Abstract
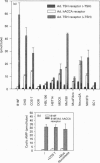
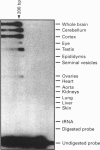
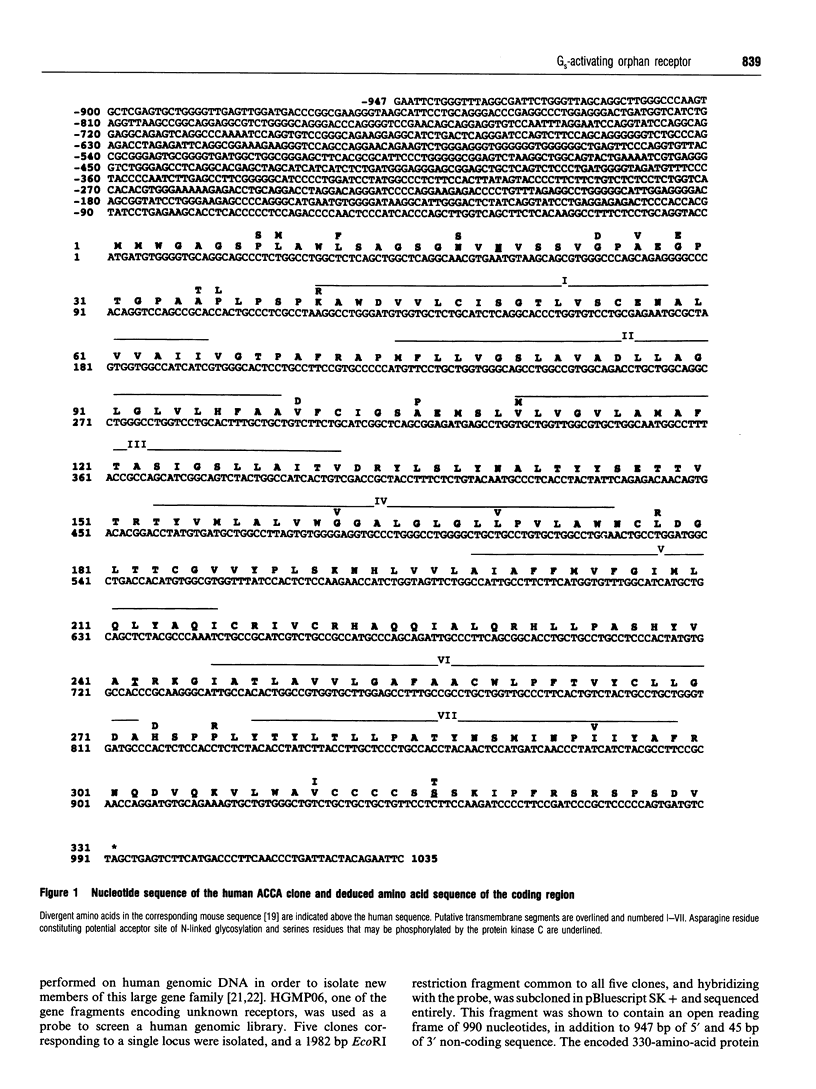
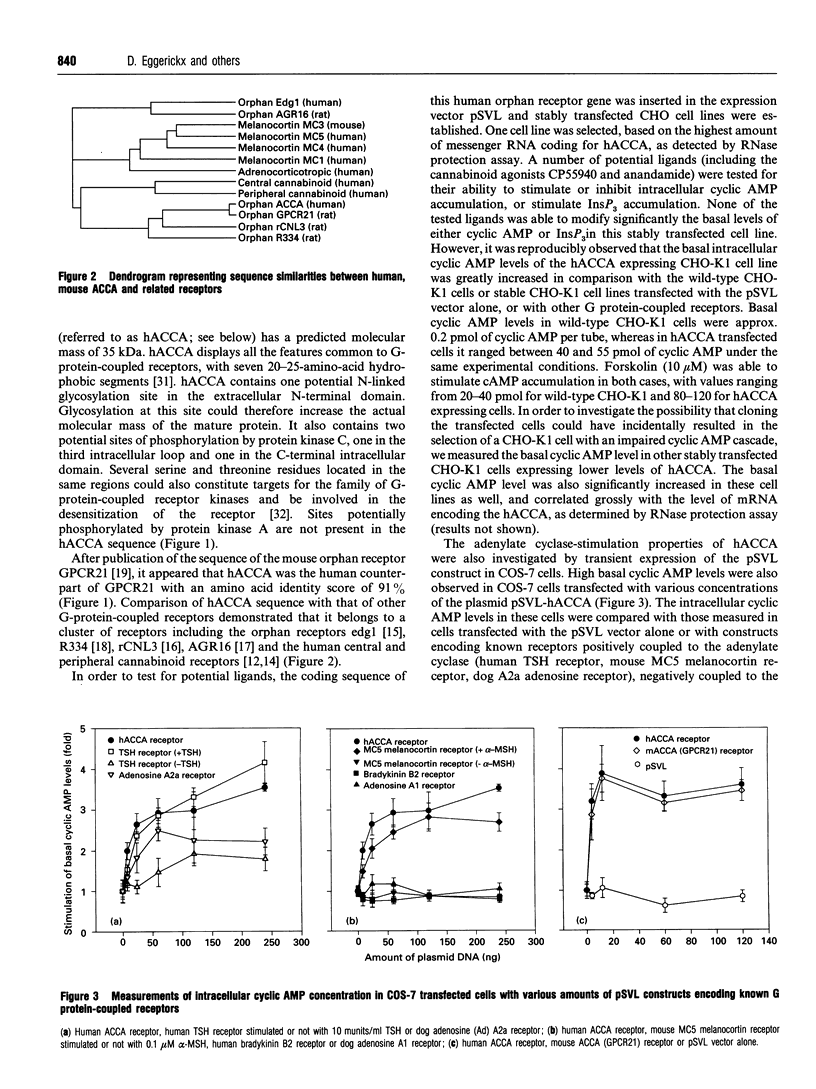
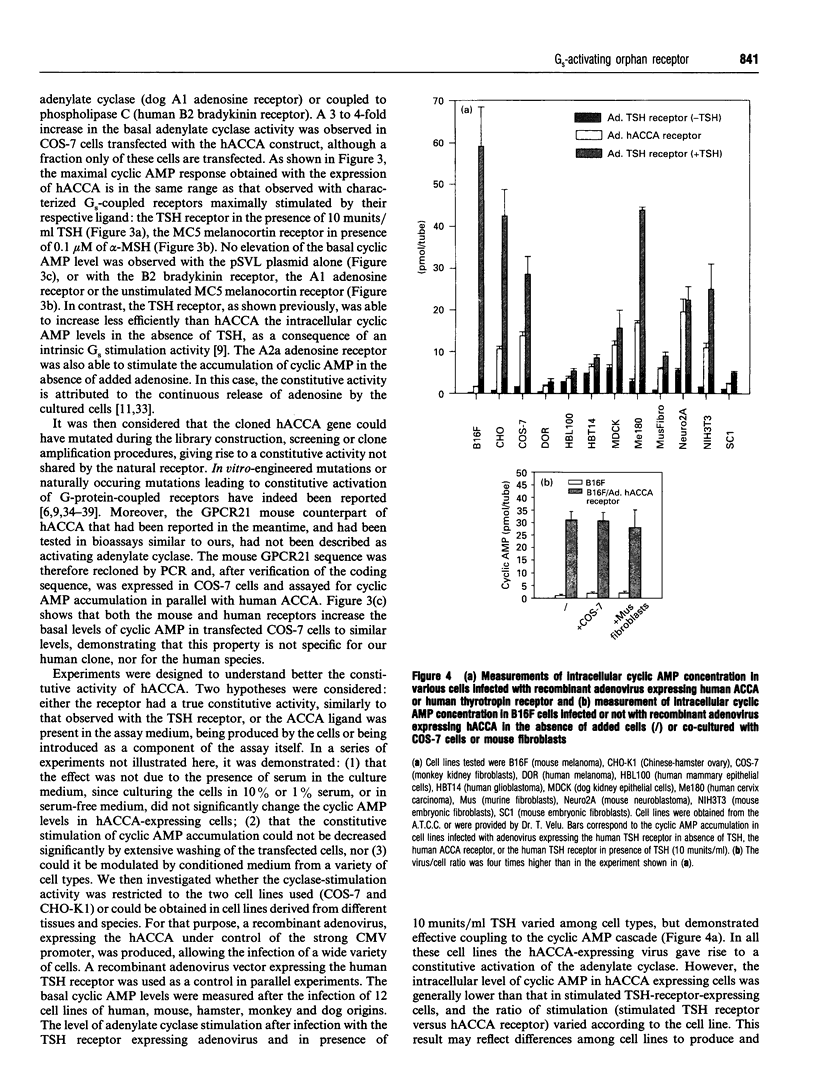
A human gene encoding an orphan G-protein-coupled receptor named ACCA (adenylate cyclase constitutive activator) was isolated from a genomic library using as a probe a DNA fragment obtained by low-stringency PCR. Human ACCA (hACCA) is a protein of 330 amino acids that exhibits all the structural hallmarks of the main family of G-protein-coupled receptors. Expression of hACCA resulted in a dramatic stimulation of adenylate cyclase, similar in amplitude to that obtained with other Gs-coupled receptors fully activated by their respective ligands. This stimulation was obtained in a large variety of stable cell lines derived from various organs, and originating from different mammalian species. hACCA was found to be the human homologue of a recently reported mouse orphan receptor (GPCR21). The mouse ACCA (mACCA) was therefore recloned by PCR, and expression of mACCA in Cos-7 cells demonstrated that the mouse receptor behaved similarly as a constitutive activator of adenylate cyclase. It is not known presently whether the stimulation of adenylate cyclase is the result of a true constitutive activity of the receptor or, alternatively, is the consequence of a permanent stimulation by a ubiquitous ligand. The tissue distribution of mACCA was determined by RNase protection assay. Abundant transcripts were found in the brain, whereas lower amounts were detected in testis, ovary and eye. Various hypotheses concerning the constitutive activity of ACCA and their potential biological significance are discussed.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen L. F., Lefkowitz R. J., Caron M. G., Cotecchia S. G-protein-coupled receptor genes as protooncogenes: constitutively activating mutation of the alpha 1B-adrenergic receptor enhances mitogenesis and tumorigenicity. Proc Natl Acad Sci U S A. 1991 Dec 15;88(24):11354–11358. doi: 10.1073/pnas.88.24.11354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aruffo A., Seed B. Molecular cloning of a CD28 cDNA by a high-efficiency COS cell expression system. Proc Natl Acad Sci U S A. 1987 Dec;84(23):8573–8577. doi: 10.1073/pnas.84.23.8573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker E. L., Westphal R. S., Schmidt D., Sanders-Bush E. Constitutively active 5-hydroxytryptamine2C receptors reveal novel inverse agonist activity of receptor ligands. J Biol Chem. 1994 Apr 22;269(16):11687–11690. [PubMed] [Google Scholar]
