Abstract
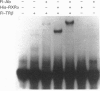
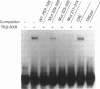
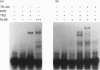
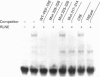
Transcription of the gene for phosphoenolpyruvate carboxy-kinase (PEPCK) is stimulated by thyroid hormone (T3), glucagon (via cyclic AMP) and glucocorticoids. A region of the PEPCK promoter between -332 and -308 mediates the induction of transcription by T3. To characterize this region further, mutations were introduced into this region of the PEPCK promoter and the modified promoters ligated to the chloramphenicol acetyltransferase (CAT) reporter gene. Using these PEPCK-CAT vectors in transient transfections in HepG2 cells, it was found that T3 stimulates PEPCK transcription through two direct repeats of the AGGTCA motif located between nucleotides -330 and -319 [PEPCK-thyroid-hormone-responsive element (TRE)]. The beta form of the T3 receptor (TR beta) bound PEPCK-TRE as a homodimer but bound far more efficiently as a heterodimeric complex with the retinoid X receptor (RXR). An additional region called P3(I) (-250 to -234) is required for T3 responsiveness and binds members of the CCAAT-enhancer-binding protein (C/EBP) family. P3(I) contains an AGGTCA-like motif that can bind the TR beta-RXR heterodimer. Mutagenesis of this motif abolished TR beta-RXR binding without reducing T3 induction. Mutation of the C/EBP-binding site or insertion of a cyclic AMP-responsive-binding-protein site at P3(I) eliminated the T3 response. Our results indicate that T3 stimulation of PEPCK transcription is mediated by TR beta bound to PEPCK-TRE and requires C/EBP to be bound at the P3(I) site.
Full text
PDF


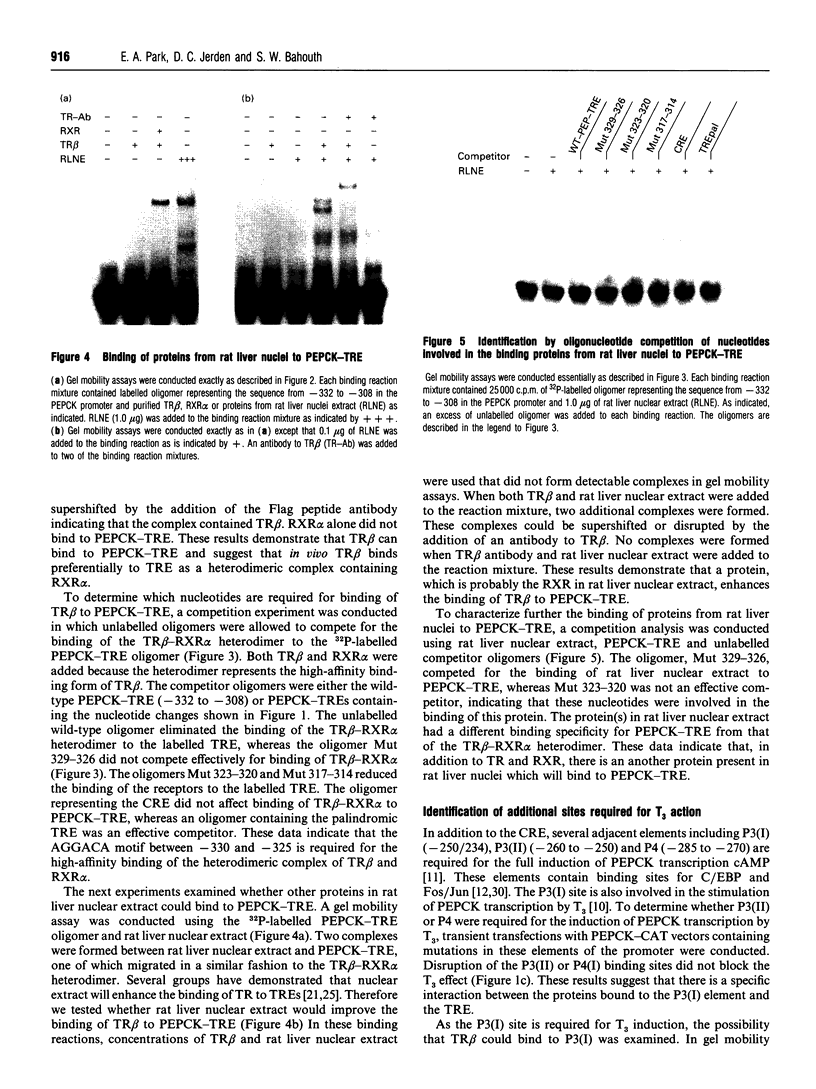
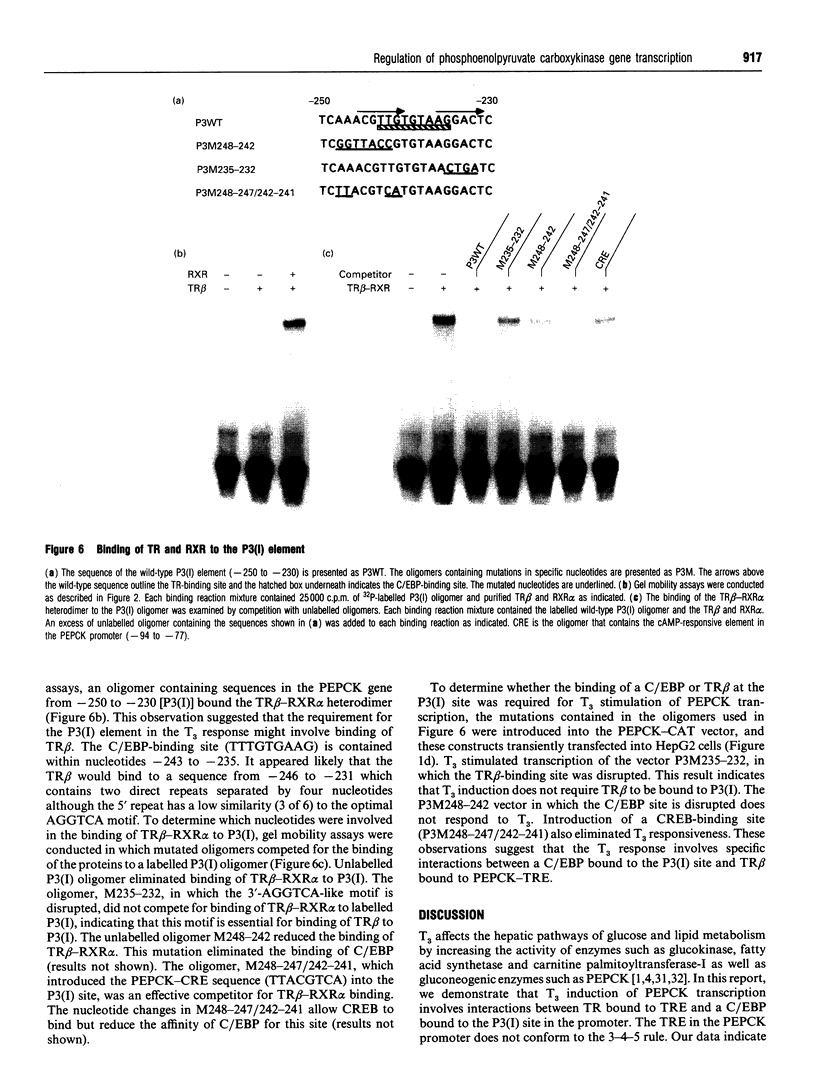


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Birkenmeier E. H., Gwynn B., Howard S., Jerry J., Gordon J. I., Landschulz W. H., McKnight S. L. Tissue-specific expression, developmental regulation, and genetic mapping of the gene encoding CCAAT/enhancer binding protein. Genes Dev. 1989 Aug;3(8):1146–1156. doi: 10.1101/gad.3.8.1146. [DOI] [PubMed] [Google Scholar]
- Cao Z., Umek R. M., McKnight S. L. Regulated expression of three C/EBP isoforms during adipose conversion of 3T3-L1 cells. Genes Dev. 1991 Sep;5(9):1538–1552. doi: 10.1101/gad.5.9.1538. [DOI] [PubMed] [Google Scholar]
