Abstract
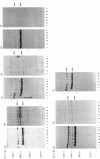
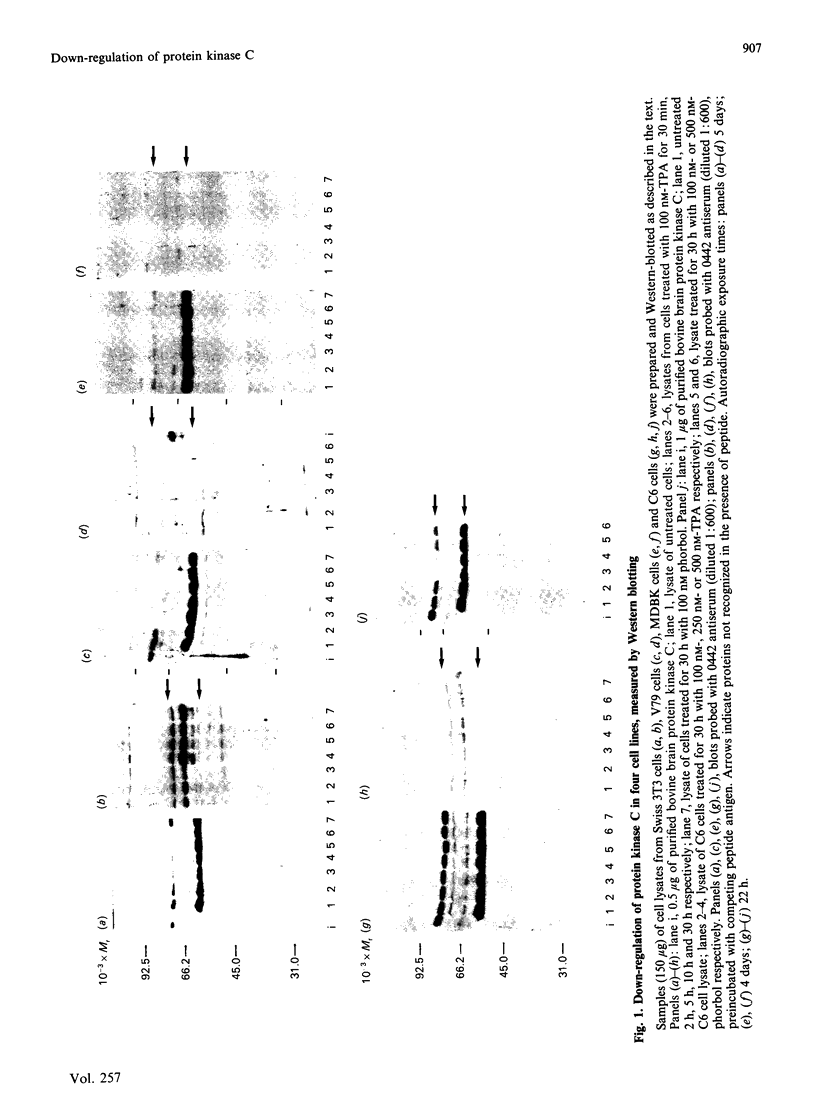
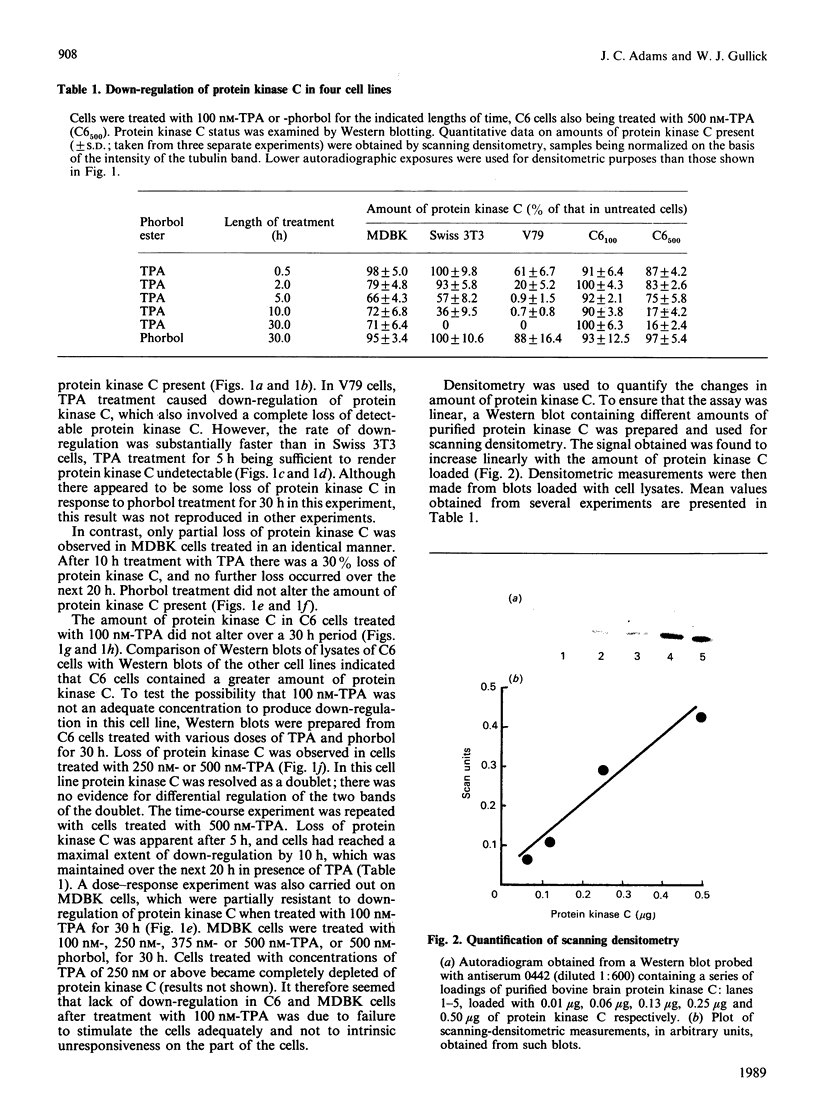
Down-regulation of protein kinase C induced by 12-O-tetradecanoylphorbol 13-acetate (TPA) was examined in Swiss 3T3, V79, MDBK and C6 cells by Western blotting. Variations in the rate of down-regulation caused by treatment with 100 nM-TPA were observed; TPA treatment for 5 h caused maximal down-regulation in V79 cells, whereas TPA treatment for 10 h or 30 h was needed for maximal down-regulation of protein kinase C in MDBK or Swiss 3T3 cells respectively. The decrease in amount of immunologically detectable protein kinase C was 30% in MDBK cells and 100% in V79 and Swiss 3T3 cells. MDBK and C6 cells could be completely depleted of protein kinase C by treatment with 250 nM-TPA. In C6 cells, after treatment with 500 nM-TPA, an 80% loss of protein kinase C was seen over 10 h. Measurement of the numbers of phorbol-ester-binding sites remaining in each cell line when protein kinase C was maximally down-regulated indicated that in MDBK and Swiss 3T3 cells loss of phorbol-ester-binding sites paralleled loss of protein kinase C, whereas in V79 and C6 cells no such correlation was observed.
Full text
PDF



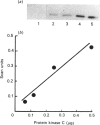
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ashendel C. L., Staller J. M., Boutwell R. K. Protein kinase activity associated with a phorbol ester receptor purified from mouse brain. Cancer Res. 1983 Sep;43(9):4333–4337. [PubMed] [Google Scholar]
- Ballester R., Rosen O. M. Fate of immunoprecipitable protein kinase C in GH3 cells treated with phorbol 12-myristate 13-acetate. J Biol Chem. 1985 Dec 5;260(28):15194–15199. [PubMed] [Google Scholar]
- Batteiger B., Newhall W. J., 5th, Jones R. B. The use of Tween 20 as a blocking agent in the immunological detection of proteins transferred to nitrocellulose membranes. J Immunol Methods. 1982 Dec 30;55(3):297–307. doi: 10.1016/0022-1759(82)90089-8. [DOI] [PubMed] [Google Scholar]
- Blackshear P. J., Witters L. A., Girard P. R., Kuo J. F., Quamo S. N. Growth factor-stimulated protein phosphorylation in 3T3-L1 cells. Evidence for protein kinase C-dependent and -independent pathways. J Biol Chem. 1985 Oct 25;260(24):13304–13315. [PubMed] [Google Scholar]
- Blumberg P. M. In vitro studies on the mode of action of the phorbol esters, potent tumor promoters: part 1. Crit Rev Toxicol. 1980 Dec;8(2):153–197. doi: 10.3109/10408448009037493. [DOI] [PubMed] [Google Scholar]
