Abstract
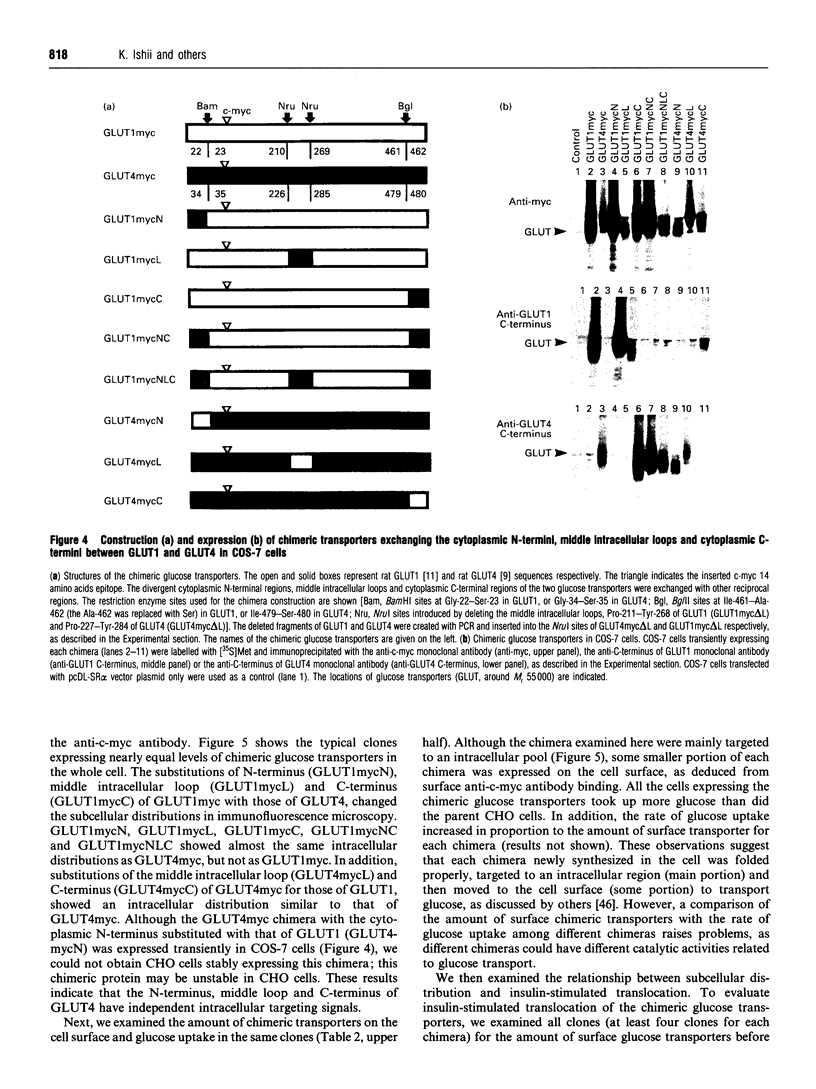
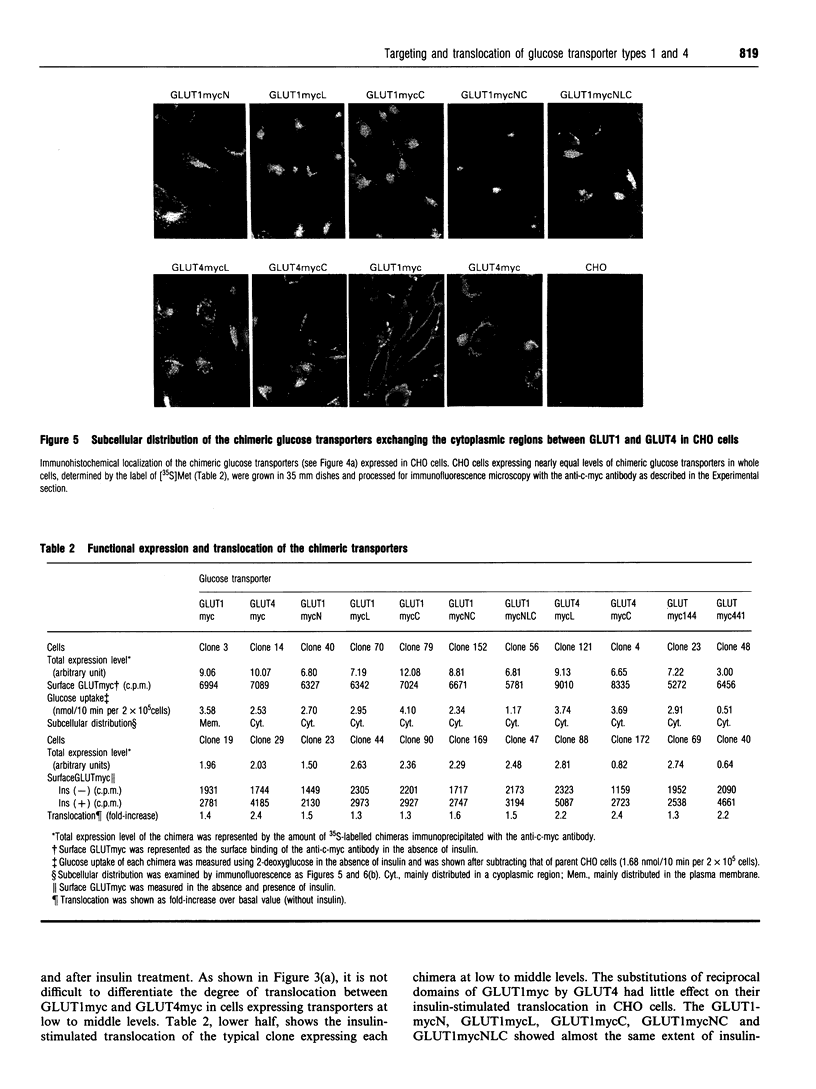
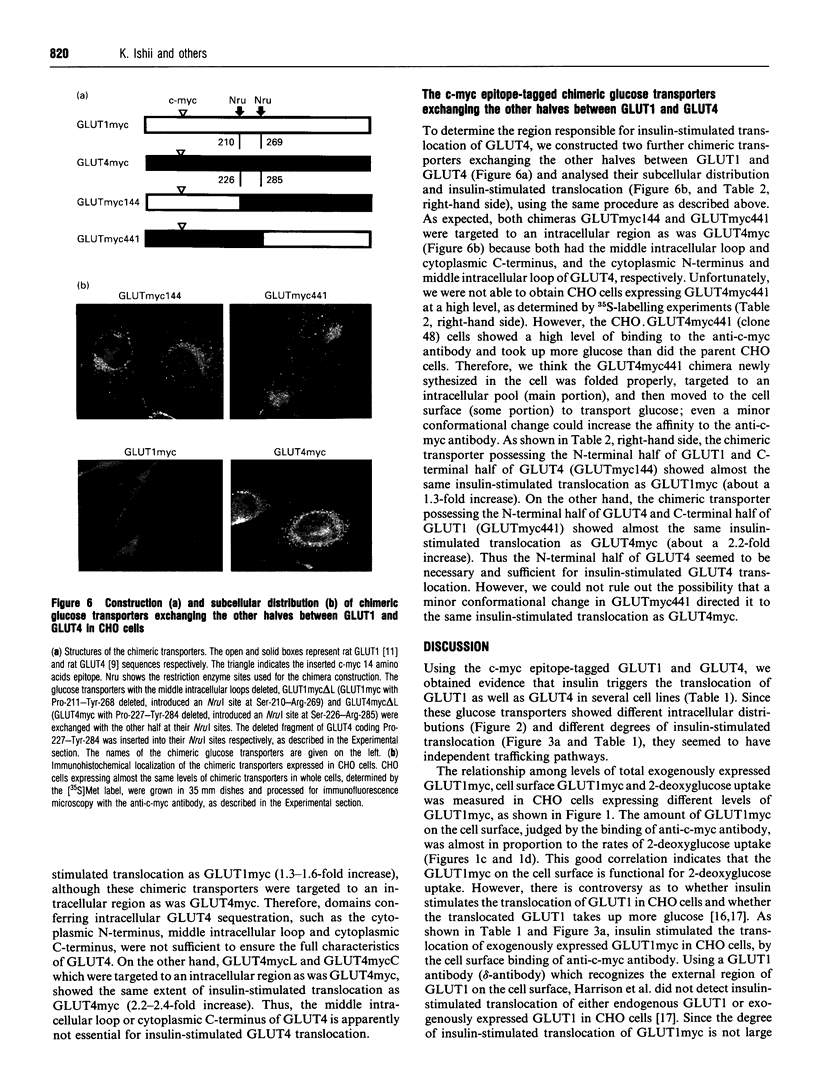
Translocation of the type 4 glucose transporter (GLUT4) to the cell surface from an intracellular pool is the major mechanism of insulin-stimulated glucose uptake in insulin-target cells. We developed a highly sensitive and quantitative method to detect GLUT4 immunologically on the surface of intact cells, using c-myc epitope-tagged GLUT4 (GLUT4myc). We constructed c-myc epitope-tagged glucose transporter type 1 (GLUT1myc) and found that the GLUT1myc was also translocated to the cell surface of Chinese hamster ovary cells, 3T3-L1 fibroblasts and NIH 3T3 cells, in response to insulin, but the degree of translocation was less than that of GLUT4myc. Since GLUT1 and GLUT4 have different intracellular distributions and different degrees of insulin-stimulated translocation, we examined the domains of GLUT4, using c-myc epitope-tagged chimeric glucose transporters between these two isoforms. The results indicated that, (1) all the cytoplasmic N-terminal region, middle intracellular loop and cytoplasmic C-terminal region of GLUT4 have independent intracellular targeting signals, (2) these sequences for intracellular targeting of GLUT4 were not sufficient to determine GLUT4 translocation in response to insulin, and (3) the N-terminal half of GLUT4 devoid both of cytoplasmic N-terminus and of middle intracellular loop seems to be necessary for insulin-stimulated GLUT4 translocation.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allard W. J., Gibbs E. M., Witters L. A., Lienhard G. E. The glucose transporter in human fibroblasts is phosphorylated in response to phorbol ester but not in response to growth factors. Biochim Biophys Acta. 1987 Jul 29;929(3):288–295. doi: 10.1016/0167-4889(87)90255-2. [DOI] [PubMed] [Google Scholar]
- Asano T., Shibasaki Y., Ohno S., Taira H., Lin J. L., Kasuga M., Kanazawa Y., Akanuma Y., Takaku F., Oka Y. Rabbit brain glucose transporter responds to insulin when expressed in insulin-sensitive Chinese hamster ovary cells. J Biol Chem. 1989 Feb 25;264(6):3416–3420. [PubMed] [Google Scholar]