- Forman B. M., Casanova J., Raaka B. M., Ghysdael J., Samuels H. H. Half-site spacing and orientation determines whether thyroid hormone and retinoic acid receptors and related factors bind to DNA response elements as monomers, homodimers, or heterodimers. Mol Endocrinol. 1992 Mar;6(3):429–442. doi: 10.1210/mend.6.3.1316541. [DOI] [PubMed] [Google Scholar]
- Giralt M., Park E. A., Gurney A. L., Liu J. S., Hakimi P., Hanson R. W. Identification of a thyroid hormone response element in the phosphoenolpyruvate carboxykinase (GTP) gene. Evidence for synergistic interaction between thyroid hormone and cAMP cis-regulatory elements. J Biol Chem. 1991 Nov 15;266(32):21991–21996. [PubMed] [Google Scholar]
- Goodridge A. G. Dietary regulation of gene expression: enzymes involved in carbohydrate and lipid metabolism. Annu Rev Nutr. 1987;7:157–185. doi: 10.1146/annurev.nu.07.070187.001105. [DOI] [PubMed] [Google Scholar]
- Gorski K., Carneiro M., Schibler U. Tissue-specific in vitro transcription from the mouse albumin promoter. Cell. 1986 Dec 5;47(5):767–776. doi: 10.1016/0092-8674(86)90519-2. [DOI] [PubMed] [Google Scholar]
- Gurney A. L., Park E. A., Giralt M., Liu J., Hanson R. W. Opposing actions of Fos and Jun on transcription of the phosphoenolpyruvate carboxykinase (GTP) gene. Dominant negative regulation by Fos. J Biol Chem. 1992 Sep 5;267(25):18133–18139. [PubMed] [Google Scholar]
- Hall R. K., Scott D. K., Noisin E. L., Lucas P. C., Granner D. K. Activation of the phosphoenolpyruvate carboxykinase gene retinoic acid response element is dependent on a retinoic acid receptor/coregulator complex. Mol Cell Biol. 1992 Dec;12(12):5527–5535. doi: 10.1128/mcb.12.12.5527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartong R., Wiersinga W. M., Lamers W. H. Nuclear 3,5,3'-triiodothyronine receptor occupancy, phosphoenolpyruvate carboxykinase (PEPck) messenger ribonucleic acid levels and PEPck enzyme activity in rat liver. Endocrinology. 1987 Jun;120(6):2460–2467. doi: 10.1210/endo-120-6-2460. [DOI] [PubMed] [Google Scholar]
- Höppner W., Seitz H. J. Effect of thyroid hormones on glucokinase gene transcription in rat liver. J Biol Chem. 1989 Dec 5;264(34):20643–20647. [PubMed] [Google Scholar]
- Imai E., Stromstedt P. E., Quinn P. G., Carlstedt-Duke J., Gustafsson J. A., Granner D. K. Characterization of a complex glucocorticoid response unit in the phosphoenolpyruvate carboxykinase gene. Mol Cell Biol. 1990 Sep;10(9):4712–4719. doi: 10.1128/mcb.10.9.4712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iynedjian P. B., Salavert A. Effects of glucagon, dexamethasone and triiodothyronine on phosphoenolpyruvate carboxykinase (GTP) synthesis and mRNA level in rat liver cells. Eur J Biochem. 1984 Dec 17;145(3):489–497. doi: 10.1111/j.1432-1033.1984.tb08583.x. [DOI] [PubMed] [Google Scholar]
