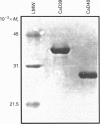
Abstract
We have reported previously that each smooth-muscle caldesmon binds predominantly to a region within residues 142-227 of tropomyosin, but a weaker binding site also exists at the N-terminal region of tropomyosin [Watson, Kuhn, Novy, Lin and Mak (1990) J. Biol. Chem. 265, 18860-18866]. In view of recent evidence for the presence of tropomyosin-binding sites at both the N- and C-terminal domains of caldesmon, we have studied the binding of the N- and C-terminal fragments of human fibroblast caldesmon expressed in Escherichia coli to tropomyosin and its CNBr fragments. The N-terminal fragment, CaD40 (residues 1-152), binds tropomyosin, but the interaction is mostly abolished in the presence of actin. CaD40 binds strongly to Cn1B(142-281) of tropomyosin, but weakly to Cn1A(11-127). The C-terminal fragment, CaD39, which corresponds to residues 443-736 of gizzard caldesmon, binds tropomyosin, and the interaction is enhanced by actin. CaD39 binds to both Cn1A(11-127) and Cn1B(142-281) of tropomyosin. Our results suggest that the N-terminal domain of caldesmon interacts with the C-terminal half of one tropomyosin molecule, whereas the C-terminal domain binds to both N- and C-terminal regions of the adjacent tropomyosin molecule along the actin filament. In addition, the binding of the N-terminal domain of caldesmon to the actin-tropomyosin filament is weak, which may allow this domain to project off the thin filament to interact with myosin.
Full text
PDF

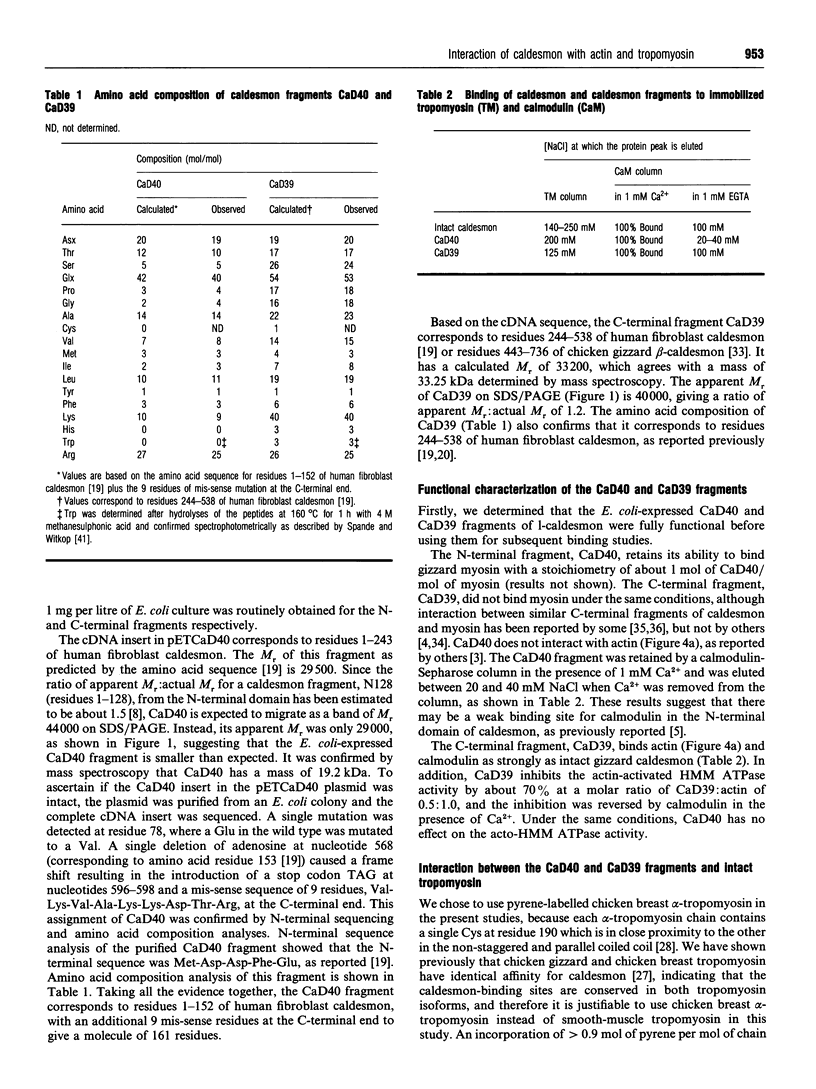


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ball E. H., Kovala T. Mapping of caldesmon: relationship between the high and low molecular weight forms. Biochemistry. 1988 Aug 9;27(16):6093–6098. doi: 10.1021/bi00416a039. [DOI] [PubMed] [Google Scholar]
- Bartegi A., Fattoum A., Derancourt J., Kassab R. Characterization of the carboxyl-terminal 10-kDa cyanogen bromide fragment of caldesmon as an actin-calmodulin-binding region. J Biol Chem. 1990 Sep 5;265(25):15231–15238. [PubMed] [Google Scholar]