- Brooker G., Harper J. F., Terasaki W. L., Moylan R. D. Radioimmunoassay of cyclic AMP and cyclic GMP. Adv Cyclic Nucleotide Res. 1979;10:1–33. [PubMed] [Google Scholar]
- Chidiac P., Hebert T. E., Valiquette M., Dennis M., Bouvier M. Inverse agonist activity of beta-adrenergic antagonists. Mol Pharmacol. 1994 Mar;45(3):490–499. [PubMed] [Google Scholar]
- Chou Y. H., Brown E. M., Levi T., Crowe G., Atkinson A. B., Arnqvist H. J., Toss G., Fuleihan G. E., Seidman J. G., Seidman C. E. The gene responsible for familial hypocalciuric hypercalcemia maps to chromosome 3q in four unrelated families. Nat Genet. 1992 Jul;1(4):295–300. doi: 10.1038/ng0792-295. [DOI] [PubMed] [Google Scholar]
- Costa T., Ogino Y., Munson P. J., Onaran H. O., Rodbard D. Drug efficacy at guanine nucleotide-binding regulatory protein-linked receptors: thermodynamic interpretation of negative antagonism and of receptor activity in the absence of ligand. Mol Pharmacol. 1992 Mar;41(3):549–560. [PubMed] [Google Scholar]
- Cotecchia S., Exum S., Caron M. G., Lefkowitz R. J. Regions of the alpha 1-adrenergic receptor involved in coupling to phosphatidylinositol hydrolysis and enhanced sensitivity of biological function. Proc Natl Acad Sci U S A. 1990 Apr;87(8):2896–2900. doi: 10.1073/pnas.87.8.2896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dohlman H. G., Thorner J., Caron M. G., Lefkowitz R. J. Model systems for the study of seven-transmembrane-segment receptors. Annu Rev Biochem. 1991;60:653–688. doi: 10.1146/annurev.bi.60.070191.003253. [DOI] [PubMed] [Google Scholar]
- Duprez L., Parma J., Van Sande J., Allgeier A., Leclère J., Schvartz C., Delisle M. J., Decoulx M., Orgiazzi J., Dumont J. Germline mutations in the thyrotropin receptor gene cause non-autoimmune autosomal dominant hyperthyroidism. Nat Genet. 1994 Jul;7(3):396–401. doi: 10.1038/ng0794-396. [DOI] [PubMed] [Google Scholar]
- Eidne K. A., Zabavnik J., Peters T., Yoshida S., Anderson L., Taylor P. L. Cloning, sequencing and tissue distribution of a candidate G protein-coupled receptor from rat pituitary gland. FEBS Lett. 1991 Nov 4;292(1-2):243–248. doi: 10.1016/0014-5793(91)80876-5. [DOI] [PubMed] [Google Scholar]