- Janknecht R., de Martynoff G., Lou J., Hipskind R. A., Nordheim A., Stunnenberg H. G. Rapid and efficient purification of native histidine-tagged protein expressed by recombinant vaccinia virus. Proc Natl Acad Sci U S A. 1991 Oct 15;88(20):8972–8976. doi: 10.1073/pnas.88.20.8972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz R. W., Koenig R. J. Nonbiased identification of DNA sequences that bind thyroid hormone receptor alpha 1 with high affinity. J Biol Chem. 1993 Sep 15;268(26):19392–19397. [PubMed] [Google Scholar]
- Lamers W. H., Hanson R. W., Meisner H. M. cAMP stimulates transcription of the gene for cytosolic phosphoenolpyruvate carboxykinase in rat liver nuclei. Proc Natl Acad Sci U S A. 1982 Sep;79(17):5137–5141. doi: 10.1073/pnas.79.17.5137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazar M. A., Berrodin T. J., Harding H. P. Differential DNA binding by monomeric, homodimeric, and potentially heteromeric forms of the thyroid hormone receptor. Mol Cell Biol. 1991 Oct;11(10):5005–5015. doi: 10.1128/mcb.11.10.5005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazar M. A. Thyroid hormone receptors: multiple forms, multiple possibilities. Endocr Rev. 1993 Apr;14(2):184–193. doi: 10.1210/edrv-14-2-184. [DOI] [PubMed] [Google Scholar]
- Liu J. S., Park E. A., Gurney A. L., Roesler W. J., Hanson R. W. Cyclic AMP induction of phosphoenolpyruvate carboxykinase (GTP) gene transcription is mediated by multiple promoter elements. J Biol Chem. 1991 Oct 5;266(28):19095–19102. [PubMed] [Google Scholar]
- Loose D. S., Cameron D. K., Short H. P., Hanson R. W. Thyroid hormone regulates transcription of the gene for cytosolic phosphoenolpyruvate carboxykinase (GTP) in rat liver. Biochemistry. 1985 Aug 13;24(17):4509–4512. doi: 10.1021/bi00338a004. [DOI] [PubMed] [Google Scholar]
- Lucas P. C., Forman B. M., Samuels H. H., Granner D. K. Specificity of a retinoic acid response element in the phosphoenolpyruvate carboxykinase gene promoter: consequences of both retinoic acid and thyroid hormone receptor binding. Mol Cell Biol. 1991 Oct;11(10):5164–5170. doi: 10.1128/mcb.11.10.5164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mangelsdorf D. J., Ong E. S., Dyck J. A., Evans R. M. Nuclear receptor that identifies a novel retinoic acid response pathway. Nature. 1990 May 17;345(6272):224–229. doi: 10.1038/345224a0. [DOI] [PubMed] [Google Scholar]
