Abstract
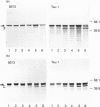
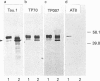
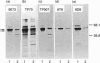
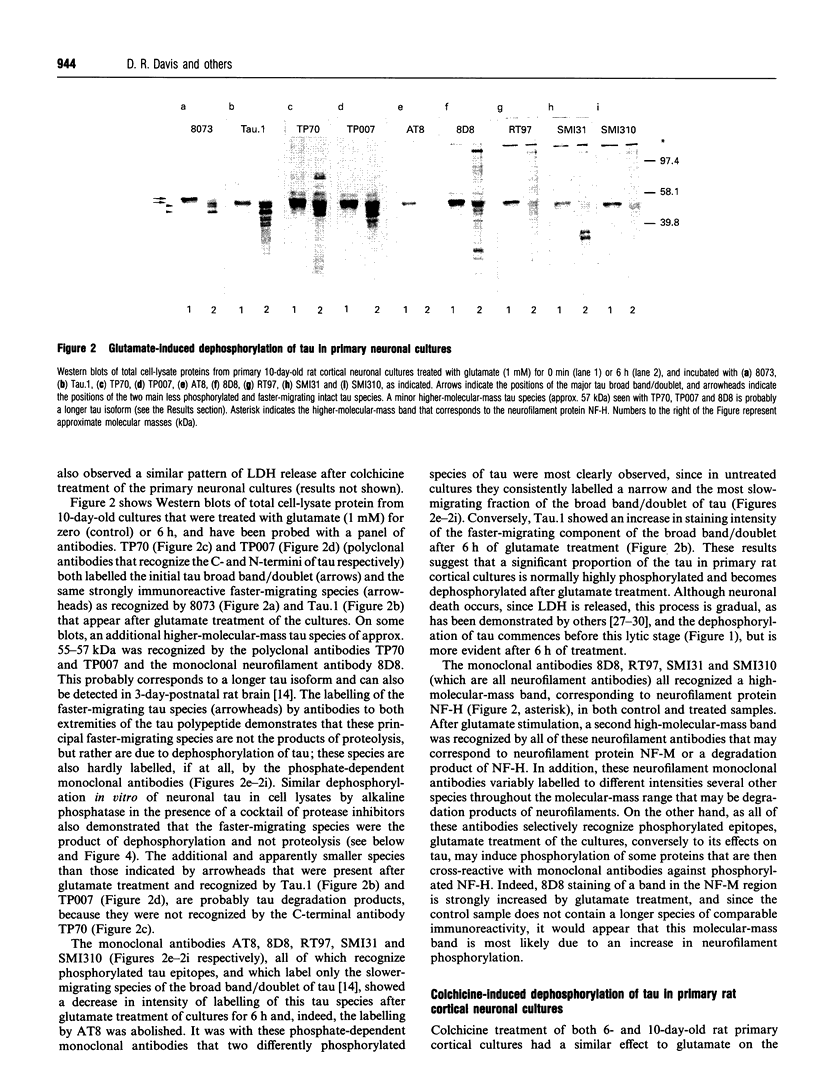
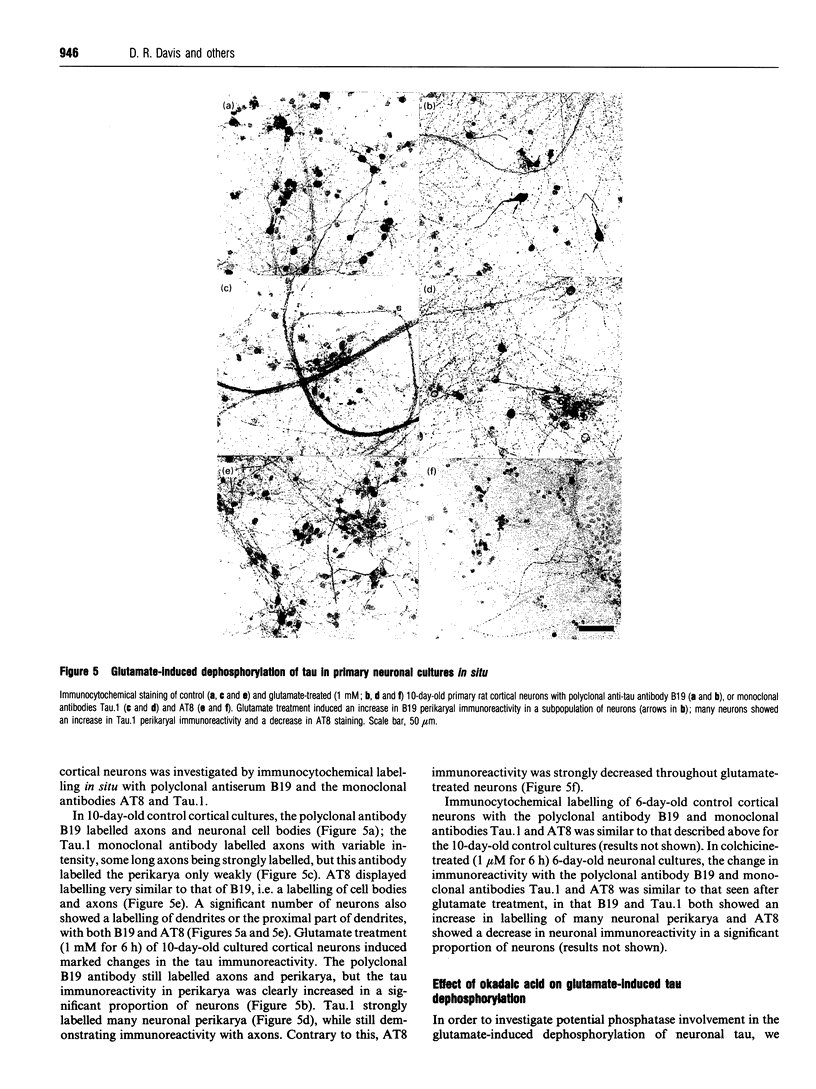
The effects of the excitatory amino acid glutamate, the microtubule destabilizing agent colchicine, and beta 25-35-amyloid peptide on the phosphorylation state of tau were studied in rat cortical neurons in primary culture. Using immunocytochemistry and Western-blot analysis, we demonstrated that a proportion of tau in these cultures is normally highly phosphorylated, but most of this tau fraction is dephosphorylated after treatment of the cultures with glutamate or colchicine, but not with beta-amyloid; the glutamate- and colchicine-induced changes in tau phosphorylation commenced before cell death, as assessed by release of lactate dehydrogenase. Dephosphorylation of tau was readily revealed by using the monoclonal antibodies Tau.1 and AT8, which have phosphate-sensitive epitopes that both centre around serine-199 and -202 (numbering of the largest tau isoform). On Western blots and by immunocytochemistry, AT8 labelling strongly decreased after glutamate and colchicine treatments, whereas Tau.1 staining was more intense. Neurofilament monoclonal antibodies, including RT97, 8D8, SMI31 and SMI310, all additionally known to recognize tau in a phosphorylation-dependent manner, also demonstrated that glutamate and colchicine treatments of the cultures induced a dephosphorylation of tau. We also showed immunocytochemically that there is an increase in tau immunoreactivity in neuronal perikarya in response to glutamate and colchicine treatment, and this occurs concomitantly with the dephosphorylation of tau. Treatment of the primary rat cortical neuronal cultures with beta 25-35-amyloid peptide, under conditions which induce neuronal degeneration, did not induce a change in tau phosphorylation, and failed to act synergistically with glutamate to produce an increase in dephosphorylation of tau over that produced by glutamate treatment alone. These findings demonstrate that glutamate and colchicine induce tau dephosphorylation, as opposed to increased tau phosphorylation, which would be more indicative of Alzheimer-type neurodegeneration.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bancher C., Brunner C., Lassmann H., Budka H., Jellinger K., Wiche G., Seitelberger F., Grundke-Iqbal I., Iqbal K., Wisniewski H. M. Accumulation of abnormally phosphorylated tau precedes the formation of neurofibrillary tangles in Alzheimer's disease. Brain Res. 1989 Jan 16;477(1-2):90–99. doi: 10.1016/0006-8993(89)91396-6. [DOI] [PubMed] [Google Scholar]