- Bogatcheva N. V., Vorotnikov A. V., Birukov K. G., Shirinsky V. P., Gusev N. B. Phosphorylation by casein kinase II affects the interaction of caldesmon with smooth muscle myosin and tropomyosin. Biochem J. 1993 Mar 1;290(Pt 2):437–442. doi: 10.1042/bj2900437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bretscher A. Smooth muscle caldesmon. Rapid purification and F-actin cross-linking properties. J Biol Chem. 1984 Oct 25;259(20):12873–12880. [PubMed] [Google Scholar]
- Bryan J., Imai M., Lee R., Moore P., Cook R. G., Lin W. G. Cloning and expression of a smooth muscle caldesmon. J Biol Chem. 1989 Aug 15;264(23):13873–13879. [PubMed] [Google Scholar]
- Childs T. J., Watson M. H., Sanghera J. S., Campbell D. L., Pelech S. L., Mak A. S. Phosphorylation of smooth muscle caldesmon by mitogen-activated protein (MAP) kinase and expression of MAP kinase in differentiated smooth muscle cells. J Biol Chem. 1992 Nov 15;267(32):22853–22859. [PubMed] [Google Scholar]
- Graceffa P., Lehrer S. S. The excimer fluorescence of pyrene-labeled tropomyosin. A probe of conformational dynamics. J Biol Chem. 1980 Dec 10;255(23):11296–11300. [PubMed] [Google Scholar]
- Graceffa P., Wang C. L., Stafford W. F. Caldesmon. Molecular weight and subunit composition by analytical ultracentrifugation. J Biol Chem. 1988 Oct 5;263(28):14196–14202. [PubMed] [Google Scholar]
- Hayashi K., Fujio Y., Kato I., Sobue K. Structural and functional relationships between h- and l-caldesmons. J Biol Chem. 1991 Jan 5;266(1):355–361. [PubMed] [Google Scholar]
- Hemric M. E., Chalovich J. M. Characterization of caldesmon binding to myosin. J Biol Chem. 1990 Nov 15;265(32):19672–19678. [PMC free article] [PubMed] [Google Scholar]
- Hemric M. E., Freedman M. V., Chalovich J. M. Inhibition of actin stimulation of skeletal muscle (A1)S-1 ATPase activity by caldesmon. Arch Biochem Biophys. 1993 Oct;306(1):39–43. doi: 10.1006/abbi.1993.1477. [DOI] [PubMed] [Google Scholar]
