Abstract
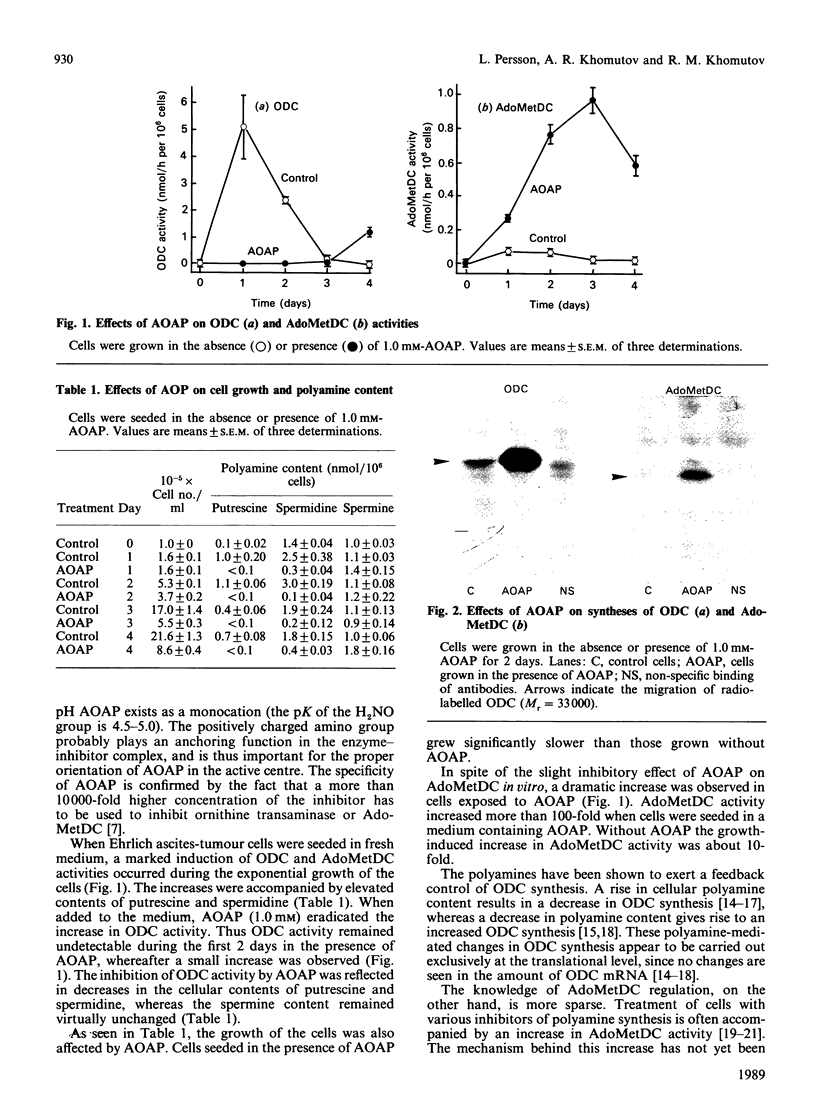
Treatment of Ehrlich ascites-tumour cells with 1-amino-oxy-3-aminopropane (AOAP), a potent inhibitor of ornithine decarboxylase, resulted in a marked decrease in cellular contents of putrescine and spermidine, concomitant with an arrest of cell growth. The activity of S-adenosylmethionine decarboxylase (AdoMetDC) was greatly increased in cells treated with AOAP. This increase in AdoMetDC activity was shown to be, at least partly, caused by enhanced synthesis of the enzyme, which most likely was induced by the change in cellular polyamine content.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alhonen-Hongisto L. Regulation of S-adenosylmethionine decarboxylase by polyamines in Ehrlich ascites-carcinoma cells grown in culture. Biochem J. 1980 Sep 15;190(3):747–754. doi: 10.1042/bj1900747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heby O. Role of polyamines in the control of cell proliferation and differentiation. Differentiation. 1981;19(1):1–20. doi: 10.1111/j.1432-0436.1981.tb01123.x. [DOI] [PubMed] [Google Scholar]
- Hölttä E., Pohjanpelto P. Control of ornithine decarboxylase in Chinese hamster ovary cells by polyamines. Translational inhibition of synthesis and acceleration of degradation of the enzyme by putrescine, spermidine, and spermine. J Biol Chem. 1986 Jul 15;261(20):9502–9508. [PubMed] [Google Scholar]
- Jänne J., Williams-Ashman H. G. Dissociation of putrescine-activated decarboxylation of S-adenosyl-L-methionine from the enzymic synthesis of spermidine and spermine by purified prostatic enzyme preparations. Biochem Biophys Res Commun. 1971 Jan 22;42(2):222–229. [PubMed] [Google Scholar]
- Kahana C., Nathans D. Translational regulation of mammalian ornithine decarboxylase by polyamines. J Biol Chem. 1985 Dec 15;260(29):15390–15393. [PubMed] [Google Scholar]
- Khomutov R. M., Denisova G. F., Khomutov A. R., Belostotskaia K. M., Shlosman R. B. Aminooksipropilamin--éffektivnyi ingibitor ornitindekarboksilazy in vitro i in vivo. Bioorg Khim. 1985 Nov;11(11):1574–1576. [PubMed] [Google Scholar]
- Mamont P. S., Joder-Ohlenbusch A. M., Nussli M., Grove J. Indirect evidence for a strict negative control of S-adenosyl-L-methionine decarboxylase by spermidine in rat hepatoma cells. Biochem J. 1981 May 15;196(2):411–422. doi: 10.1042/bj1960411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pegg A. E. Polyamine metabolism and its importance in neoplastic growth and a target for chemotherapy. Cancer Res. 1988 Feb 15;48(4):759–774. [PubMed] [Google Scholar]
