Abstract
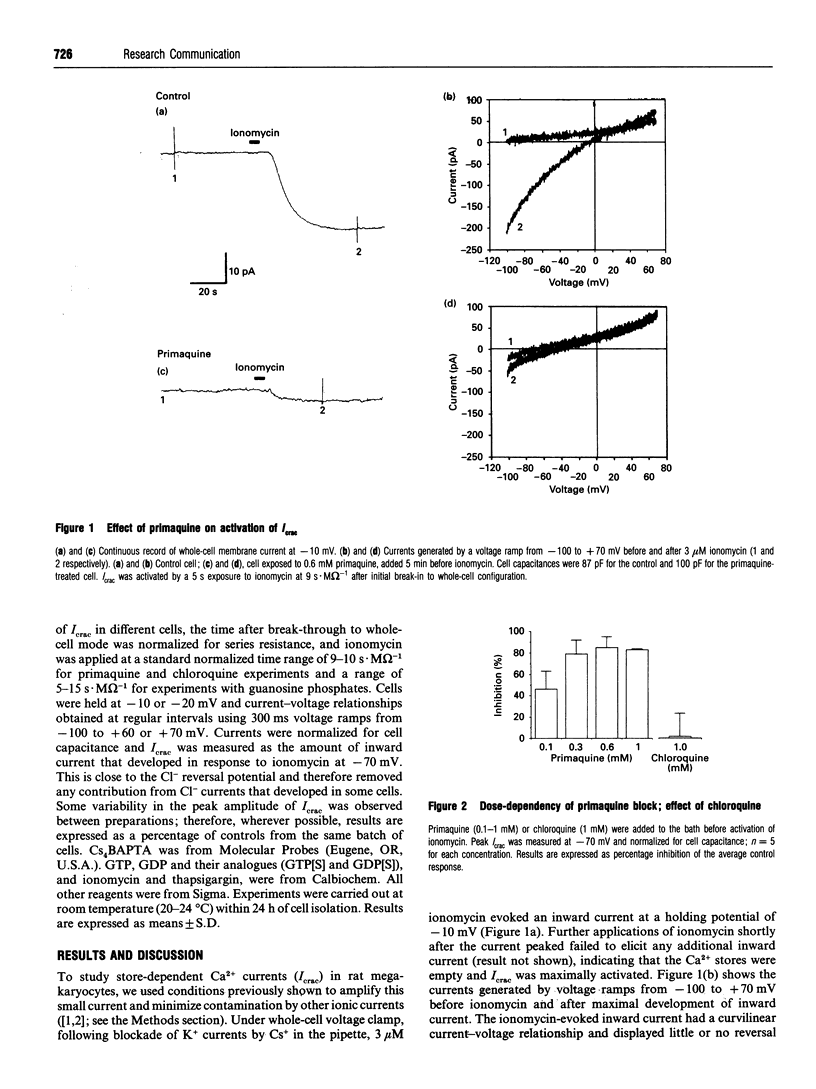
The whole-cell patch-clamp technique was used to study the effect of primaquine, an inhibitor of vesicular transport, on the calcium-release-activated current (Icrac) in rat megakaryocytes. Addition of primaquine, before emptying of internal Ca2+ stores by ionomycin, prevented the development of Icrac, with a half-maximal concentration of near 100 microM. Maximal inhibition (> or = 83%) was observed at 0.6-1 mM primaquine. At 1 mM, chloroquine, a related compound which is less effective at blocking vesicular secretion, had no effect on Icrac. Primaquine (0.8 mM) added after sustained activation of Icrac caused a gradual block of current, with maximal inhibition of 50% observed after 2-3 min. At 1 mM, internal guanosine 5'-[gamma-thio]triphosphate reduced Icrac by 65 +/- 13%. Neither 1 mM GTP nor 2 mM guanosine 5'-[beta-thio]diphosphate had any significant effect on Icrac. The recognized role of GTPases in the regulation of vesicular trafficking, together with block of Icrac activation by primaquine, provide evidence that the channels carrying Icrac may be stored in a vesicular membrane compartment and transferred to the plasma membrane following store depletion.