- Horiuchi K. Y., Chacko S. Interaction between caldesmon and tropomyosin in the presence and absence of smooth muscle actin. Biochemistry. 1988 Nov 1;27(22):8388–8393. doi: 10.1021/bi00422a014. [DOI] [PubMed] [Google Scholar]
- Horiuchi K. Y., Samuel M., Chacko S. Mechanism for the inhibition of acto-heavy meromyosin ATPase by the actin/calmodulin binding domain of caldesmon. Biochemistry. 1991 Jan 22;30(3):712–717. doi: 10.1021/bi00217a019. [DOI] [PubMed] [Google Scholar]
- Huber P. A., Redwood C. S., Avent N. D., Tanner M. J., Marston S. B. Identification of functioning regulatory sites and a new myosin binding site in the C-terminal 288 amino acids of caldesmon expressed from a human clone. J Muscle Res Cell Motil. 1993 Aug;14(4):385–391. doi: 10.1007/BF00121289. [DOI] [PubMed] [Google Scholar]
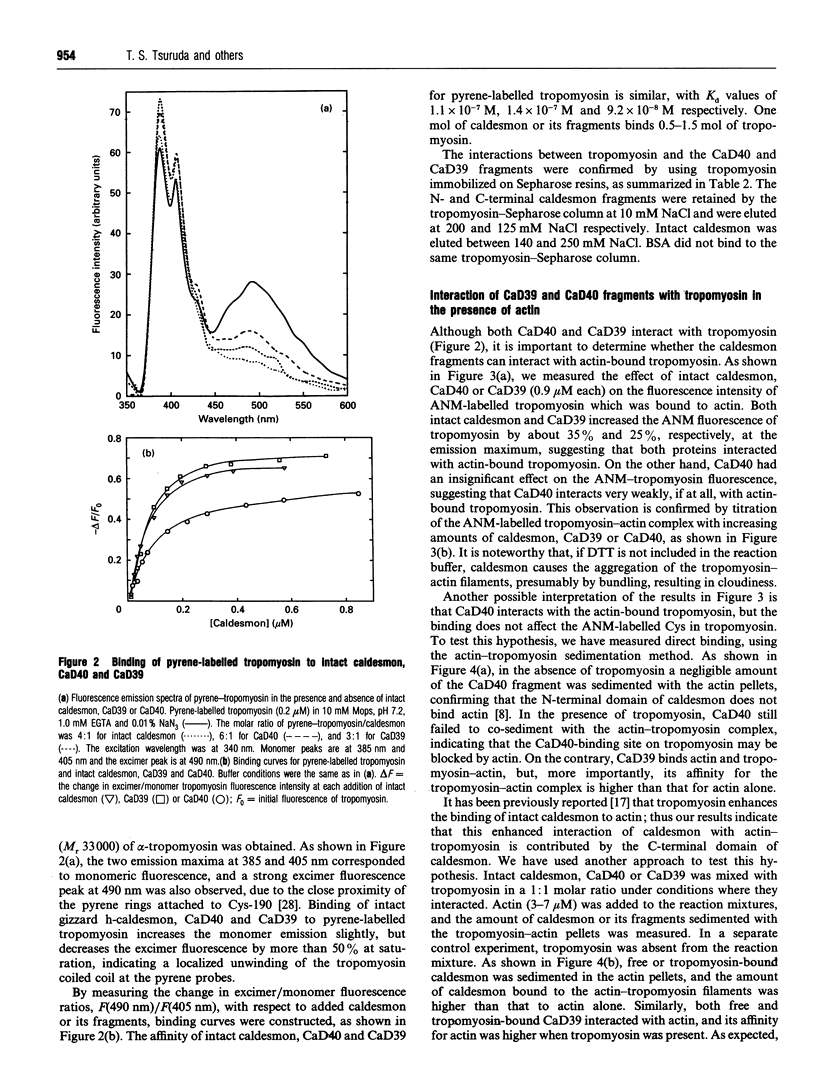
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Mak A. S., Smillie L. B. Structural interpretation of the two-site binding of troponin on the muscle thin filament. J Mol Biol. 1981 Jul 5;149(3):541–550. doi: 10.1016/0022-2836(81)90486-1. [DOI] [PubMed] [Google Scholar]
- Mak A. S., Watson M. H., Litwin C. M., Wang J. H. Phosphorylation of caldesmon by cdc2 kinase. J Biol Chem. 1991 Apr 15;266(11):6678–6681. [PubMed] [Google Scholar]
- Margossian S. S., Lowey S. Preparation of myosin and its subfragments from rabbit skeletal muscle. Methods Enzymol. 1982;85(Pt B):55–71. doi: 10.1016/0076-6879(82)85009-x. [DOI] [PubMed] [Google Scholar]
- Marston S. B., Fraser I. D., Huber P. A., Pritchard K., Gusev N. B., Torok K. Location of two contact sites between human smooth muscle caldesmon and Ca(2+)-calmodulin. J Biol Chem. 1994 Mar 18;269(11):8134–8139. [PubMed] [Google Scholar]
- Marston S. B., Redwood C. S. The essential role of tropomyosin in cooperative regulation of smooth muscle thin filament activity by caldesmon. J Biol Chem. 1993 Jun 15;268(17):12317–12320. [PubMed] [Google Scholar]