- Baudier J., Cole R. D. Phosphorylation of tau proteins to a state like that in Alzheimer's brain is catalyzed by a calcium/calmodulin-dependent kinase and modulated by phospholipids. J Biol Chem. 1987 Dec 25;262(36):17577–17583. [PubMed] [Google Scholar]
- Binder L. I., Frankfurter A., Rebhun L. I. The distribution of tau in the mammalian central nervous system. J Cell Biol. 1985 Oct;101(4):1371–1378. doi: 10.1083/jcb.101.4.1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bramblett G. T., Goedert M., Jakes R., Merrick S. E., Trojanowski J. Q., Lee V. M. Abnormal tau phosphorylation at Ser396 in Alzheimer's disease recapitulates development and contributes to reduced microtubule binding. Neuron. 1993 Jun;10(6):1089–1099. doi: 10.1016/0896-6273(93)90057-x. [DOI] [PubMed] [Google Scholar]
- Brion J. P., Couck A. M., Passareiro E., Flament-Durand J. Neurofibrillary tangles of Alzheimer's disease: an immunohistochemical study. J Submicrosc Cytol. 1985 Jan;17(1):89–96. [PubMed] [Google Scholar]
- Brion J. P., Couck A. M., Robertson J., Loviny T. L., Anderton B. H. Neurofilament monoclonal antibodies RT97 and 8D8 recognize different modified epitopes in paired helical filament-tau in Alzheimer's disease. J Neurochem. 1993 Apr;60(4):1372–1382. doi: 10.1111/j.1471-4159.1993.tb03298.x. [DOI] [PubMed] [Google Scholar]
- Brion J. P., Hanger D. P., Couck A. M., Anderton B. H. A68 proteins in Alzheimer's disease are composed of several tau isoforms in a phosphorylated state which affects their electrophoretic mobilities. Biochem J. 1991 Nov 1;279(Pt 3):831–836. doi: 10.1042/bj2790831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brion J. P., Smith C., Couck A. M., Gallo J. M., Anderton B. H. Developmental changes in tau phosphorylation: fetal tau is transiently phosphorylated in a manner similar to paired helical filament-tau characteristic of Alzheimer's disease. J Neurochem. 1993 Dec;61(6):2071–2080. doi: 10.1111/j.1471-4159.1993.tb07444.x. [DOI] [PubMed] [Google Scholar]
- Choi D. W. Glutamate neurotoxicity and diseases of the nervous system. Neuron. 1988 Oct;1(8):623–634. doi: 10.1016/0896-6273(88)90162-6. [DOI] [PubMed] [Google Scholar]
- Choi D. W. Ionic dependence of glutamate neurotoxicity. J Neurosci. 1987 Feb;7(2):369–379. doi: 10.1523/JNEUROSCI.07-02-00369.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman M. P., Anderton B. H. Phosphate-dependent monoclonal antibodies to neurofilaments and Alzheimer neurofibrillary tangles recognize a synthetic phosphopeptide. J Neurochem. 1990 May;54(5):1548–1555. doi: 10.1111/j.1471-4159.1990.tb01203.x. [DOI] [PubMed] [Google Scholar]
