Abstract
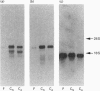
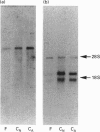
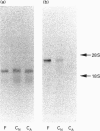
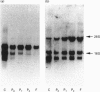
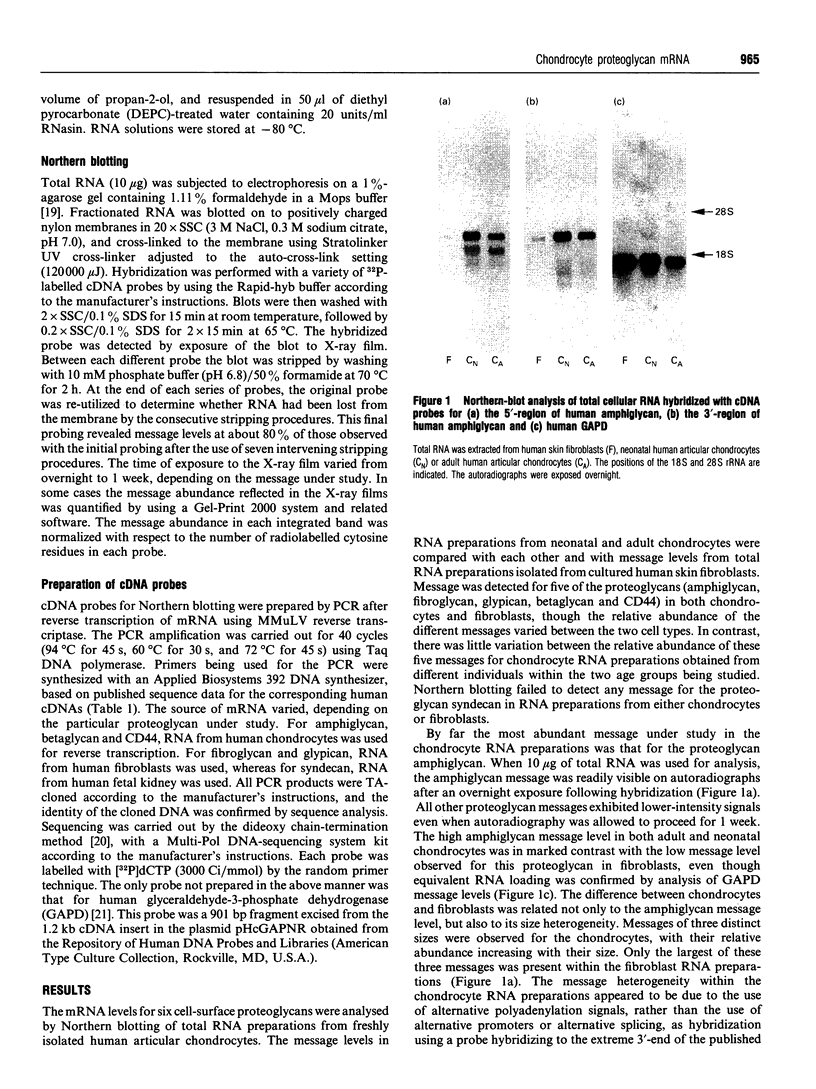
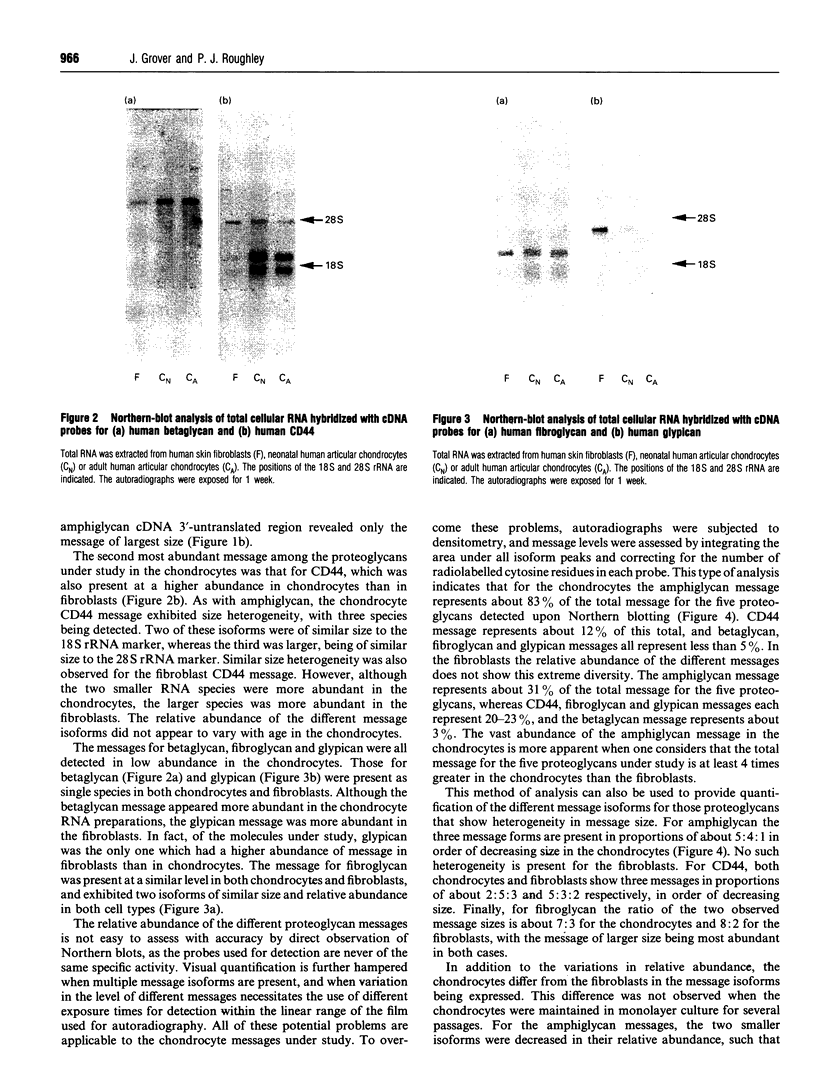
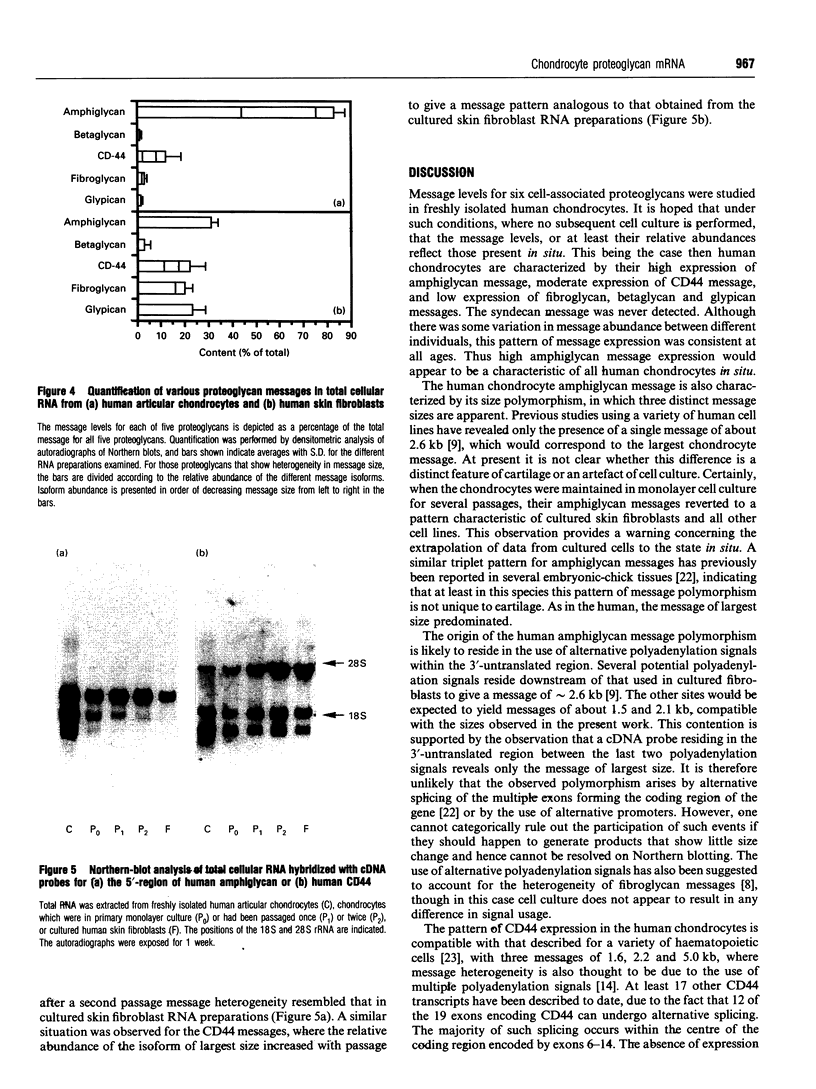
The expression of six cell-surface proteoglycans (syndecan, fibroglycan, amphiglycan, glypican, betaglycan and CD44) was studied at the mRNA level. Analysis was performed by Northern blotting using total RNA preparations from freshly isolated articular chondrocytes obtained from both juveniles and adults. Similar results were obtained for both age groups. By far the most abundant message was that for amphiglycan, CD44 message was next in relative abundance, and the messages for fibroglycan, glypican and betaglycan were all expressed at low levels. Syndecan message could not be detected by this technique. This pattern of expression was different to that observed in cultured skin fibroblasts, where the messages for amphiglycan, CD44, fibroglycan and glypican were all expressed at a similar level. In contrast with the fibroblasts, where the amphiglycan message exhibits no size polymorphism, the chondrocyte amphiglycan message is present in three polymorphic forms, due to the use of alternative polyadenylation signals. When the newly isolated chondrocytes are maintained in monolayer culture for several passages, the amphiglycan message heterogeneity reverts to that characteristic of the fibroblasts. Thus human articular chondrocytes are characterized by both their high level of amphiglycan message expression and their use of alternative polyadenylation signals.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andres J. L., DeFalcis D., Noda M., Massagué J. Binding of two growth factor families to separate domains of the proteoglycan betaglycan. J Biol Chem. 1992 Mar 25;267(9):5927–5930. [PubMed] [Google Scholar]
- Andres J. L., Rönnstrand L., Cheifetz S., Massagué J. Purification of the transforming growth factor-beta (TGF-beta) binding proteoglycan betaglycan. J Biol Chem. 1991 Dec 5;266(34):23282–23287. [PubMed] [Google Scholar]