- Borner C., Eppenberger U., Wyss R., Fabbro D. Continuous synthesis of two protein-kinase-C-related proteins after down-regulation by phorbol esters. Proc Natl Acad Sci U S A. 1988 Apr;85(7):2110–2114. doi: 10.1073/pnas.85.7.2110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Chida K., Kato N., Kuroki T. Down regulation of phorbol diester receptors by proteolytic degradation of protein kinase C in a cultured cell line of fetal rat skin keratinocytes. J Biol Chem. 1986 Oct 5;261(28):13013–13018. [PubMed] [Google Scholar]
- Coussens L., Parker P. J., Rhee L., Yang-Feng T. L., Chen E., Waterfield M. D., Francke U., Ullrich A. Multiple, distinct forms of bovine and human protein kinase C suggest diversity in cellular signaling pathways. Science. 1986 Aug 22;233(4766):859–866. doi: 10.1126/science.3755548. [DOI] [PubMed] [Google Scholar]
- Driedger P. E., Blumberg P. M. Specific binding of phorbol ester tumor promoters. Proc Natl Acad Sci U S A. 1980 Jan;77(1):567–571. doi: 10.1073/pnas.77.1.567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunphy W. G., Delclos K. B., Blumberg P. M. Characterization of specific binding of [3H]phorbol 12,13-dibutyrate and [3H]phorbol 12-myristate 13-acetate to mouse brain. Cancer Res. 1980 Oct;40(10):3635–3641. [PubMed] [Google Scholar]
- Fournier A., Murray A. W. Application of phorbol ester to mouse skin causes a rapid and sustained loss of protein kinase C. Nature. 1987 Dec 24;330(6150):767–769. doi: 10.1038/330767a0. [DOI] [PubMed] [Google Scholar]
- Glenney J. Antibody probing of western blots which have been stained with india ink. Anal Biochem. 1986 Aug 1;156(2):315–319. doi: 10.1016/0003-2697(86)90259-9. [DOI] [PubMed] [Google Scholar]
- Hoshijima M., Kikuchi A., Tanimoto T., Kaibuchi K., Takai Y. Formation of a phorbol ester-binding fragment from protein kinase C by proteolytic digestion. Cancer Res. 1986 Jun;46(6):3000–3004. [PubMed] [Google Scholar]
- Housey G. M., O'Brian C. A., Johnson M. D., Kirschmeier P., Weinstein I. B. Isolation of cDNA clones encoding protein kinase C: evidence for a protein kinase C-related gene family. Proc Natl Acad Sci U S A. 1987 Feb;84(4):1065–1069. doi: 10.1073/pnas.84.4.1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hovis J. G., Stumpo D. J., Halsey D. L., Blackshear P. J. Effects of mitogens on ornithine decarboxylase activity and messenger RNA levels in normal and protein kinase C-deficient NIH-3T3 fibroblasts. J Biol Chem. 1986 Aug 5;261(22):10380–10386. [PubMed] [Google Scholar]
- Huang K. P., Huang F. L. Immunochemical characterization of rat brain protein kinase C. J Biol Chem. 1986 Nov 5;261(31):14781–14787. [PubMed] [Google Scholar]