- McKnight S. L., Lane M. D., Gluecksohn-Waelsch S. Is CCAAT/enhancer-binding protein a central regulator of energy metabolism? Genes Dev. 1989 Dec;3(12B):2021–2024. doi: 10.1101/gad.3.12b.2021. [DOI] [PubMed] [Google Scholar]
- Mitchell J., Noisin E., Hall R., O'Brien R., Imai E., Granner D. Integration of multiple signals through a complex hormone response unit in the phosphoenolpyruvate carboxykinase gene promoter. Mol Endocrinol. 1994 May;8(5):585–594. doi: 10.1210/mend.8.5.8058068. [DOI] [PubMed] [Google Scholar]
- Murray M. B., Zilz N. D., McCreary N. L., MacDonald M. J., Towle H. C. Isolation and characterization of rat cDNA clones for two distinct thyroid hormone receptors. J Biol Chem. 1988 Sep 5;263(25):12770–12777. [PubMed] [Google Scholar]
- Mynatt R. L., Park E. A., Thorngate F. E., Das H. K., Cook G. A. Changes in carnitine palmitoyltransferase-I mRNA abundance produced by hyperthyroidism and hypothyroidism parallel changes in activity. Biochem Biophys Res Commun. 1994 Jun 15;201(2):932–937. doi: 10.1006/bbrc.1994.1791. [DOI] [PubMed] [Google Scholar]
- Park E. A., Gurney A. L., Nizielski S. E., Hakimi P., Cao Z., Moorman A., Hanson R. W. Relative roles of CCAAT/enhancer-binding protein beta and cAMP regulatory element-binding protein in controlling transcription of the gene for phosphoenolpyruvate carboxykinase (GTP). J Biol Chem. 1993 Jan 5;268(1):613–619. [PubMed] [Google Scholar]
- Park E. A., Roesler W. J., Liu J., Klemm D. J., Gurney A. L., Thatcher J. D., Shuman J., Friedman A., Hanson R. W. The role of the CCAAT/enhancer-binding protein in the transcriptional regulation of the gene for phosphoenolpyruvate carboxykinase (GTP). Mol Cell Biol. 1990 Dec;10(12):6264–6272. doi: 10.1128/mcb.10.12.6264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel Y. M., Yun J. S., Liu J., McGrane M. M., Hanson R. W. An analysis of regulatory elements in the phosphoenolpyruvate carboxykinase (GTP) gene which are responsible for its tissue-specific expression and metabolic control in transgenic mice. J Biol Chem. 1994 Feb 25;269(8):5619–5628. [PubMed] [Google Scholar]
- Roesler W. J., McFie P. J., Dauvin C. The liver-enriched transcription factor D-site-binding protein activates the promoter of the phosphoenolpyruvate carboxykinase gene in hepatoma cells. J Biol Chem. 1992 Oct 15;267(29):21235–21243. [PubMed] [Google Scholar]