- Gérard C. M., Mollereau C., Vassart G., Parmentier M. Molecular cloning of a human cannabinoid receptor which is also expressed in testis. Biochem J. 1991 Oct 1;279(Pt 1):129–134. doi: 10.1042/bj2790129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gómez-Foix A. M., Coats W. S., Baqué S., Alam T., Gerard R. D., Newgard C. B. Adenovirus-mediated transfer of the muscle glycogen phosphorylase gene into hepatocytes confers altered regulation of glycogen metabolism. J Biol Chem. 1992 Dec 15;267(35):25129–25134. [PubMed] [Google Scholar]
- Hayashi Y., DePaoli A. M., Burant C. F., Refetoff S. Expression of a thyroid hormone-responsive recombinant gene introduced into adult mice livers by replication-defective adenovirus can be regulated by endogenous thyroid hormone receptor. J Biol Chem. 1994 Sep 30;269(39):23872–23875. [PubMed] [Google Scholar]
- Hla T., Maciag T. An abundant transcript induced in differentiating human endothelial cells encodes a polypeptide with structural similarities to G-protein-coupled receptors. J Biol Chem. 1990 Jun 5;265(16):9308–9313. [PubMed] [Google Scholar]
- Kemp B. E., Pearson R. B. Protein kinase recognition sequence motifs. Trends Biochem Sci. 1990 Sep;15(9):342–346. doi: 10.1016/0968-0004(90)90073-k. [DOI] [PubMed] [Google Scholar]
- Kjelsberg M. A., Cotecchia S., Ostrowski J., Caron M. G., Lefkowitz R. J. Constitutive activation of the alpha 1B-adrenergic receptor by all amino acid substitutions at a single site. Evidence for a region which constrains receptor activation. J Biol Chem. 1992 Jan 25;267(3):1430–1433. [PubMed] [Google Scholar]
- Ledent C., Dumont J. E., Vassart G., Parmentier M. Thyroid expression of an A2 adenosine receptor transgene induces thyroid hyperplasia and hyperthyroidism. EMBO J. 1992 Feb;11(2):537–542. doi: 10.1002/j.1460-2075.1992.tb05084.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefkowitz R. J. G-protein-coupled receptors. Turned on to ill effect. Nature. 1993 Oct 14;365(6447):603–604. doi: 10.1038/365603a0. [DOI] [PubMed] [Google Scholar]
- Libert F., Parmentier M., Lefort A., Dinsart C., Van Sande J., Maenhaut C., Simons M. J., Dumont J. E., Vassart G. Selective amplification and cloning of four new members of the G protein-coupled receptor family. Science. 1989 May 5;244(4904):569–572. doi: 10.1126/science.2541503. [DOI] [PubMed] [Google Scholar]
