [DOI] [PubMed] [Google Scholar]
- Salmon DP, Butters N, Chan AS. 1999. The deterioration of semantic memory in Alzheimer’s disease. Can J Exp Psychol. 53:108–117. doi: 10.1037/h0087303 [DOI] [PubMed] [Google Scholar]
- Schalley AC, Zaefferer D. 2007. Ontolinguistics: How Ontological Status Shapes the Linguistic Coding of Concepts. Berlin, Germany: Walter de Gruyter; 10.1515/9783110197792 [DOI] [Google Scholar]
- Sclan SG, Reisberg B. 1992. Functional assessment staging (FAST) in Alzheimer's disease: reliability, validity, and ordinality. Int Psychogeriatr. 4:55–69. doi: 10.1017/s1041610292001157 [DOI] [PubMed] [Google Scholar]
- Silveri MC, Ciccarelli N. 2009. Semantic memory in object use. Neuropsychologia. 47:2634–2641. doi: 10.1016/j.neuropsychologia.2009.05.013 [DOI] [PubMed] [Google Scholar]
- Silveri MC, Daniele A, Giustolisi L, et al. 1991. Dissociation between knowledge of living and nonliving things in dementia of the Alzheimer type. Neurology. 41:545–546. doi: 10.1212/wnl.41.4.545 [DOI] [PubMed] [Google Scholar]
- Simoes Loureiro I, Lefebvre L. 2016. Retrogenesis of semantic knowledge: comparative approach of acquisition and deterioration of concepts in semantic memory. Neuropsychology. 30:853–859. doi: 10.1037/neu0000272 [DOI] [PubMed] [Google Scholar]
- Snowden JS. 2015. Semantic memory. In: Wright JD, ed. International Encyclopedia of the Social & Behavioral Sciences, 2nd ed. Amsterdam, Netherlands: Elsevier; 572–578. [Google Scholar]
- Sperber D. 1994. The modularity of thought and the epidemiology of representations. In: Hirschfeld LA, Gelman SA, eds. Mapping the Mind: Domain Specificity in Cognition and Culture. Cambridge, United Kingdom: Cambridge University Press; 39–67. doi: 10.1017/CBO9780511752902.003 [DOI] [Google Scholar]
- Strauss E, Sherman EM, Spreen O. 2006. A Compendium of Neuropsychological Tests: Administration, Norms, and Commentary, 3rd ed. Oxford, United Kingdom: Oxford University Press. [Google Scholar]
- Sturtevant WC. 1964. Studies in ethnoscience. Am Anthropol. 66:99–131. doi: 10.1525/aa.1964.66.3.02a00850 [DOI] [Google Scholar]
- Taler V, Phillips NA. 2008. Language performance in Alzheimer’s disease and mild cognitive impairment: a comparative review. J Clin Exp Neuropsychol. 30:501–556. doi: 10.1080/13803390701550128 [DOI] [PubMed] [Google Scholar]
- Tulving E. 1972. Episodic and semantic memory. In: Tulving E, Donaldson W, eds. Organization of Memory. Cambridge, Massachusetts: Academic Press; 382–402. [Google Scholar]
- Ulep MG, Saraon SK, McLea S. 2018. Alzheimer disease. J Nurse Pract. 14:129–135. doi: 10.1016/j.nurpra.2017.10.014 [DOI] [Google Scholar]
- Venneri A, Mitolo M, Beltrachini L, et al. 2019. Beyond episodic memory: semantic processing as independent predictor of hippocampal/perirhinal volume in aging and mild cognitive impairment due to Alzheimer’s disease. Neuropsychology. 33:523–533. doi: 10.1037/neu0000534 [DOI] [PubMed] [Google Scholar]
- Verma M, Howard RJ. 2012. Semantic memory and language dysfunction in early Alzheimer's disease: a review. Int J Geriatr Psychiatry. 27:1209–1217. doi: 10.1002/gps.3766 [DOI] [PubMed] [Google Scholar]
