Abstract
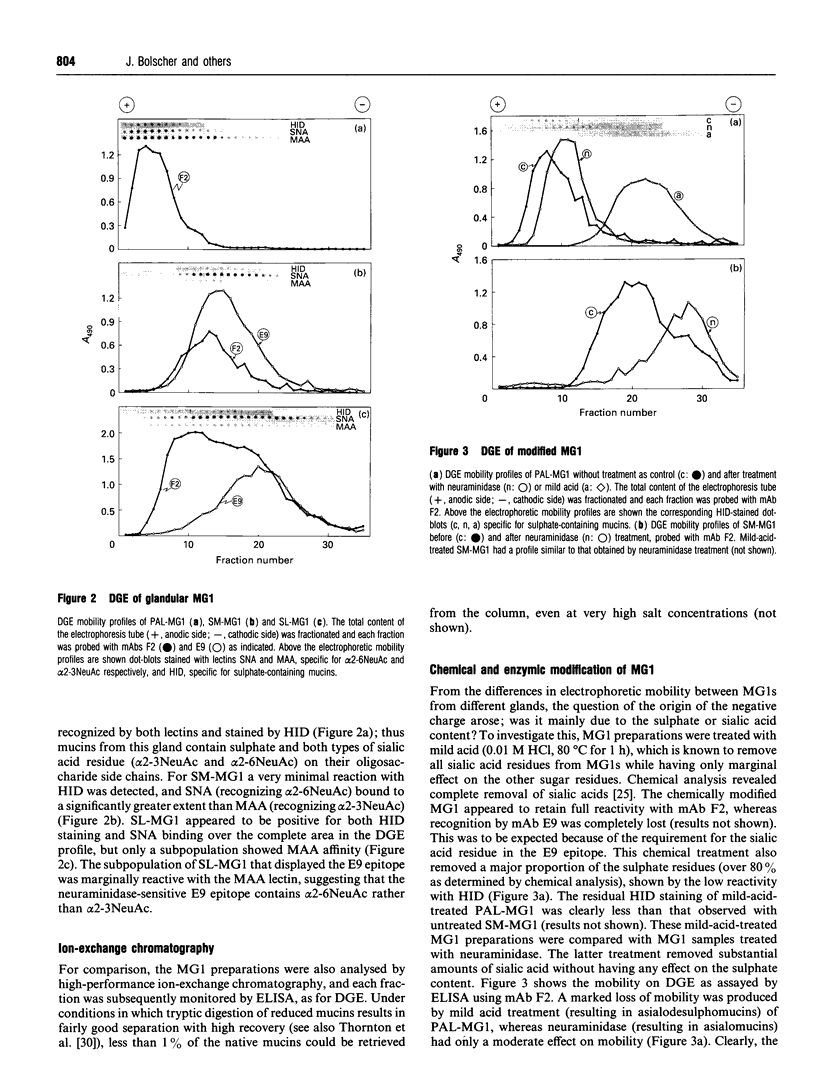
High-M(r) mucins [mucin glycoprotein 1 (MG1)] isolated from human saliva from the individual salivary glands were chemically characterized. The carbohydrate content of MG1 derived from palatal (PAL), submandibular (SM) and sublingual (SL) saliva was typical of mucins but showed heterogeneity, especially in the amount of sialic acid and sulphated sugar residues. The physicochemical properties of native MG1s make conventional SDS/PAGE and ion-exchange chromatography unsuitable for investigating differences between individual samples. Recently a density-gradient electrophoresis (DGE) device has been developed, primarily for separation based on the charge of entire cells or cell organelles [Tulp, Verwoerd and Pieters (1993) Electrophoresis 14, 1295-1301]. We have used this apparatus to study the high-M(r) salivary mucins. Using DGE, the MG1s of individual glands were seen to have clearly distinct electrophoretic mobilities, as monitored by ELISA using MG1-specific monoclonal antibodies. Even within a particular MG1 preparation, subpopulations could be distinguished. DGE analysis of a chemically and enzymically modified MG1 series, followed by ELISA and dot-blot detection using specific monoclonal antibodies, lectins and high-iron diamine staining, suggests that the high electrophoretic mobility of PAL-MG1 is mainly the result of a high sulphate content, whereas the SL subpopulations differ mainly in binding type and amount of sialic acid. SM-MG1 most resembles the low-mobility subpopulation of SL-MG1, except that it has a lower sulphate content. In conclusion, DGE appears to be a powerful method for analysis of native mucin; it has been used to demonstrate that MG1s from the various salivary glands are biochemically much more diverse than was previously assumed.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bobek L. A., Tsai H., Biesbrock A. R., Levine M. J. Molecular cloning, sequence, and specificity of expression of the gene encoding the low molecular weight human salivary mucin (MUC7). J Biol Chem. 1993 Sep 25;268(27):20563–20569. [PubMed] [Google Scholar]
- Cheng P. W., Boat T. F., Cranfill K., Yankaskas J. R., Boucher R. C. Increased sulfation of glycoconjugates by cultured nasal epithelial cells from patients with cystic fibrosis. J Clin Invest. 1989 Jul;84(1):68–72. doi: 10.1172/JCI114171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crepin M., Porchet N., Aubert J. P., Degand P. Diversity of the peptide moiety of human airway mucins. Biorheology. 1990;27(3-4):471–484. doi: 10.3233/bir-1990-273-426. [DOI] [PubMed] [Google Scholar]