- Correas I., Díaz-Nido J., Avila J. Microtubule-associated protein tau is phosphorylated by protein kinase C on its tubulin binding domain. J Biol Chem. 1992 Aug 5;267(22):15721–15728. [PubMed] [Google Scholar]
- Dawson V. L., Dawson T. M., Bartley D. A., Uhl G. R., Snyder S. H. Mechanisms of nitric oxide-mediated neurotoxicity in primary brain cultures. J Neurosci. 1993 Jun;13(6):2651–2661. doi: 10.1523/JNEUROSCI.13-06-02651.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drechsel D. N., Hyman A. A., Cobb M. H., Kirschner M. W. Modulation of the dynamic instability of tubulin assembly by the microtubule-associated protein tau. Mol Biol Cell. 1992 Oct;3(10):1141–1154. doi: 10.1091/mbc.3.10.1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drewes G., Mandelkow E. M., Baumann K., Goris J., Merlevede W., Mandelkow E. Dephosphorylation of tau protein and Alzheimer paired helical filaments by calcineurin and phosphatase-2A. FEBS Lett. 1993 Dec 28;336(3):425–432. doi: 10.1016/0014-5793(93)80850-t. [DOI] [PubMed] [Google Scholar]
- Gallo J. M., Hanger D. P., Twist E. C., Kosik K. S., Anderton B. H. Expression and phosphorylation of a three-repeat isoform of tau in transfected non-neuronal cells. Biochem J. 1992 Sep 1;286(Pt 2):399–404. doi: 10.1042/bj2860399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goedert M., Spillantini M. G., Cairns N. J., Crowther R. A. Tau proteins of Alzheimer paired helical filaments: abnormal phosphorylation of all six brain isoforms. Neuron. 1992 Jan;8(1):159–168. doi: 10.1016/0896-6273(92)90117-v. [DOI] [PubMed] [Google Scholar]
- Goedert M., Spillantini M. G., Jakes R., Rutherford D., Crowther R. A. Multiple isoforms of human microtubule-associated protein tau: sequences and localization in neurofibrillary tangles of Alzheimer's disease. Neuron. 1989 Oct;3(4):519–526. doi: 10.1016/0896-6273(89)90210-9. [DOI] [PubMed] [Google Scholar]
- Gong C. X., Grundke-Iqbal I., Damuni Z., Iqbal K. Dephosphorylation of microtubule-associated protein tau by protein phosphatase-1 and -2C and its implication in Alzheimer disease. FEBS Lett. 1994 Mar 14;341(1):94–98. doi: 10.1016/0014-5793(94)80247-5. [DOI] [PubMed] [Google Scholar]
- Gong C. X., Grundke-Iqbal I., Iqbal K. Dephosphorylation of Alzheimer's disease abnormally phosphorylated tau by protein phosphatase-2A. Neuroscience. 1994 Aug;61(4):765–772. doi: 10.1016/0306-4522(94)90400-6. [DOI] [PubMed] [Google Scholar]