- Vonk JM, Bouteloup V, Mangin JF, et al. 2020. Semantic loss marks early Alzheimer’s disease‐related neurodegeneration in older adults without dementia. Alzheimers Dement (Amst). 12:e12066. doi: 10.1002/dad2.12066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warrington EK. 1975. The selective impairment of semantic memory. Q J Exp Psychol. 27:635–657. doi: 10.1080/14640747508400525 [DOI] [PubMed] [Google Scholar]
- Warrington EK, McCarthy R. 1983. Category specific access dysphasia. Brain. 106:859–878. doi: 10.1093/brain/106.4.859 [DOI] [PubMed] [Google Scholar]
- Warrington EK, McCarthy RA. 1987. Categories of knowledge: further fractionations and an attempted integration. Brain. 110:1273–1296. doi: 10.1093/brain/110.5.1273 [DOI] [PubMed] [Google Scholar]
- Warrington EK, Shallice T. 1984. Category specific semantic impairments. Brain. 107:829–854. doi: 10.1093/brain/107.3.829 [DOI] [PubMed] [Google Scholar]
- Waxman S, Gelman R. 1986. Preschoolers’ use of superordinate relations in classification and language. Cogn Dev. 1:139–156. doi: 10.1016/S0885-2014(86)80016-8 [DOI] [Google Scholar]
- Waxman SR. 1999. The dubbing ceremony revisited: object naming and categorization in infancy and early childhood. In: Medin DL, Atran S, eds. Folkbiology. Cambridge, Massachusetts: MIT Press; 233–284. [Google Scholar]
- Weintraub S, Wicklund AH, Salmon DP. 2012. The neuropsychological profile of Alzheimer disease. Cold Spring Harb Perspect Med. 2:a006171. doi: 10.1101/cshperspect.a006171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weller SC. 2007. Cultural consensus theory: applications and frequently asked questions. Field Method. 19:339–368. doi: 10.1177/1525822X07303502 [DOI] [Google Scholar]
- Weller SC, Romney AK. 1988. Systematic Data Collection. New York, New York: Sage. [Google Scholar]
- Weller SC, Vickers B, Bernard HR, et al. 2018. Open-ended interview questions and saturation. PLoS One. 13:e0198606. doi: 10.1371/journal.pone.0198606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wencelius J, Garine E, Raimond C, et al. FLARES. 2017. Accessed May 16, 2022. www.anthrocogs.com/shiny/flares/ [Google Scholar]
- Whatmough C, Chertkow H, Murtha S, et al. 2003. The semantic category effect increases with worsening anomia in Alzheimer’s type dementia. Brain Lang. 84:134–147. doi: 10.1016/s0093-934x(02)00524-2 [DOI] [PubMed] [Google Scholar]
- Wisniewski EJ, Murphy GL. 1989. Superordinate and basic category names in discourse: a textual analysis. Discourse Process. 12:245–261. doi: 10.1080/01638538909544728 [DOI] [Google Scholar]
- World Health Organization . 2021. Global Status Report on the Public Health Response to Dementia [online report]. Accessed May 16, 2022. https://www.who.int/publications/i/item/9789240033245
- Zaitchik D, Solomon GE. 2008. Animist thinking in the elderly and in patients with Alzheimer’s disease. Cogn Neuropsychol. 25:27–37. doi: 10.1080/02643290801904059 [DOI] [PubMed] [Google Scholar]
- Zannino GD, Caltagirone C, Carlesimo GA. 2015. The contribution of neurodegenerative diseases to the modelling of semantic memory: a new proposal and a review of the literature. Neuropsychologia. 75:274–290. doi: 10.1016/j.neuropsychologia.2015.06.023 [DOI] [PubMed] [Google Scholar]
- Zannino GD, Perri R, Pasqualetti P, et al. 2006. (Category-specific) semantic deficit in Alzheimer's patients: the role of semantic distance. Neuropsychologia. 44:52–61. doi: 10.1016/j.neuropsychologia.2005.04.008 [DOI] [PubMed] [Google Scholar]