- Asano T., Takata K., Katagiri H., Tsukuda K., Lin J. L., Ishihara H., Inukai K., Hirano H., Yazaki Y., Oka Y. Domains responsible for the differential targeting of glucose transporter isoforms. J Biol Chem. 1992 Sep 25;267(27):19636–19641. [PubMed] [Google Scholar]
- Baldini G., Hohman R., Charron M. J., Lodish H. F. Insulin and nonhydrolyzable GTP analogs induce translocation of GLUT 4 to the plasma membrane in alpha-toxin-permeabilized rat adipose cells. J Biol Chem. 1991 Mar 5;266(7):4037–4040. [PubMed] [Google Scholar]
- Bell G. I., Burant C. F., Takeda J., Gould G. W. Structure and function of mammalian facilitative sugar transporters. J Biol Chem. 1993 Sep 15;268(26):19161–19164. [PubMed] [Google Scholar]
- Birnbaum M. J., Haspel H. C., Rosen O. M. Cloning and characterization of a cDNA encoding the rat brain glucose-transporter protein. Proc Natl Acad Sci U S A. 1986 Aug;83(16):5784–5788. doi: 10.1073/pnas.83.16.5784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calderhead D. M., Kitagawa K., Lienhard G. E., Gould G. W. Translocation of the brain-type glucose transporter largely accounts for insulin stimulation of glucose transport in BC3H-1 myocytes. Biochem J. 1990 Aug 1;269(3):597–601. doi: 10.1042/bj2690597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark A. E., Holman G. D. Exofacial photolabelling of the human erythrocyte glucose transporter with an azitrifluoroethylbenzoyl-substituted bismannose. Biochem J. 1990 Aug 1;269(3):615–622. doi: 10.1042/bj2690615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cushman S. W., Wardzala L. J. Potential mechanism of insulin action on glucose transport in the isolated rat adipose cell. Apparent translocation of intracellular transport systems to the plasma membrane. J Biol Chem. 1980 May 25;255(10):4758–4762. [PubMed] [Google Scholar]