- Gendler S., Taylor-Papadimitriou J., Duhig T., Rothbard J., Burchell J. A highly immunogenic region of a human polymorphic epithelial mucin expressed by carcinomas is made up of tandem repeats. J Biol Chem. 1988 Sep 15;263(26):12820–12823. [PubMed] [Google Scholar]
- Gum J. R., Byrd J. C., Hicks J. W., Toribara N. W., Lamport D. T., Kim Y. S. Molecular cloning of human intestinal mucin cDNAs. Sequence analysis and evidence for genetic polymorphism. J Biol Chem. 1989 Apr 15;264(11):6480–6487. [PubMed] [Google Scholar]
- Gum J. R., Hicks J. W., Swallow D. M., Lagace R. L., Byrd J. C., Lamport D. T., Siddiki B., Kim Y. S. Molecular cloning of cDNAs derived from a novel human intestinal mucin gene. Biochem Biophys Res Commun. 1990 Aug 31;171(1):407–415. doi: 10.1016/0006-291x(90)91408-k. [DOI] [PubMed] [Google Scholar]
- Gupta R., Jentoft N. The structure of tracheobronchial mucins from cystic fibrosis and control patients. J Biol Chem. 1992 Feb 15;267(5):3160–3167. [PubMed] [Google Scholar]
- Hannig K., Kowalski M., Klöck G., Zimmermann U., Mang V. Free-flow electrophoresis under microgravity: evidence for enhanced resolution of cell separation. Electrophoresis. 1990 Aug;11(8):600–604. doi: 10.1002/elps.1150110803. [DOI] [PubMed] [Google Scholar]
- Hilkens J., Buijs F. Biosynthesis of MAM-6, an epithelial sialomucin. Evidence for involvement of a rare proteolytic cleavage step in the endoplasmic reticulum. J Biol Chem. 1988 Mar 25;263(9):4215–4222. [PubMed] [Google Scholar]
- Jacobs L. R., Huber P. W. Regional distribution and alterations of lectin binding to colorectal mucin in mucosal biopsies from controls and subjects with inflammatory bowel diseases. J Clin Invest. 1985 Jan;75(1):112–118. doi: 10.1172/JCI111662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein A., Carnoy C., Wieruszeski J. M., Strecker G., Strang A. M., van Halbeek H., Roussel P., Lamblin G. The broad diversity of neutral and sialylated oligosaccharides derived from human salivary mucins. Biochemistry. 1992 Jul 7;31(26):6152–6165. doi: 10.1021/bi00141a028. [DOI] [PubMed] [Google Scholar]
- Levine M. J., Reddy M. S., Tabak L. A., Loomis R. E., Bergey E. J., Jones P. C., Cohen R. E., Stinson M. W., Al-Hashimi I. Structural aspects of salivary glycoproteins. J Dent Res. 1987 Feb;66(2):436–441. doi: 10.1177/00220345870660020901. [DOI] [PubMed] [Google Scholar]
- Ligtenberg M. J., Vos H. L., Gennissen A. M., Hilkens J. Episialin, a carcinoma-associated mucin, is generated by a polymorphic gene encoding splice variants with alternative amino termini. J Biol Chem. 1990 Apr 5;265(10):5573–5578. [PubMed] [Google Scholar]
- Loomis R. E., Prakobphol A., Levine M. J., Reddy M. S., Jones P. C. Biochemical and biophysical comparison of two mucins from human submandibular-sublingual saliva. Arch Biochem Biophys. 1987 Nov 1;258(2):452–464. doi: 10.1016/0003-9861(87)90366-3. [DOI] [PubMed] [Google Scholar]