- Inoue M., Kishimoto A., Takai Y., Nishizuka Y. Studies on a cyclic nucleotide-independent protein kinase and its proenzyme in mammalian tissues. II. Proenzyme and its activation by calcium-dependent protease from rat brain. J Biol Chem. 1977 Nov 10;252(21):7610–7616. [PubMed] [Google Scholar]
- Jaken S., Tashjian A. H., Jr, Blumberg P. M. Relationship between biological responsiveness to phorbol esters and receptor levels in GH4C1 rat pituitary cells. Cancer Res. 1981 Dec;41(12 Pt 1):4956–4960. [PubMed] [Google Scholar]
- Kajikawa N., Kishimoto A., Shiota M., Nishizuka Y. Ca2+ -dependent neutral protease and proteolytic activation of Ca2+-activated, phospholipid-dependent protein kinase. Methods Enzymol. 1983;102:279–290. doi: 10.1016/s0076-6879(83)02028-5. [DOI] [PubMed] [Google Scholar]
- Kikkawa U., Takai Y., Tanaka Y., Miyake R., Nishizuka Y. Protein kinase C as a possible receptor protein of tumor-promoting phorbol esters. J Biol Chem. 1983 Oct 10;258(19):11442–11445. [PubMed] [Google Scholar]
- Knopf J. L., Lee M. H., Sultzman L. A., Kriz R. W., Loomis C. R., Hewick R. M., Bell R. M. Cloning and expression of multiple protein kinase C cDNAs. Cell. 1986 Aug 15;46(4):491–502. doi: 10.1016/0092-8674(86)90874-3. [DOI] [PubMed] [Google Scholar]
- Kraft A. S., Anderson W. B. Phorbol esters increase the amount of Ca2+, phospholipid-dependent protein kinase associated with plasma membrane. Nature. 1983 Feb 17;301(5901):621–623. doi: 10.1038/301621a0. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lee M. H., Bell R. M. The lipid binding, regulatory domain of protein kinase C. A 32-kDa fragment contains the calcium- and phosphatidylserine-dependent phorbol diester binding activity. J Biol Chem. 1986 Nov 15;261(32):14867–14870. [PubMed] [Google Scholar]
- Melloni E., Pontremoli S., Michetti M., Sacco O., Sparatore B., Salamino F., Horecker B. L. Binding of protein kinase C to neutrophil membranes in the presence of Ca2+ and its activation by a Ca2+-requiring proteinase. Proc Natl Acad Sci U S A. 1985 Oct;82(19):6435–6439. doi: 10.1073/pnas.82.19.6435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niedel J. E., Kuhn L. J., Vandenbark G. R. Phorbol diester receptor copurifies with protein kinase C. Proc Natl Acad Sci U S A. 1983 Jan;80(1):36–40. doi: 10.1073/pnas.80.1.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohno S., Kawasaki H., Imajoh S., Suzuki K., Inagaki M., Yokokura H., Sakoh T., Hidaka H. Tissue-specific expression of three distinct types of rabbit protein kinase C. Nature. 1987 Jan 8;325(7000):161–166. doi: 10.1038/325161a0. [DOI] [PubMed] [Google Scholar]