- Baciu P. C., Acaster C., Goetinck P. F. Molecular cloning and genomic organization of chicken syndecan-4. J Biol Chem. 1994 Jan 7;269(1):696–703. [PubMed] [Google Scholar]
- Carey D. J., Evans D. M., Stahl R. C., Asundi V. K., Conner K. J., Garbes P., Cizmeci-Smith G. Molecular cloning and characterization of N-syndecan, a novel transmembrane heparan sulfate proteoglycan. J Cell Biol. 1992 Apr;117(1):191–201. doi: 10.1083/jcb.117.1.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Culty M., Miyake K., Kincade P. W., Sikorski E., Butcher E. C., Underhill C., Silorski E. The hyaluronate receptor is a member of the CD44 (H-CAM) family of cell surface glycoproteins. J Cell Biol. 1990 Dec;111(6 Pt 1):2765–2774. doi: 10.1083/jcb.111.6.2765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- David G., Bai X. M., Van der Schueren B., Marynen P., Cassiman J. J., Van den Berghe H. Spatial and temporal changes in the expression of fibroglycan (syndecan-2) during mouse embryonic development. Development. 1993 Nov;119(3):841–854. doi: 10.1242/dev.119.3.841. [DOI] [PubMed] [Google Scholar]
- David G. Integral membrane heparan sulfate proteoglycans. FASEB J. 1993 Aug;7(11):1023–1030. doi: 10.1096/fasebj.7.11.8370471. [DOI] [PubMed] [Google Scholar]
- David G., Lories V., Decock B., Marynen P., Cassiman J. J., Van den Berghe H. Molecular cloning of a phosphatidylinositol-anchored membrane heparan sulfate proteoglycan from human lung fibroblasts. J Cell Biol. 1990 Dec;111(6 Pt 2):3165–3176. doi: 10.1083/jcb.111.6.3165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- David G., van der Schueren B., Marynen P., Cassiman J. J., van den Berghe H. Molecular cloning of amphiglycan, a novel integral membrane heparan sulfate proteoglycan expressed by epithelial and fibroblastic cells. J Cell Biol. 1992 Aug;118(4):961–969. doi: 10.1083/jcb.118.4.961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grover J., Roughley P. J. Versican gene expression