- Muramatsu T. Carbohydrate signals in metastasis and prognosis of human carcinomas. Glycobiology. 1993 Aug;3(4):291–296. doi: 10.1093/glycob/3.4.291. [DOI] [PubMed] [Google Scholar]
- Nieuw Amerongen A. V., Oderkerk C. H., Roukema P. A., Wolf J. H., Lisman J. J., Overdijk B., Fournet B., Montreuil J., Van Halbeek H., Mutsaers H. G. Primary structure of O- and N-glycosylic carbohydrate chains derived from murine submandibular mucin (MSM). Carbohydr Res. 1987 Jul 1;164:43–48. doi: 10.1016/0008-6215(87)80117-9. [DOI] [PubMed] [Google Scholar]
- Porchet N., Nguyen V. C., Dufosse J., Audie J. P., Guyonnet-Duperat V., Gross M. S., Denis C., Degand P., Bernheim A., Aubert J. P. Molecular cloning and chromosomal localization of a novel human tracheo-bronchial mucin cDNA containing tandemly repeated sequences of 48 base pairs. Biochem Biophys Res Commun. 1991 Mar 15;175(2):414–422. doi: 10.1016/0006-291x(91)91580-6. [DOI] [PubMed] [Google Scholar]
- SPICER S. S. DIAMINE METHODS FOR DIFFERENTIALING MUCOSUBSTANCES HISTOCHEMICALLY. J Histochem Cytochem. 1965 Mar;13:211–234. doi: 10.1177/13.3.211. [DOI] [PubMed] [Google Scholar]
- Savage A. V., Koppen P. L., Schiphorst W. E., Trippelvitz L. A., Van Halbeek H., Vliegenthart J. F., Van den Eijnden D. H. Porcine submaxillary mucin contains alpha 2----3- and alpha 2----6-linked N-acetyl- and N-glycolylneuraminic acid. Eur J Biochem. 1986 Oct 1;160(1):123–129. doi: 10.1111/j.1432-1033.1986.tb09948.x. [DOI] [PubMed] [Google Scholar]
- Shibuya N., Goldstein I. J., Broekaert W. F., Nsimba-Lubaki M., Peeters B., Peumans W. J. The elderberry (Sambucus nigra L.) bark lectin recognizes the Neu5Ac(alpha 2-6)Gal/GalNAc sequence. J Biol Chem. 1987 Feb 5;262(4):1596–1601. [PubMed] [Google Scholar]
- Slomiany B. L., Murty V. L., Slomiany A. Structural features of carbohydrate chains in human salivary mucins. Int J Biochem. 1993 Feb;25(2):259–265. doi: 10.1016/0020-711x(93)90015-7. [DOI] [PubMed] [Google Scholar]
- Strous G. J., Dekker J. Mucin-type glycoproteins. Crit Rev Biochem Mol Biol. 1992;27(1-2):57–92. doi: 10.3109/10409239209082559. [DOI] [PubMed] [Google Scholar]
- Terho T. T., Hartiala K. Method for determination of the sulfate content of glycosaminoglycans. Anal Biochem. 1971 Jun;41(2):471–476. doi: 10.1016/0003-2697(71)90167-9. [DOI] [PubMed] [Google Scholar]
- Therkildsen M. H., Mandel U., Christensen M., Dabelsteen E. Simple mucin-type Tn and sialosyl-Tn carbohydrate antigens in salivary gland carcinomas. Cancer. 1993 Aug 15;72(4):1147–1154. doi: 10.1002/1097-0142(19930815)72:4<1147::aid-cncr2820720403>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- Thornton D. J., Devine P. L., Hanski C., Howard M., Sheehan J. K. Identification of two major populations of mucins in respiratory secretions. Am J Respir Crit Care Med. 1994 Sep;150(3):823–832. doi: 10.1164/ajrccm.150.3.8087358. [DOI] [PubMed] [Google Scholar]