- Marston S. B., Redwood C. S. The molecular anatomy of caldesmon. Biochem J. 1991 Oct 1;279(Pt 1):1–16. doi: 10.1042/bj2790001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumura F., Yamashiro S. Caldesmon. Curr Opin Cell Biol. 1993 Feb;5(1):70–76. doi: 10.1016/s0955-0674(05)80010-9. [DOI] [PubMed] [Google Scholar]
- Mezgueldi M., Derancourt J., Calas B., Kassab R., Fattoum A. Precise identification of the regulatory F-actin- and calmodulin-binding sequences in the 10-kDa carboxyl-terminal domain of caldesmon. J Biol Chem. 1994 Apr 29;269(17):12824–12832. [PubMed] [Google Scholar]
- Moody C., Lehman W., Craig R. Caldesmon and the structure of smooth muscle thin filaments: electron microscopy of isolated thin filaments. J Muscle Res Cell Motil. 1990 Apr;11(2):176–185. doi: 10.1007/BF01766496. [DOI] [PubMed] [Google Scholar]
- Novy R. E., Lin J. L., Lin J. J. Characterization of cDNA clones encoding a human fibroblast caldesmon isoform and analysis of caldesmon expression in normal and transformed cells. J Biol Chem. 1991 Sep 5;266(25):16917–16924. [PubMed] [Google Scholar]
- Novy R. E., Sellers J. R., Liu L. F., Lin J. J. In vitro functional characterization of bacterially expressed human fibroblast tropomyosin isoforms and their chimeric mutants. Cell Motil Cytoskeleton. 1993;26(3):248–261. doi: 10.1002/cm.970260308. [DOI] [PubMed] [Google Scholar]
- Pardee J. D., Spudich J. A. Purification of muscle actin. Methods Enzymol. 1982;85(Pt B):164–181. doi: 10.1016/0076-6879(82)85020-9. [DOI] [PubMed] [Google Scholar]
- Persechini A., Hartshorne D. J. Ordered phosphorylation of the two 20 000 molecular weight light chains of smooth muscle myosin. Biochemistry. 1983 Jan 18;22(2):470–476. doi: 10.1021/bi00271a033. [DOI] [PubMed] [Google Scholar]
- Pollard T. D. Assays for myosin. Methods Enzymol. 1982;85(Pt B):123–130. doi: 10.1016/0076-6879(82)85015-5. [DOI] [PubMed] [Google Scholar]
- Redwood C. S., Marston S. B. Binding and regulatory properties of expressed functional domains of chicken gizzard smooth muscle caldesmon. J Biol Chem. 1993 May 25;268(15):10969–10976. [PubMed] [Google Scholar]