- Czech M. P., Chawla A., Woon C. W., Buxton J., Armoni M., Tang W., Joly M., Corvera S. Exofacial epitope-tagged glucose transporter chimeras reveal COOH-terminal sequences governing cellular localization. J Cell Biol. 1993 Oct;123(1):127–135. doi: 10.1083/jcb.123.1.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czech M. P., Clancy B. M., Pessino A., Woon C. W., Harrison S. A. Complex regulation of simple sugar transport in insulin-responsive cells. Trends Biochem Sci. 1992 May;17(5):197–201. doi: 10.1016/0968-0004(92)90266-c. [DOI] [PubMed] [Google Scholar]
- Ebina Y., Araki E., Taira M., Shimada F., Mori M., Craik C. S., Siddle K., Pierce S. B., Roth R. A., Rutter W. J. Replacement of lysine residue 1030 in the putative ATP-binding region of the insulin receptor abolishes insulin- and antibody-stimulated glucose uptake and receptor kinase activity. Proc Natl Acad Sci U S A. 1987 Feb;84(3):704–708. doi: 10.1073/pnas.84.3.704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebina Y., Edery M., Ellis L., Standring D., Beaudoin J., Roth R. A., Rutter W. J. Expression of a functional human insulin receptor from a cloned cDNA in Chinese hamster ovary cells. Proc Natl Acad Sci U S A. 1985 Dec;82(23):8014–8018. doi: 10.1073/pnas.82.23.8014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evan G. I., Lewis G. K., Ramsay G., Bishop J. M. Isolation of monoclonal antibodies specific for human c-myc proto-oncogene product. Mol Cell Biol. 1985 Dec;5(12):3610–3616. doi: 10.1128/mcb.5.12.3610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould G. W., Lienhard G. E. Expression of a functional glucose transporter in Xenopus oocytes. Biochemistry. 1989 Nov 28;28(24):9447–9452. doi: 10.1021/bi00450a030. [DOI] [PubMed] [Google Scholar]
- Haney P. M., Slot J. W., Piper R. C., James D. E., Mueckler M. Intracellular targeting of the insulin-regulatable glucose transporter (GLUT4) is isoform specific and independent of cell type. J Cell Biol. 1991 Aug;114(4):689–699. doi: 10.1083/jcb.114.4.689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison S. A., Buxton J. M., Clancy B. M., Czech M. P. Insulin regulation of hexose transport in mouse 3T3-L1 cells expressing the human HepG2 glucose transporter. J Biol Chem. 1990 Nov 25;265(33):20106–20116. [PubMed] [Google Scholar]