in human articular cartilage and comparison of mRNA splicing variation with aggrecan. Biochem J. 1993 Apr 15;291(Pt 2):361–367. doi: 10.1042/bj2910361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim C. W., Goldberger O. A., Gallo R. L., Bernfield M. Members of the syndecan family of heparan sulfate proteoglycans are expressed in distinct cell-, tissue-, and development-specific patterns. Mol Biol Cell. 1994 Jul;5(7):797–805. doi: 10.1091/mbc.5.7.797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kokenyesi R., Bernfield M. Core protein structure and sequence determine the site and presence of heparan sulfate and chondroitin sulfate on syndecan-1. J Biol Chem. 1994 Apr 22;269(16):12304–12309. [PubMed] [Google Scholar]
- Lesley J., Hyman R., Kincade P. W. CD44 and its interaction with extracellular matrix. Adv Immunol. 1993;54:271–335. doi: 10.1016/s0065-2776(08)60537-4. [DOI] [PubMed] [Google Scholar]
- Lories V., Cassiman J. J., Van den Berghe H., David G. Differential expression of cell surface heparan sulfate proteoglycans in human mammary epithelial cells and lung fibroblasts. J Biol Chem. 1992 Jan 15;267(2):1116–1122. [PubMed] [Google Scholar]
- López-Casillas F., Cheifetz S., Doody J., Andres J. L., Lane W. S., Massagué J. Structure and expression of the membrane proteoglycan betaglycan, a component of the TGF-beta receptor system. Cell. 1991 Nov 15;67(4):785–795. doi: 10.1016/0092-8674(91)90073-8. [DOI] [PubMed] [Google Scholar]
- López-Casillas F., Payne H. M., Andres J. L., Massagué J. Betaglycan can act as a dual modulator of TGF-beta access to signaling receptors: mapping of ligand binding and GAG attachment sites. J Cell Biol. 1994 Feb;124(4):557–568. doi: 10.1083/jcb.124.4.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mali M., Jaakkola P., Arvilommi A. M., Jalkanen M. Sequence of human syndecan indicates a novel gene family of integral membrane proteoglycans. J Biol Chem. 1990 Apr 25;265(12):6884–6889. [PubMed] [Google Scholar]