- Rosella G., Zajac J. D., Kaczmarczyk S. J., Andrikopoulos S., Proietto J. Impaired suppression of gluconeogenesis induced by overexpression of a noninsulin-responsive phosphoenolpyruvate carboxykinase gene. Mol Endocrinol. 1993 Nov;7(11):1456–1462. doi: 10.1210/mend.7.11.8114759. [DOI] [PubMed] [Google Scholar]
- Sasaki K., Cripe T. P., Koch S. R., Andreone T. L., Petersen D. D., Beale E. G., Granner D. K. Multihormonal regulation of phosphoenolpyruvate carboxykinase gene transcription. The dominant role of insulin. J Biol Chem. 1984 Dec 25;259(24):15242–15251. [PubMed] [Google Scholar]
- Schaufele F., West B. L., Baxter J. D. Synergistic activation of the rat growth hormone promoter by Pit-1 and the thyroid hormone receptor. Mol Endocrinol. 1992 Apr;6(4):656–665. doi: 10.1210/mend.6.4.1584227. [DOI] [PubMed] [Google Scholar]
- Schmidt E. D., van Beeren M., Glass C. K., Wiersinga W. M., Lamers W. H. Interaction between the thyroid hormone receptor and co-factors on the promoter of the gene encoding phospho enol pyruvate carboxykinase. Biochim Biophys Acta. 1993 Feb 20;1172(1-2):82–88. doi: 10.1016/0167-4781(93)90272-f. [DOI] [PubMed] [Google Scholar]
- Tontonoz P., Hu E., Devine J., Beale E. G., Spiegelman B. M. PPAR gamma 2 regulates adipose expression of the phosphoenolpyruvate carboxykinase gene. Mol Cell Biol. 1995 Jan;15(1):351–357. doi: 10.1128/mcb.15.1.351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umesono K., Murakami K. K., Thompson C. C., Evans R. M. Direct repeats as selective response elements for the thyroid hormone, retinoic acid, and vitamin D3 receptors. Cell. 1991 Jun 28;65(7):1255–1266. doi: 10.1016/0092-8674(91)90020-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voz M. L., Peers B., Wiedig M. J., Jacquemin P., Belayew A., Martial J. A. Transcriptional regulation by triiodothyronine requires synergistic action of the thyroid receptor with another trans-acting factor. Mol Cell Biol. 1992 Sep;12(9):3991–3997. doi: 10.1128/mcb.12.9.3991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams G. R., Harney J. W., Moore D. D., Larsen P. R., Brent G. A. Differential capacity of wild type promoter elements for binding and trans-activation by retinoic acid and thyroid hormone receptors. Mol Endocrinol. 1992 Oct;6(10):1527–1537. doi: 10.1210/mend.6.10.1333048. [DOI] [PubMed] [Google Scholar]