- Harrison S. A., Buxton J. M., Helgerson A. L., MacDonald R. G., Chlapowski F. J., Carruthers A., Czech M. P. Insulin action on activity and cell surface disposition of human HepG2 glucose transporters expressed in Chinese hamster ovary cells. J Biol Chem. 1990 Apr 5;265(10):5793–5801. [PubMed] [Google Scholar]
- Hayashi H., Kamohara S., Nishioka Y., Kanai F., Miyake N., Fukui Y., Shibasaki F., Takenawa T., Ebina Y. Insulin treatment stimulates the tyrosine phosphorylation of the alpha-type 85-kDa subunit of phosphatidylinositol 3-kinase in vivo. J Biol Chem. 1992 Nov 5;267(31):22575–22580. [PubMed] [Google Scholar]
- Hayashi H., Owada M. K., Sonobe S., Domae K., Yamanouchi T., Kakunaga T., Kitajima Y., Yaoita H. Monoclonal antibodies specific to a Ca2(+)-bound form of lipocortin I distinguish its Ca2(+)-dependent phospholipid-binding ability from its ability to inhibit phospholipase A2. Biochem J. 1990 Aug 1;269(3):709–715. doi: 10.1042/bj2690709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higuchi R., Krummel B., Saiki R. K. A general method of in vitro preparation and specific mutagenesis of DNA fragments: study of protein and DNA interactions. Nucleic Acids Res. 1988 Aug 11;16(15):7351–7367. doi: 10.1093/nar/16.15.7351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho S. N., Hunt H. D., Horton R. M., Pullen J. K., Pease L. R. Site-directed mutagenesis by overlap extension using the polymerase chain reaction. Gene. 1989 Apr 15;77(1):51–59. doi: 10.1016/0378-1119(89)90358-2. [DOI] [PubMed] [Google Scholar]
- Hresko R. C., Kruse M., Strube M., Mueckler M. Topology of the Glut 1 glucose transporter deduced from glycosylation scanning mutagenesis. J Biol Chem. 1994 Aug 12;269(32):20482–20488. [PubMed] [Google Scholar]
- James D. E., Brown R., Navarro J., Pilch P. F. Insulin-regulatable tissues express a unique insulin-sensitive glucose transport protein. Nature. 1988 May 12;333(6169):183–185. doi: 10.1038/333183a0. [DOI] [PubMed] [Google Scholar]
