Abstract
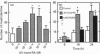
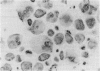
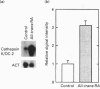
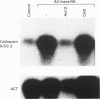
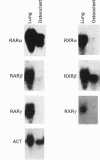
Vitamin A metabolites such as all-trans-retinoic acid (all-trans-RA) affect several steps of metabolic processes in vertebrates. In the last few years, several studies have shown the effect of RA on bone formation and metabolism. However, mechanisms of its action still remain unclear, especially with respect to the regulation of bone cells. Therefore, this study was carried out to clarify how RA regulates the activity of osteoclasts. Using a pit assay involving unfractionated bone cells, including osteoclasts obtained from rabbits, we found that RA stimulated an increase in the bone-resorbing activity in a dose- and time-dependent manner. Furthermore, this effect occurred more rapidly than that of treatments with 1 alpha,25-dihydroxyvitamin D3. However, this effect of RA may be partly related to cross-talk between osteoclasts and other types of cells. Therefore we studied the effect of RA on isolated osteoclasts. We found that all-trans-RA regulates the gene expression of cathepsin K/OC-2, a dominant cysteine proteinase, at the transcriptional level in mature osteoclasts isolated from rabbits. Moreover, retinoic acid-receptor alpha mRNA and retinoid X-receptor beta mRNA were expressed in these mature osteoclasts. Our results indicate that osteoclasts are target cells for RA and that RA might regulate a part of bone formation and metabolism through osteoclasts.
Full text
PDF
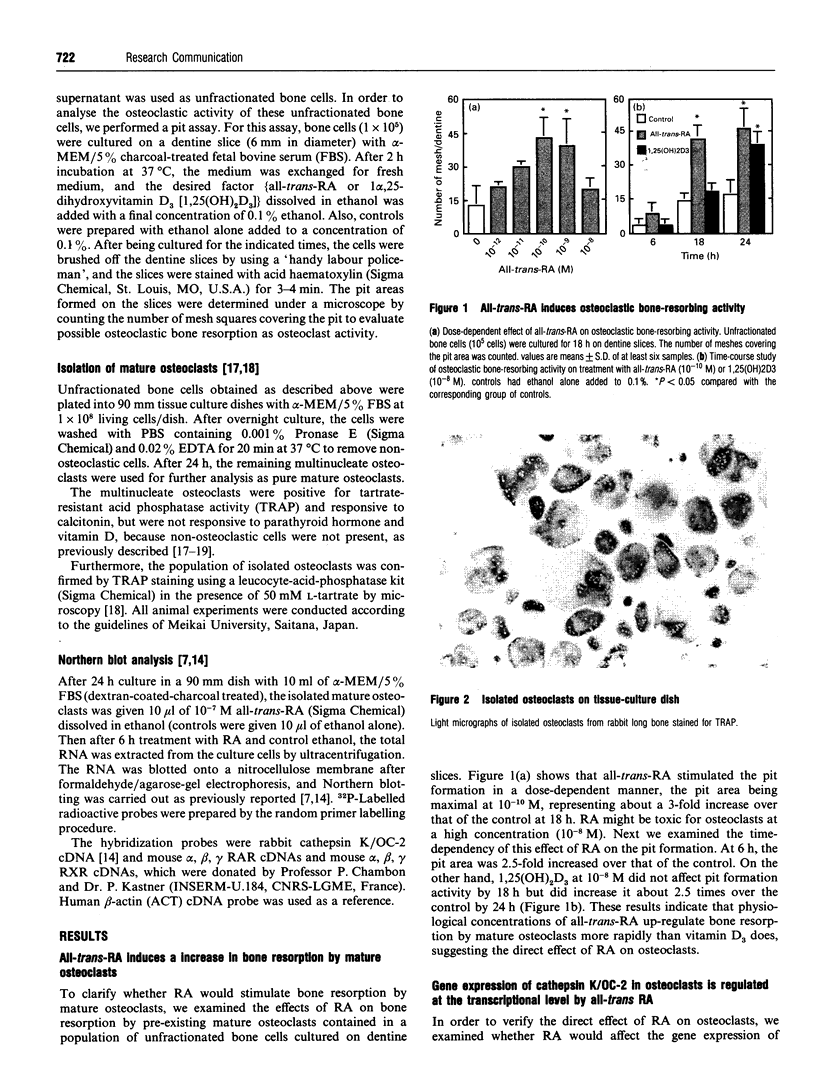


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Breitman T. R., Selonick S. E., Collins S. J. Induction of differentiation of the human promyelocytic leukemia cell line (HL-60) by retinoic acid. Proc Natl Acad Sci U S A. 1980 May;77(5):2936–2940. doi: 10.1073/pnas.77.5.2936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickson I., Walls J. Vitamin A and bone formation. Effect of an excess of retinol on bone collagen synthesis in vitro. Biochem J. 1985 Mar 15;226(3):789–795. doi: 10.1042/bj2260789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hata K., Kukita T., Akamine A., Kukita A., Kurisu K. Trypsinized osteoclast-like multinucleated cells formed in rat bone marrow cultures efficiently form resorption lacunae on dentine. Bone. 1992;13(2):139–146. doi: 10.1016/8756-3282(92)90003-f. [DOI] [PubMed] [Google Scholar]