- Sanders C., Smillie L. B. Chicken gizzard tropomyosin: head-to-tail assembly and interaction with F-actin and troponin. Can J Biochem Cell Biol. 1984 Jun;62(6):443–448. doi: 10.1139/o84-060. [DOI] [PubMed] [Google Scholar]
- Shirinsky V. P., Biryukov K. G., Hettasch J. M., Sellers J. R. Inhibition of the relative movement of actin and myosin by caldesmon and calponin. J Biol Chem. 1992 Aug 5;267(22):15886–15892. [PubMed] [Google Scholar]
- Sobue K., Sellers J. R. Caldesmon, a novel regulatory protein in smooth muscle and nonmuscle actomyosin systems. J Biol Chem. 1991 Jul 5;266(19):12115–12118. [PubMed] [Google Scholar]
- Sutherland C., Walsh M. P. Phosphorylation of caldesmon prevents its interaction with smooth muscle myosin. J Biol Chem. 1989 Jan 5;264(1):578–583. [PubMed] [Google Scholar]
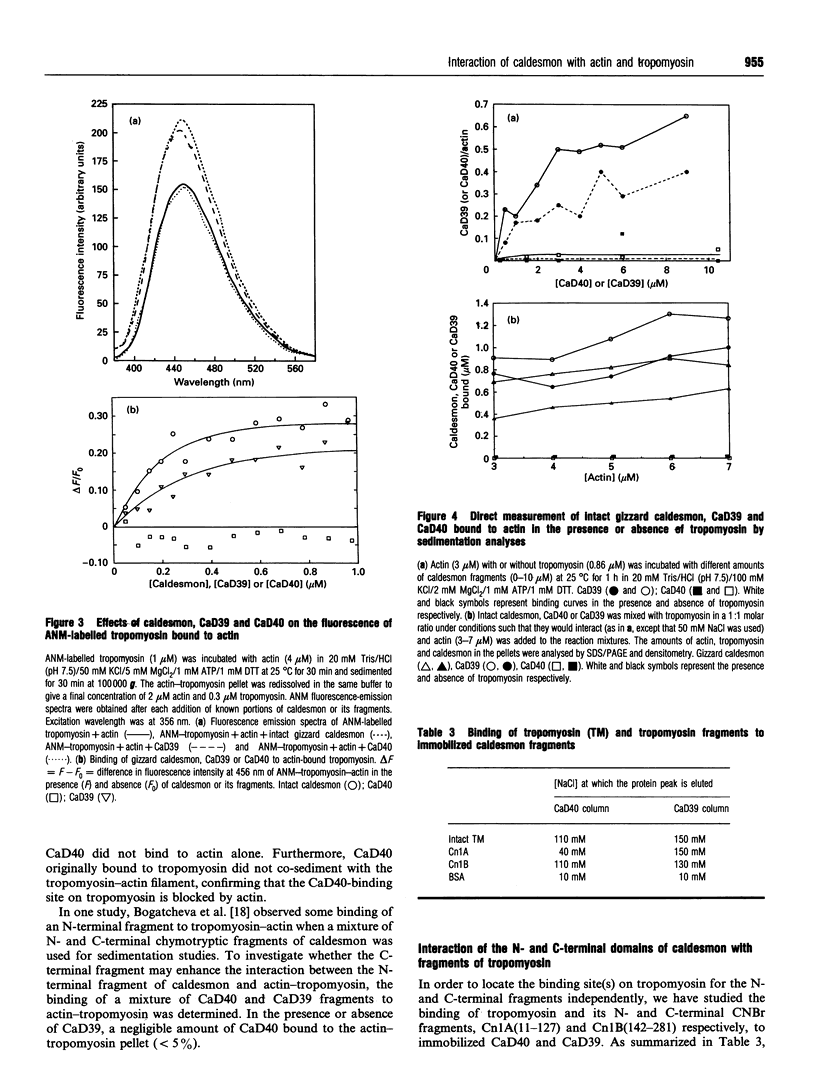
- Velaz L., Chen Y. D., Chalovich J. M. Characterization of a caldesmon fragment that competes with myosin-ATP binding to actin. Biophys J. 1993 Aug;65(2):892–898. doi: 10.1016/S0006-3495(93)81113-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velaz L., Ingraham R. H., Chalovich J. M. Dissociation of the effect of caldesmon on the ATPase activity and on the binding of smooth heavy meromyosin to actin by partial digestion of caldesmon. J Biol Chem. 1990 Feb 15;265(5):2929–2934. [PubMed] [Google Scholar]
- Wang C. L., Wang L. W., Lu R. C. Caldesmon has two calmodulin-binding domains. Biochem Biophys Res Commun. 1989 Jul 31;162(2):746–752. doi: 10.1016/0006-291x(89)92373-5. [DOI] [PubMed] [Google Scholar]
- Watson M. H., Kuhn A. E., Mak A. S. Caldesmon, calmodulin and tropomyosin interactions. Biochim Biophys Acta. 1990 Aug 13;1054(1):103–113. doi: 10.1016/0167-4889(90)90211-u. [DOI] [PubMed] [Google Scholar]
- Watson M. H., Kuhn A. E., Novy R. E., Lin J. J., Mak A. S. Caldesmon-binding sites on tropomyosin. J Biol Chem. 1990 Nov 5;265(31):18860–18866. [PubMed] [Google Scholar]
- Zhan Q. Q., Wong S. S., Wang C. L. A calmodulin-binding peptide of caldesmon. J Biol Chem. 1991 Nov 15;266(32):21810–21814. [PubMed] [Google Scholar]