- Greenberg S. G., Davies P., Schein J. D., Binder L. I. Hydrofluoric acid-treated tau PHF proteins display the same biochemical properties as normal tau. J Biol Chem. 1992 Jan 5;267(1):564–569. [PubMed] [Google Scholar]
- Hanger D. P., Hughes K., Woodgett J. R., Brion J. P., Anderton B. H. Glycogen synthase kinase-3 induces Alzheimer's disease-like phosphorylation of tau: generation of paired helical filament epitopes and neuronal localisation of the kinase. Neurosci Lett. 1992 Nov 23;147(1):58–62. doi: 10.1016/0304-3940(92)90774-2. [DOI] [PubMed] [Google Scholar]
- Johnson G. V. Differential phosphorylation of tau by cyclic AMP-dependent protein kinase and Ca2+/calmodulin-dependent protein kinase II: metabolic and functional consequences. J Neurochem. 1992 Dec;59(6):2056–2062. doi: 10.1111/j.1471-4159.1992.tb10094.x. [DOI] [PubMed] [Google Scholar]
- Kanemaru K., Ihara Y. [Abnormal proteins in the brain in Alzheimer's disease]. Tanpakushitsu Kakusan Koso. 1991 Jan;36(1):2–11. [PubMed] [Google Scholar]
- Kenessey A., Yen S. H. The extent of phosphorylation of fetal tau is comparable to that of PHF-tau from Alzheimer paired helical filaments. Brain Res. 1993 Nov 26;629(1):40–46. doi: 10.1016/0006-8993(93)90478-6. [DOI] [PubMed] [Google Scholar]
- Koh J. Y., Choi D. W. Quantitative determination of glutamate mediated cortical neuronal injury in cell culture by lactate dehydrogenase efflux assay. J Neurosci Methods. 1987 May;20(1):83–90. doi: 10.1016/0165-0270(87)90041-0. [DOI] [PubMed] [Google Scholar]
- Koh J. Y., Goldberg M. P., Hartley D. M., Choi D. W. Non-NMDA receptor-mediated neurotoxicity in cortical culture. J Neurosci. 1990 Feb;10(2):693–705. doi: 10.1523/JNEUROSCI.10-02-00693.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koh J. Y., Yang L. L., Cotman C. W. Beta-amyloid protein increases the vulnerability of cultured cortical neurons to excitotoxic damage. Brain Res. 1990 Nov 19;533(2):315–320. doi: 10.1016/0006-8993(90)91355-k. [DOI] [PubMed] [Google Scholar]
- Kojima M., Takahashi N., Ikeuchi T., Hatanaka H. Nerve growth factor (NGF)-mediated up-regulation of low-affinity NGF receptor gene expression in cultured basal forebrain cholinergic neurons from postnatal 3-day-old rats. Brain Res Mol Brain Res. 1992 Dec;16(3-4):267–273. doi: 10.1016/0169-328x(92)90235-4. [DOI] [PubMed] [Google Scholar]
- Kosik K. S., Orecchio L. D., Bakalis S., Duffy L., Neve R. L. Partial sequence of MAP2 in the region of a shared epitope with Alzheimer neurofibrillary tangles. J Neurochem. 1988 Aug;51(2):587–598. doi: 10.1111/j.1471-4159.1988.tb01079.x. [DOI] [PubMed] [Google Scholar]