- James D. E., Strube M., Mueckler M. Molecular cloning and characterization of an insulin-regulatable glucose transporter. Nature. 1989 Mar 2;338(6210):83–87. doi: 10.1038/338083a0. [DOI] [PubMed] [Google Scholar]
- Janicot M., Lane M. D. Activation of glucose uptake by insulin and insulin-like growth factor I in Xenopus oocytes. Proc Natl Acad Sci U S A. 1989 Apr;86(8):2642–2646. doi: 10.1073/pnas.86.8.2642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamohara S., Hayashi H., Todaka M., Kanai F., Ishii K., Imanaka T., Escobedo J. A., Williams L. T., Ebina Y. Platelet-derived growth factor triggers translocation of the insulin-regulatable glucose transporter (type 4) predominantly through phosphatidylinositol 3-kinase binding sites on the receptor. Proc Natl Acad Sci U S A. 1995 Feb 14;92(4):1077–1081. doi: 10.1073/pnas.92.4.1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
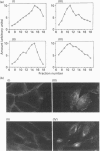
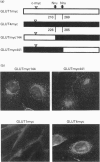
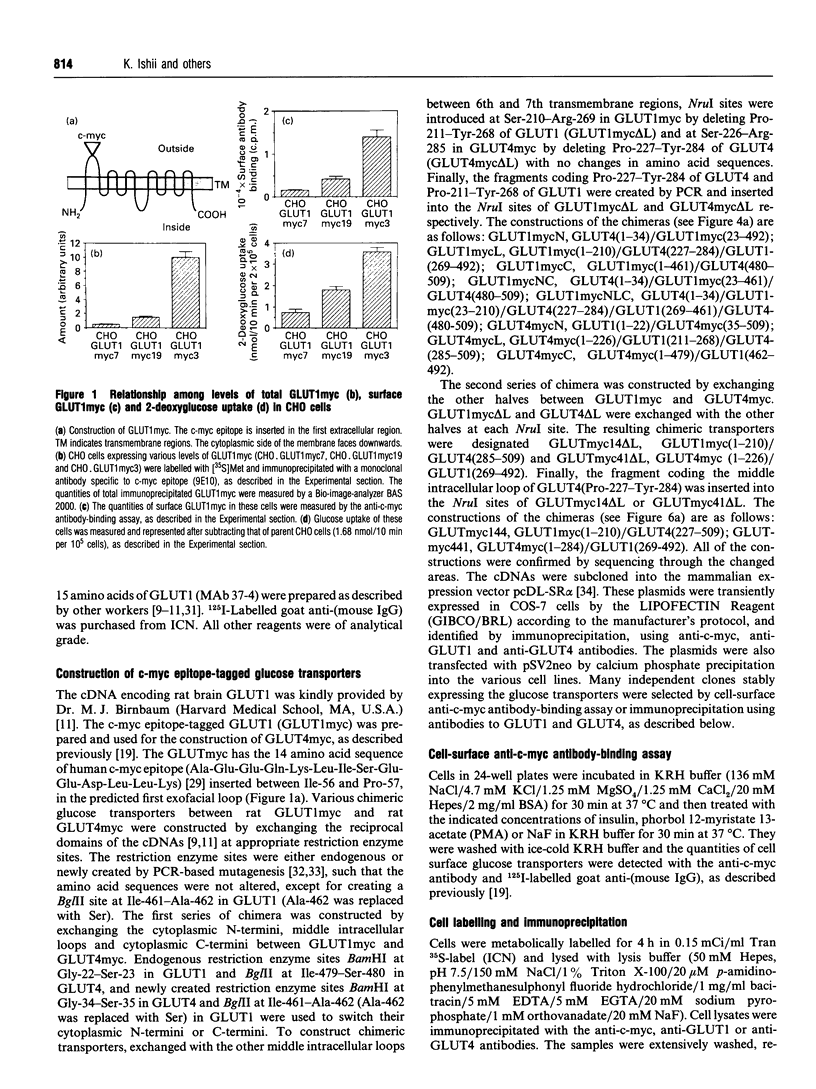
- Kanai F., Nishioka Y., Hayashi H., Kamohara S., Todaka M., Ebina Y. Direct demonstration of insulin-induced GLUT4 translocation to the surface of intact cells by insertion of a c-myc epitope into an exofacial GLUT4 domain. J Biol Chem. 1993 Jul 5;268(19):14523–14526. [PubMed] [Google Scholar]
- Kasanicki M. A., Pilch P. F. Regulation of glucose-transporter function. Diabetes Care. 1990 Mar;13(3):219–227. doi: 10.2337/diacare.13.3.219. [DOI] [PubMed] [Google Scholar]
- Marshall B. A., Murata H., Hresko R. C., Mueckler M. Domains that confer intracellular sequestration of the Glut4 glucose transporter in Xenopus oocytes. J Biol Chem. 1993 Dec 15;268(35):26193–26199. [PubMed] [Google Scholar]
- Mueckler M., Caruso C., Baldwin S. A., Panico M., Blench I., Morris H. R., Allard W. J., Lienhard G. E., Lodish H. F. Sequence and structure of a human glucose transporter. Science. 1985 Sep 6;229(4717):941–945. doi: 10.1126/science.3839598. [DOI] [PubMed] [Google Scholar]