- Parker P. J., Coussens L., Totty N., Rhee L., Young S., Chen E., Stabel S., Waterfield M. D., Ullrich A. The complete primary structure of protein kinase C--the major phorbol ester receptor. Science. 1986 Aug 22;233(4766):853–859. doi: 10.1126/science.3755547. [DOI] [PubMed] [Google Scholar]
- Parker P. J., Stabel S., Waterfield M. D. Purification to homogeneity of protein kinase C from bovine brain--identity with the phorbol ester receptor. EMBO J. 1984 May;3(5):953–959. doi: 10.1002/j.1460-2075.1984.tb01913.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasti G., Lacal J. C., Warren B. S., Aaronson S. A., Blumberg P. M. Loss of mouse fibroblast cell response to phorbol esters restored by microinjected protein kinase C. 1986 Nov 27-Dec 3Nature. 324(6095):375–377. doi: 10.1038/324375a0. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Pena A., Rozengurt E. Disappearance of Ca2+-sensitive, phospholipid-dependent protein kinase activity in phorbol ester-treated 3T3 cells. Biochem Biophys Res Commun. 1984 May 16;120(3):1053–1059. doi: 10.1016/s0006-291x(84)80213-2. [DOI] [PubMed] [Google Scholar]
- Salomon D. S. Inhibition of epidermal growth factor binding to mouse embryonal carcinoma cells by phorbol esters mediated by specific phorbol ester receptors. J Biol Chem. 1981 Aug 10;256(15):7958–7966. [PubMed] [Google Scholar]
- Solanki V., Slaga T. J., Callaham M., Huberman E. Down regulation of specific binding of [20-3H]phorbol 12,13-dibutyrate and phorbol ester-induced differentiation of human promyelocytic leukemia cells. Proc Natl Acad Sci U S A. 1981 Mar;78(3):1722–1725. doi: 10.1073/pnas.78.3.1722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stabel S., Rodriguez-Pena A., Young S., Rozengurt E., Parker P. J. Quantitation of protein kinase C by immunoblot--expression in different cell lines and response to phorbol esters. J Cell Physiol. 1987 Jan;130(1):111–117. doi: 10.1002/jcp.1041300116. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchida T., Filburn C. R. Affinity chromatography of protein kinase C-phorbol ester receptor on polyacrylamide-immobilized phosphatidylserine. J Biol Chem. 1984 Oct 25;259(20):12311–12314. [PubMed] [Google Scholar]
- Woodgett J. R., Hunter T. Immunological evidence for two physiological forms of protein kinase C. Mol Cell Biol. 1987 Jan;7(1):85–96. doi: 10.1128/mcb.7.1.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young S., Parker P. J., Ullrich A., Stabel S. Down-regulation of protein kinase C is due to an increased rate of degradation. Biochem J. 1987 Jun 15;244(3):775–779. doi: 10.1042/bj2440775. [DOI] [PMC free article] [PubMed] [Google Scholar]