- Hayes K. C., Nielsen S. W., Eaton H. D. Pathogenesis of the optic nerve lesion in vitamin A-deficient calves. Arch Ophthalmol. 1968 Dec;80(6):777–787. doi: 10.1001/archopht.1968.00980050779019. [DOI] [PubMed] [Google Scholar]
- Hough S., Avioli L. V., Muir H., Gelderblom D., Jenkins G., Kurasi H., Slatopolsky E., Bergfeld M. A., Teitelbaum S. L. Effects of hypervitaminosis A on the bone and mineral metabolism of the rat. Endocrinology. 1988 Jun;122(6):2933–2939. doi: 10.1210/endo-122-6-2933. [DOI] [PubMed] [Google Scholar]
- Inaoka T., Bilbe G., Ishibashi O., Tezuka K., Kumegawa M., Kokubo T. Molecular cloning of human cDNA for cathepsin K: novel cysteine proteinase predominantly expressed in bone. Biochem Biophys Res Commun. 1995 Jan 5;206(1):89–96. doi: 10.1006/bbrc.1995.1013. [DOI] [PubMed] [Google Scholar]
- Jetten A. M., Jetten M. E., Sherman M. I. Stimulation of differentiation of several murine embryonal carcinoma cell lines by retinoic acid. Exp Cell Res. 1979 Dec;124(2):381–391. doi: 10.1016/0014-4827(79)90213-1. [DOI] [PubMed] [Google Scholar]
- Kaji H., Sugimoto T., Miyauchi A., Fukase M., Tezuka K., Hakeda Y., Kumegawa M., Chihara K. Calcitonin inhibits osteopontin mRNA expression in isolated rabbit osteoclasts. Endocrinology. 1994 Jul;135(1):484–487. doi: 10.1210/endo.135.1.8013390. [DOI] [PubMed] [Google Scholar]
- Kindmark A., Törmä H., Johansson A., Ljunghall S., Melhus H. Reverse transcription-polymerase chain reaction assay demonstrates that the 9-cis retinoic acid receptor alpha is expressed in human osteoblasts. Biochem Biophys Res Commun. 1993 May 14;192(3):1367–1372. doi: 10.1006/bbrc.1993.1567. [DOI] [PubMed] [Google Scholar]
- Lohnes D., Mark M., Mendelsohn C., Dollé P., Dierich A., Gorry P., Gansmuller A., Chambon P. Function of the retinoic acid receptors (RARs) during development (I). Craniofacial and skeletal abnormalities in RAR double mutants. Development. 1994 Oct;120(10):2723–2748. doi: 10.1242/dev.120.10.2723. [DOI] [PubMed] [Google Scholar]
- MacDonald B. R., Takahashi N., McManus L. M., Holahan J., Mundy G. R., Roodman G. D. Formation of multinucleated cells that respond to osteotropic hormones in long term human bone marrow cultures. Endocrinology. 1987 Jun;120(6):2326–2333. doi: 10.1210/endo-120-6-2326. [DOI] [PubMed] [Google Scholar]
- Mano H., Mori R., Ozawa T., Takeyama K., Yoshizawa Y., Kojima R., Arao Y., Masushige S., Kato S. Positive and negative regulation of retinoid X receptor gene expression by thyroid hormone in the rat. Transcriptional and post-transcriptional controls by thyroid hormone. J Biol Chem. 1994 Jan 21;269(3):1591–1594. [PubMed] [Google Scholar]