- Pegg A. E. Recent advances in the biochemistry of polyamines in eukaryotes. Biochem J. 1986 Mar 1;234(2):249–262. doi: 10.1042/bj2340249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pegg A. E., Wechter R., Pajunen A. Increase in S-adenosylmethionine decarboxylase in SV-3T3 cells treated with S-methyl-5'-methylthioadenosine. Biochem J. 1987 May 15;244(1):49–54. doi: 10.1042/bj2440049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pegg A. E., Williams-Ashman H. G. On the role of S-adenosyl-L-methionine in the biosynthesis of spermidine by rat prostate. J Biol Chem. 1969 Feb 25;244(4):682–693. [PubMed] [Google Scholar]
- Persson L. Antibodies to ornithine decarboxylase. Immunochemical cross-reactivity. Acta Chem Scand B. 1982;36(10):685–688. doi: 10.3891/acta.chem.scand.36b-0685. [DOI] [PubMed] [Google Scholar]
- Persson L., Holm I., Heby O. Regulation of ornithine decarboxylase mRNA translation by polyamines. Studies using a cell-free system and a cell line with an amplified ornithine decarboxylase gene. J Biol Chem. 1988 Mar 5;263(7):3528–3533. [PubMed] [Google Scholar]
- Persson L., Holm I., Heby O. Translational regulation of ornithine decarboxylase by polyamines. FEBS Lett. 1986 Sep 15;205(2):175–178. doi: 10.1016/0014-5793(86)80892-4. [DOI] [PubMed] [Google Scholar]
- Persson L., Oredsson S. M., Anehus S., Heby O. Ornithine decarboxylase inhibitors increase the cellular content of the enzyme: implications for translational regulation. Biochem Biophys Res Commun. 1985 Aug 30;131(1):239–245. doi: 10.1016/0006-291x(85)91794-2. [DOI] [PubMed] [Google Scholar]
- Persson L., Seely J. E., Pegg A. E. Investigation of structure and rate of synthesis of ornithine decarboxylase protein in mouse kidney. Biochemistry. 1984 Jul 31;23(16):3777–3783. doi: 10.1021/bi00311a033. [DOI] [PubMed] [Google Scholar]
- Pösö H., Pegg A. E. Comparison of S-adenosylmethionine decarboxylases from rat liver and muscle. Biochemistry. 1982 Jun 22;21(13):3116–3122. doi: 10.1021/bi00256a013. [DOI] [PubMed] [Google Scholar]
- Shirahata A., Pegg A. E. Increased content of mRNA for a precursor of S-adenosylmethionine decarboxylase in rat prostate after treatment with 2-difluoromethylornithine. J Biol Chem. 1986 Oct 15;261(29):13833–13837. [PubMed] [Google Scholar]
- Shirahata A., Pegg A. E. Regulation of S-adenosylmethionine decarboxylase activity in rat liver and prostate. J Biol Chem. 1985 Aug 15;260(17):9583–9588. [PubMed] [Google Scholar]
- Tabor C. W., Tabor H. Polyamines. Annu Rev Biochem. 1984;53:749–790. doi: 10.1146/annurev.bi.53.070184.003533. [DOI] [PubMed] [Google Scholar]