- Mühlbacher C., Karnieli E., Schaff P., Obermaier B., Mushack J., Rattenhuber E., Häring H. U. Phorbol esters imitate in rat fat-cells the full effect of insulin on glucose-carrier translocation, but not on 3-O-methylglucose-transport activity. Biochem J. 1988 Feb 1;249(3):865–870. doi: 10.1042/bj2490865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pessin J. E., Bell G. I. Mammalian facilitative glucose transporter family: structure and molecular regulation. Annu Rev Physiol. 1992;54:911–930. doi: 10.1146/annurev.ph.54.030192.004403. [DOI] [PubMed] [Google Scholar]
- Piper R. C., Hess L. J., James D. E. Differential sorting of two glucose transporters expressed in insulin-sensitive cells. Am J Physiol. 1991 Mar;260(3 Pt 1):C570–C580. doi: 10.1152/ajpcell.1991.260.3.C570. [DOI] [PubMed] [Google Scholar]
- Piper R. C., Tai C., Kulesza P., Pang S., Warnock D., Baenziger J., Slot J. W., Geuze H. J., Puri C., James D. E. GLUT-4 NH2 terminus contains a phenylalanine-based targeting motif that regulates intracellular sequestration. J Cell Biol. 1993 Jun;121(6):1221–1232. doi: 10.1083/jcb.121.6.1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piper R. C., Tai C., Slot J. W., Hahn C. S., Rice C. M., Huang H., James D. E. The efficient intracellular sequestration of the insulin-regulatable glucose transporter (GLUT-4) is conferred by the NH2 terminus. J Cell Biol. 1992 May;117(4):729–743. doi: 10.1083/jcb.117.4.729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quon M. J., Guerre-Millo M., Zarnowski M. J., Butte A. J., Em M., Cushman S. W., Taylor S. I. Tyrosine kinase-deficient mutant human insulin receptors (Met1153-->Ile) overexpressed in transfected rat adipose cells fail to mediate translocation of epitope-tagged GLUT4. Proc Natl Acad Sci U S A. 1994 Jun 7;91(12):5587–5591. doi: 10.1073/pnas.91.12.5587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibasaki Y., Asano T., Lin J. L., Tsukuda K., Katagiri H., Ishihara H., Yazaki Y., Oka Y. Two glucose transporter isoforms are sorted differentially and are expressed in distinct cellular compartments. Biochem J. 1992 Feb 1;281(Pt 3):829–834. doi: 10.1042/bj2810829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki K., Kono T. Evidence