- Kosik K. S., Orecchio L. D., Binder L., Trojanowski J. Q., Lee V. M., Lee G. Epitopes that span the tau molecule are shared with paired helical filaments. Neuron. 1988 Nov;1(9):817–825. doi: 10.1016/0896-6273(88)90129-8. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lichtenberg-Kraag B., Mandelkow E. M., Biernat J., Steiner B., Schröter C., Gustke N., Meyer H. E., Mandelkow E. Phosphorylation-dependent epitopes of neurofilament antibodies on tau protein and relationship with Alzheimer tau. Proc Natl Acad Sci U S A. 1992 Jun 15;89(12):5384–5388. doi: 10.1073/pnas.89.12.5384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindwall G., Cole R. D. Phosphorylation affects the ability of tau protein to promote microtubule assembly. J Biol Chem. 1984 Apr 25;259(8):5301–5305. [PubMed] [Google Scholar]
- Mattson M. P. Antigenic changes similar to those seen in neurofibrillary tangles are elicited by glutamate and Ca2+ influx in cultured hippocampal neurons. Neuron. 1990 Jan;4(1):105–117. doi: 10.1016/0896-6273(90)90447-n. [DOI] [PubMed] [Google Scholar]
- Mattson M. P., Cheng B., Davis D., Bryant K., Lieberburg I., Rydel R. E. beta-Amyloid peptides destabilize calcium homeostasis and render human cortical neurons vulnerable to excitotoxicity. J Neurosci. 1992 Feb;12(2):376–389. doi: 10.1523/JNEUROSCI.12-02-00376.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattson M. P. Effects of microtubule stabilization and destabilization on tau immunoreactivity in cultured hippocampal neurons. Brain Res. 1992 Jun 5;582(1):107–118. doi: 10.1016/0006-8993(92)90323-2. [DOI] [PubMed] [Google Scholar]
- Mercken M., Vandermeeren M., Lübke U., Six J., Boons J., Van de Voorde A., Martin J. J., Gheuens J. Monoclonal antibodies with selective specificity for Alzheimer Tau are directed against phosphatase-sensitive epitopes. Acta Neuropathol. 1992;84(3):265–272. doi: 10.1007/BF00227819. [DOI] [PubMed] [Google Scholar]
- Robertson J., Loviny T. L., Goedert M., Jakes R., Murray K. J., Anderton B. H., Hanger D. P. Phosphorylation of tau by cyclic-AMP-dependent protein kinase. Dementia. 1993 Sep-Oct;4(5):256–263. doi: 10.1159/000107331. [DOI] [PubMed] [Google Scholar]
- Sandhu F. A., Porter R. H., Eller R. V., Zain S. B., Salim M., Greenamyre J. T. NMDA and AMPA receptors in transgenic mice expressing human beta-amyloid protein. J Neurochem. 1993 Dec;61(6):2286–2289. doi: 10.1111/j.1471-4159.1993.tb07471.x. [DOI] [PubMed] [Google Scholar]