- Marynen P., Zhang J., Cassiman J. J., Van den Berghe H., David G. Partial primary structure of the 48- and 90-kilodalton core proteins of cell surface-associated heparan sulfate proteoglycans of lung fibroblasts. Prediction of an integral membrane domain and evidence for multiple distinct core proteins at the cell surface of human lung fibroblasts. J Biol Chem. 1989 Apr 25;264(12):7017–7024. [PubMed] [Google Scholar]
- Rapraeger A. C. The coordinated regulation of heparan sulfate, syndecans and cell behavior. Curr Opin Cell Biol. 1993 Oct;5(5):844–853. doi: 10.1016/0955-0674(93)90034-n. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spring J., Goldberger O. A., Jenkins N. A., Gilbert D. J., Copeland N. G., Bernfield M. Mapping of the syndecan genes in the mouse: linkage with members of the myc gene family. Genomics. 1994 Jun;21(3):597–601. doi: 10.1006/geno.1994.1319. [DOI] [PubMed] [Google Scholar]
- Stamenkovic I., Amiot M., Pesando J. M., Seed B. A lymphocyte molecule implicated in lymph node homing is a member of the cartilage link protein family. Cell. 1989 Mar 24;56(6):1057–1062. doi: 10.1016/0092-8674(89)90638-7. [DOI] [PubMed] [Google Scholar]
- Tso J. Y., Sun X. H., Kao T. H., Reece K. S., Wu R. Isolation and characterization of rat and human glyceraldehyde-3-phosphate dehydrogenase cDNAs: genomic complexity and molecular evolution of the gene. Nucleic Acids Res. 1985 Apr 11;13(7):2485–2502. doi: 10.1093/nar/13.7.2485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X. F., Lin H. Y., Ng-Eaton E., Downward J., Lodish H. F., Weinberg R. A. Expression cloning and characterization of the TGF-beta type III receptor. Cell. 1991 Nov 15;67(4):797–805. doi: 10.1016/0092-8674(91)90074-9. [DOI] [PubMed] [Google Scholar]
- Wight T. N., Kinsella M. G., Qwarnström E. E. The role of proteoglycans in cell adhesion, migration and proliferation. Curr Opin Cell Biol. 1992 Oct;4(5):793–801. doi: 10.1016/0955-0674(92)90102-i. [DOI] [PubMed] [Google Scholar]
- Yanagishita M., Hascall V. C. Cell surface heparan sulfate proteoglycans. J Biol Chem. 1992 May 15;267(14):9451–9454. [PubMed] [Google Scholar]