- Maenhaut C., Van Sande J., Libert F., Abramowicz M., Parmentier M., Vanderhaegen J. J., Dumont J. E., Vassart G., Schiffmann S. RDC8 codes for an adenosine A2 receptor with physiological constitutive activity. Biochem Biophys Res Commun. 1990 Dec 31;173(3):1169–1178. doi: 10.1016/s0006-291x(05)80909-x. [DOI] [PubMed] [Google Scholar]
- Marchese A., Docherty J. M., Nguyen T., Heiber M., Cheng R., Heng H. H., Tsui L. C., Shi X., George S. R., O'Dowd B. F. Cloning of human genes encoding novel G protein-coupled receptors. Genomics. 1994 Oct;23(3):609–618. doi: 10.1006/geno.1994.1549. [DOI] [PubMed] [Google Scholar]
- Matsuda L. A., Lolait S. J., Brownstein M. J., Young A. C., Bonner T. I. Structure of a cannabinoid receptor and functional expression of the cloned cDNA. Nature. 1990 Aug 9;346(6284):561–564. doi: 10.1038/346561a0. [DOI] [PubMed] [Google Scholar]
- McGrory W. J., Bautista D. S., Graham F. L. A simple technique for the rescue of early region I mutations into infectious human adenovirus type 5. Virology. 1988 Apr;163(2):614–617. doi: 10.1016/0042-6822(88)90302-9. [DOI] [PubMed] [Google Scholar]
- Munro S., Thomas K. L., Abu-Shaar M. Molecular characterization of a peripheral receptor for cannabinoids. Nature. 1993 Sep 2;365(6441):61–65. doi: 10.1038/365061a0. [DOI] [PubMed] [Google Scholar]
- Nilsson C. L., Eriksson E. Haloperidol increases prolactin release and cyclic AMP formation in vitro: inverse agonism at dopamine D2 receptors? J Neural Transm Gen Sect. 1993;92(2-3):213–220. doi: 10.1007/BF01244880. [DOI] [PubMed] [Google Scholar]
- O'Dowd B. F., Lefkowitz R. J., Caron M. G. Structure of the adrenergic and related receptors. Annu Rev Neurosci. 1989;12:67–83. doi: 10.1146/annurev.ne.12.030189.000435. [DOI] [PubMed] [Google Scholar]
- Okazaki H., Ishizaka N., Sakurai T., Kurokawa K., Goto K., Kumada M., Takuwa Y. Molecular cloning of a novel putative G protein-coupled receptor expressed in the cardiovascular system. Biochem Biophys Res Commun. 1993 Feb 15;190(3):1104–1109. doi: 10.1006/bbrc.1993.1163. [DOI] [PubMed] [Google Scholar]
- Parma J., Duprez L., Van Sande J., Cochaux P., Gervy C., Mockel J., Dumont J., Vassart G. Somatic mutations in the thyrotropin receptor gene cause hyperfunctioning thyroid adenomas. Nature. 1993 Oct 14;365(6447):649–651. doi: 10.1038/365649a0. [DOI] [PubMed] [Google Scholar]
- Parmentier M., Libert F., Maenhaut C., Lefort A., Gérard C., Perret J., Van Sande J., Dumont J. E., Vassart G. Molecular cloning of the thyrotropin receptor. Science. 1989 Dec 22;246(4937):1620–1622. doi: 10.1126/science.2556796. [DOI] [PubMed] [Google Scholar]