- Sautière P. E., Sindou P., Couratier P., Hugon J., Wattez A., Delacourte A. Tau antigenic changes induced by glutamate in rat primary culture model: a biochemical approach. Neurosci Lett. 1992 Jun 22;140(2):206–210. doi: 10.1016/0304-3940(92)90104-f. [DOI] [PubMed] [Google Scholar]
- Scott C. W., Spreen R. C., Herman J. L., Chow F. P., Davison M. D., Young J., Caputo C. B. Phosphorylation of recombinant tau by cAMP-dependent protein kinase. Identification of phosphorylation sites and effect on microtubule assembly. J Biol Chem. 1993 Jan 15;268(2):1166–1173. [PubMed] [Google Scholar]
- Scott C. W., Vulliet P. R., Caputo C. B. Phosphorylation of tau by proline-directed protein kinase (p34cdc2/p58cyclin A) decreases tau-induced microtubule assembly and antibody SMI33 reactivity. Brain Res. 1993 May 21;611(2):237–242. doi: 10.1016/0006-8993(93)90508-k. [DOI] [PubMed] [Google Scholar]
- Sindou P., Couratier P., Barthe D., Hugon J. A dose-dependent increase of Tau immunostaining is produced by glutamate toxicity in primary neuronal cultures. Brain Res. 1992 Feb 14;572(1-2):242–246. doi: 10.1016/0006-8993(92)90476-p. [DOI] [PubMed] [Google Scholar]
- Steiner B., Mandelkow E. M., Biernat J., Gustke N., Meyer H. E., Schmidt B., Mieskes G., Söling H. D., Drechsel D., Kirschner M. W. Phosphorylation of microtubule-associated protein tau: identification of the site for Ca2(+)-calmodulin dependent kinase and relationship with tau phosphorylation in Alzheimer tangles. EMBO J. 1990 Nov;9(11):3539–3544. doi: 10.1002/j.1460-2075.1990.tb07563.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sternberger N. H., Sternberger L. A., Ulrich J. Aberrant neurofilament phosphorylation in Alzheimer disease. Proc Natl Acad Sci U S A. 1985 Jun;82(12):4274–4276. doi: 10.1073/pnas.82.12.4274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sygowski L. A., Fieles A. W., Lo M. M., Scott C. W., Caputo C. B. Phosphorylation of tau protein in tau-transfected 3T3 cells. Brain Res Mol Brain Res. 1993 Nov;20(3):221–228. doi: 10.1016/0169-328x(93)90044-p. [DOI] [PubMed] [Google Scholar]
- Watanabe A., Hasegawa M., Suzuki M., Takio K., Morishima-Kawashima M., Titani K., Arai T., Kosik K. S., Ihara Y. In vivo phosphorylation sites in fetal and adult rat tau. J Biol Chem. 1993 Dec 5;268(34):25712–25717. [PubMed] [Google Scholar]
- Wischik C. M., Novak M., Edwards P. C., Klug A., Tichelaar W., Crowther R. A. Structural characterization of the core of the paired helical filament of Alzheimer disease. Proc Natl Acad Sci U S A. 1988 Jul;85(13):4884–4888. doi: 10.1073/pnas.85.13.4884. [DOI] [PMC free article] [PubMed] [Google Scholar]