that insulin causes translocation of glucose transport activity to the plasma membrane from an intracellular storage site. Proc Natl Acad Sci U S A. 1980 May;77(5):2542–2545. doi: 10.1073/pnas.77.5.2542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takebe Y., Seiki M., Fujisawa J., Hoy P., Yokota K., Arai K., Yoshida M., Arai N. SR alpha promoter: an efficient and versatile mammalian cDNA expression system composed of the simian virus 40 early promoter and the R-U5 segment of human T-cell leukemia virus type 1 long terminal repeat. Mol Cell Biol. 1988 Jan;8(1):466–472. doi: 10.1128/mcb.8.1.466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor S. I., Accili D., Imai Y. Insulin resistance or insulin deficiency. Which is the primary cause of NIDDM? Diabetes. 1994 Jun;43(6):735–740. doi: 10.2337/diab.43.6.735. [DOI] [PubMed] [Google Scholar]
- Vera J. C., Rosen O. M. Reconstitution of an insulin signaling pathway in Xenopus laevis oocytes: coexpression of a mammalian insulin receptor and three different mammalian hexose transporters. Mol Cell Biol. 1990 Feb;10(2):743–751. doi: 10.1128/mcb.10.2.743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verhey K. J., Birnbaum M. J. A Leu-Leu sequence is essential for COOH-terminal targeting signal of GLUT4 glucose transporter in fibroblasts. J Biol Chem. 1994 Jan 28;269(4):2353–2356. [PubMed] [Google Scholar]
- Verhey K. J., Hausdorff S. F., Birnbaum M. J. Identification of the carboxy terminus as important for the isoform-specific subcellular targeting of glucose transporter proteins. J Cell Biol. 1993 Oct;123(1):137–147. doi: 10.1083/jcb.123.1.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogt B., Mushack J., Seffer E., Häring H. U. The translocation of the glucose transporter sub-types GLUT1 and GLUT4 in isolated fat cells is differently regulated by phorbol esters. Biochem J. 1991 May 1;275(Pt 3):597–600. doi: 10.1042/bj2750597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White M. F., Kahn C. R. The insulin signaling system. J Biol Chem. 1994 Jan 7;269(1):1–4. [PubMed] [Google Scholar]