- Rao V. R., Cohen G. B., Oprian D. D. Rhodopsin mutation G90D and a molecular mechanism for congenital night blindness. Nature. 1994 Feb 17;367(6464):639–642. doi: 10.1038/367639a0. [DOI] [PubMed] [Google Scholar]
- Robbins L. S., Nadeau J. H., Johnson K. R., Kelly M. A., Roselli-Rehfuss L., Baack E., Mountjoy K. G., Cone R. D. Pigmentation phenotypes of variant extension locus alleles result from point mutations that alter MSH receptor function. Cell. 1993 Mar 26;72(6):827–834. doi: 10.1016/0092-8674(93)90572-8. [DOI] [PubMed] [Google Scholar]
- Saeki Y., Ueno S., Mizuno R., Nishimura T., Fujimura H., Nagai Y., Yanagihara T. Molecular cloning of a novel putative G protein-coupled receptor (GPCR21) which is expressed predominantly in mouse central nervous system. FEBS Lett. 1993 Dec 27;336(2):317–322. doi: 10.1016/0014-5793(93)80828-i. [DOI] [PubMed] [Google Scholar]
- Samama P., Cotecchia S., Costa T., Lefkowitz R. J. A mutation-induced activated state of the beta 2-adrenergic receptor. Extending the ternary complex model. J Biol Chem. 1993 Mar 5;268(7):4625–4636. [PubMed] [Google Scholar]
- Samama P., Pei G., Costa T., Cotecchia S., Lefkowitz R. J. Negative antagonists promote an inactive conformation of the beta 2-adrenergic receptor. Mol Pharmacol. 1994 Mar;45(3):390–394. [PubMed] [Google Scholar]
- Shenker A., Laue L., Kosugi S., Merendino J. J., Jr, Minegishi T., Cutler G. B., Jr A constitutively activating mutation of the luteinizing hormone receptor in familial male precocious puberty. Nature. 1993 Oct 14;365(6447):652–654. doi: 10.1038/365652a0. [DOI] [PubMed] [Google Scholar]
- Song Z. H., Young W. S., 3rd, Brownstein M. J., Bonner T. I. Molecular cloning of a novel candidate G protein-coupled receptor from rat brain. FEBS Lett. 1994 Sep 12;351(3):375–379. doi: 10.1016/0014-5793(94)00888-4. [DOI] [PubMed] [Google Scholar]
- Tiberi M., Caron M. G. High agonist-independent activity is a distinguishing feature of the dopamine D1B receptor subtype. J Biol Chem. 1994 Nov 11;269(45):27925–27931. [PubMed] [Google Scholar]
- Velu T. J., Beguinot L., Vass W. C., Zhang K., Pastan I., Lowy D. R. Retroviruses expressing different levels of the normal epidermal growth factor receptor: biological properties and new bioassay. J Cell Biochem. 1989 Feb;39(2):153–166. doi: 10.1002/jcb.240390207. [DOI] [PubMed] [Google Scholar]