- Thornton D. J., Sheehan J. K., Carlstedt I. Heterogeneity of mucus glycoproteins from cystic fibrotic sputum. Are there different families of mucins? Biochem J. 1991 Jun 15;276(Pt 3):677–682. doi: 10.1042/bj2760677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tulp A., Verwoerd D., Dobberstein B., Ploegh H. L., Pieters J. Isolation and characterization of the intracellular MHC class II compartment. Nature. 1994 May 12;369(6476):120–126. doi: 10.1038/369120a0. [DOI] [PubMed] [Google Scholar]
- Tulp A., Verwoerd D., Pieters J. Application of an improved density gradient electrophoresis apparatus to the separation of proteins, cells and subcellular organelles. Electrophoresis. 1993 Dec;14(12):1295–1301. doi: 10.1002/elps.11501401198. [DOI] [PubMed] [Google Scholar]
- Veerman E. C., Valentijn-Benz M., Bank R. A., Nieuw Amerongen A. V. Isolation of high molecular weight mucins from human whole saliva by ultracentrifugation. J Biol Buccale. 1989 Dec;17(4):307–312. [PubMed] [Google Scholar]
- Veerman E. C., Valentijn-Benz M., van den Keybus P. A., Rathman W. M., Sheehan J. K., Nieuw Amerongen A. V. Immunochemical analysis of high molecular-weight human salivary mucins (MG1) using monoclonal antibodies. Arch Oral Biol. 1991;36(12):923–932. doi: 10.1016/0003-9969(91)90125-e. [DOI] [PubMed] [Google Scholar]
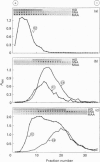
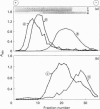
- Veerman E. C., van den Keybus P. A., Valentijn-Benz M., Nieuw Amerongen A. V. Isolation of different high-Mr mucin species from human whole saliva. Biochem J. 1992 May 1;283(Pt 3):807–811. doi: 10.1042/bj2830807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W. C., Cummings R. D. The immobilized leukoagglutinin from the seeds of Maackia amurensis binds with high affinity to complex-type Asn-linked oligosaccharides containing terminal sialic acid-linked alpha-2,3 to penultimate galactose residues. J Biol Chem. 1988 Apr 5;263(10):4576–4585. [PubMed] [Google Scholar]
- Wesley A., Forstner J., Qureshi R., Mantle M., Forstner G. Human intestinal mucin in cystic fibrosis. Pediatr Res. 1983 Jan;17(1):65–69. doi: 10.1203/00006450-198301000-00013. [DOI] [PubMed] [Google Scholar]
- Yuen C. T., Bezouska K., O'Brien J., Stoll M., Lemoine R., Lubineau A., Kiso M., Hasegawa A., Bockovich N. J., Nicolaou K. C. Sulfated blood group Lewis(a). A superior oligosaccharide ligand for human E-selectin. J Biol Chem. 1994 Jan 21;269(3):1595–1598. [PubMed] [Google Scholar]