- Mano H., Ozawa T., Takeyama K., Yoshizawa Y., Kojima R., Kato S., Masushige S. Thyroid hormone affects the gene expression of retinoid X receptors in the adult rat. Biochem Biophys Res Commun. 1993 Mar 31;191(3):943–949. doi: 10.1006/bbrc.1993.1308. [DOI] [PubMed] [Google Scholar]
- McSheehy P. M., Chambers T. J. 1,25-Dihydroxyvitamin D3 stimulates rat osteoblastic cells to release a soluble factor that increases osteoclastic bone resorption. J Clin Invest. 1987 Aug;80(2):425–429. doi: 10.1172/JCI113089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng K. W., Livesey S. A., Collier F., Gummer P. R., Martin T. J. Effect of retinoids on the growth, ultrastructure, and cytoskeletal structures of malignant rat osteoblasts. Cancer Res. 1985 Oct;45(10):5106–5113. [PubMed] [Google Scholar]
- O'Neill R. P., Jones S. J., Boyde A., Taylor M. L., Arnett T. R. Effect of retinoic acid on the resorptive activity of chick osteoclasts in vitro. Bone. 1992;13(1):23–27. doi: 10.1016/8756-3282(92)90357-3. [DOI] [PubMed] [Google Scholar]
- Petkovich M., Brand N. J., Krust A., Chambon P. A human retinoic acid receptor which belongs to the family of nuclear receptors. Nature. 1987 Dec 3;330(6147):444–450. doi: 10.1038/330444a0. [DOI] [PubMed] [Google Scholar]
- Scheven B. A., Hamilton N. J. Retinoic acid and 1,25-dihydroxyvitamin D3 stimulate osteoclast formation by different mechanisms. Bone. 1990;11(1):53–59. doi: 10.1016/8756-3282(90)90072-7. [DOI] [PubMed] [Google Scholar]
- Sporn M. B., Clamon G. H., Dunlop N. M., Newton D. L., Smith J. M., Saffiotti U. Activity of vitamin A analogues in cell cultures of mouse epidermis and organ cultures of hamster trachea. Nature. 1975 Jan 3;253(5486):47–50. doi: 10.1038/253047a0. [DOI] [PubMed] [Google Scholar]
- Takada Y., Kusuda M., Hiura K., Sato T., Mochizuki H., Nagao Y., Tomura M., Yahiro M., Hakeda Y., Kawashima H. A simple method to assess osteoclast-mediated bone resorption using unfractionated bone cells. Bone Miner. 1992 Jun;17(3):347–359. doi: 10.1016/0169-6009(92)90785-c. [DOI] [PubMed] [Google Scholar]
- Tezuka K., Tezuka Y., Maejima A., Sato T., Nemoto K., Kamioka H., Hakeda Y., Kumegawa M. Molecular cloning of a possible cysteine proteinase predominantly expressed in osteoclasts. J Biol Chem. 1994 Jan 14;269(2):1106–1109. [PubMed] [Google Scholar]