Full text
PDF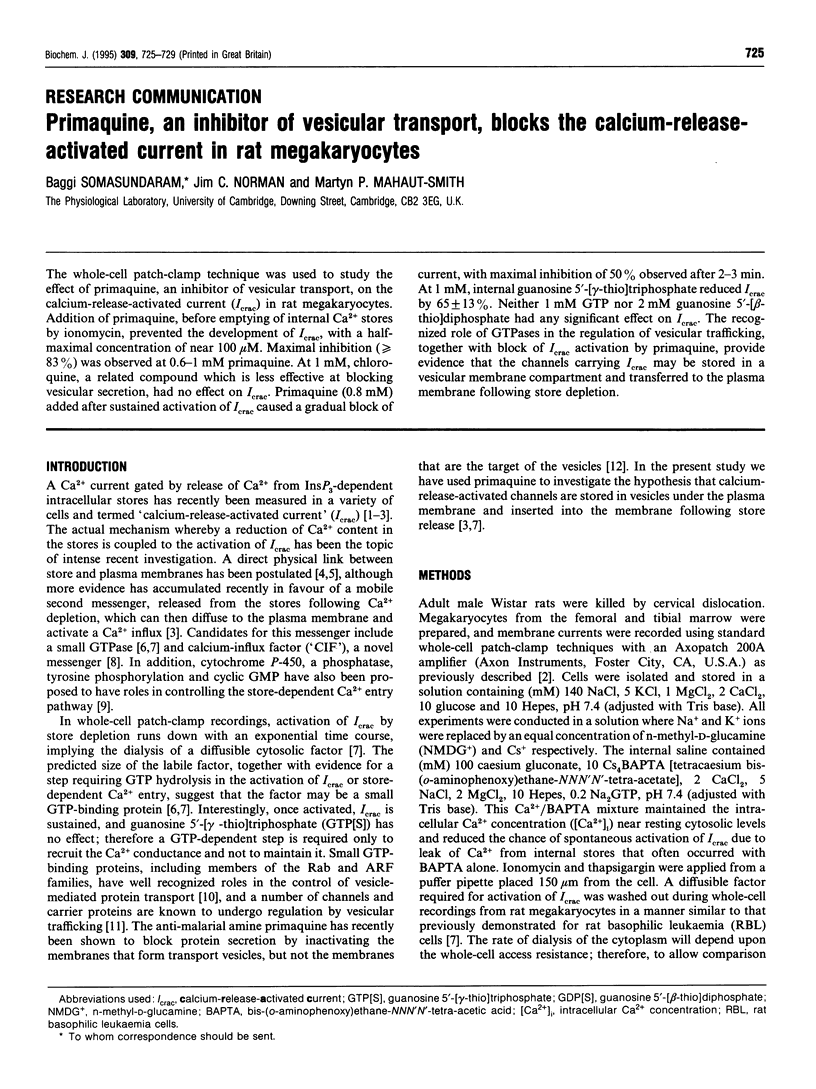



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bird G. S., Putney J. W., Jr Inhibition of thapsigargin-induced calcium entry by microinjected guanine nucleotide analogues. Evidence for the involvement of a small G-protein in capacitative calcium entry. J Biol Chem. 1993 Oct 15;268(29):21486–21488. [PubMed] [Google Scholar]
- Braulke T., Geuze H. J., Slot J. W., Hasilik A., von Figura K. On the effects of weak bases and monensin on sorting and processing of lysosomal enzymes in human cells. Eur J Cell Biol. 1987 Jun;43(3):316–321. [PubMed] [Google Scholar]
- Eckstein F., Cassel D., Levkovitz H., Lowe M., Selinger Z. Guanosine 5'-O-(2-thiodiphosphate). An inhibitor of adenylate cyclase stimulation by guanine nucleotides and fluoride ions. J Biol Chem. 1979 Oct 10;254(19):9829–9834. [PubMed] [Google Scholar]
- Fasolato C., Hoth M., Penner R. A GTP-dependent step in the activation mechanism of capacitative calcium influx. J Biol Chem. 1993 Oct 5;268(28):20737–20740. [PubMed] [Google Scholar]
- Fasolato C., Innocenti B., Pozzan T. Receptor-activated Ca2+ influx: how many mechanisms for how many channels? Trends Pharmacol Sci. 1994 Mar;15(3):77–83. doi: 10.1016/0165-6147(94)90282-8. [DOI] [PubMed] [Google Scholar]
- Harrison P. T., Davis W., Norman J. C., Hockaday A. R., Allen J. M. Binding of monomeric immunoglobulin G triggers Fc gamma RI-mediated endocytosis. J Biol Chem. 1994 Sep 30;269(39):24396–24402. [PubMed] [Google Scholar]
- Hiebsch R. R., Raub T. J., Wattenberg B. W. Primaquine blocks transport by inhibiting the formation of functional transport vesicles. Studies in a cell-free assay of protein transport through the Golgi apparatus. J Biol Chem. 1991 Oct 25;266(30):20323–20328. [PubMed] [Google Scholar]
- Hoth M., Penner R. Calcium release-activated calcium current in rat mast cells. J Physiol. 1993 Jun;465:359–386. doi: 10.1113/jphysiol.1993.sp019681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoth M., Penner R. Depletion of intracellular calcium stores activates a calcium current in mast cells. Nature. 1992 Jan 23;355(6358):353–356. doi: 10.1038/355353a0. [DOI] [PubMed] [Google Scholar]
- Irvine R. F. Inositol phosphates and Ca2+ entry: toward a proliferation or a simplification? FASEB J. 1992 Sep;6(12):3085–3091. doi: 10.1096/fasebj.6.12.1325932. [DOI] [PubMed] [Google Scholar]
- Melançon P., Glick B. S., Malhotra V., Weidman P. J., Serafini T., Gleason M. L., Orci L., Rothman J. E. Involvement of GTP-binding "G" proteins in transport through the Golgi stack. Cell. 1987 Dec 24;51(6):1053–1062. doi: 10.1016/0092-8674(87)90591-5. [DOI] [PubMed] [Google Scholar]
- Pryer N. K., Wuestehube L. J., Schekman R. Vesicle-mediated protein sorting. Annu Rev Biochem. 1992;61:471–516. doi: 10.1146/annurev.bi.61.070192.002351. [DOI] [PubMed] [Google Scholar]
- Putney J. W., Jr A model for receptor-regulated calcium entry. Cell Calcium. 1986 Feb;7(1):1–12. doi: 10.1016/0143-4160(86)90026-6. [DOI] [PubMed] [Google Scholar]
- Randriamampita C., Tsien R. Y. Emptying of intracellular Ca2+ stores releases a novel small messenger that stimulates Ca2+ influx. Nature. 1993 Aug 26;364(6440):809–814. doi: 10.1038/364809a0. [DOI] [PubMed] [Google Scholar]
- Sargeant P., Sage S. O. Calcium signalling in platelets and other nonexcitable cells. Pharmacol Ther. 1994;64(3):395–443. doi: 10.1016/0163-7258(94)90019-1. [DOI] [PubMed] [Google Scholar]
- Schwartz A. L., Bolognesi A., Fridovich S. E. Recycling of the asialoglycoprotein receptor and the effect of lysosomotropic amines in hepatoma cells. J Cell Biol. 1984 Feb;98(2):732–738. doi: 10.1083/jcb.98.2.732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sewell J. L., Kahn R. A. Sequences of the bovine and yeast ADP-ribosylation factor and comparison to other GTP-binding proteins. Proc Natl Acad Sci U S A. 1988 Jul;85(13):4620–4624. doi: 10.1073/pnas.85.13.4620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somasundaram B., Mahaut-Smith M. P. Three cation influx currents activated by purinergic receptor stimulation in rat megakaryocytes. J Physiol. 1994 Oct 15;480(Pt 2):225–231. doi: 10.1113/jphysiol.1994.sp020355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strous G. J., Du Maine A., Zijderhand-Bleekemolen J. E., Slot J. W., Schwartz A. L. Effect of lysosomotropic amines on the secretory pathway and on the recycling of the asialoglycoprotein receptor in human hepatoma cells. J Cell Biol. 1985 Aug;101(2):531–539. doi: 10.1083/jcb.101.2.531. [DOI] [PMC free article] [PubMed] [Google Scholar]


