Abstract
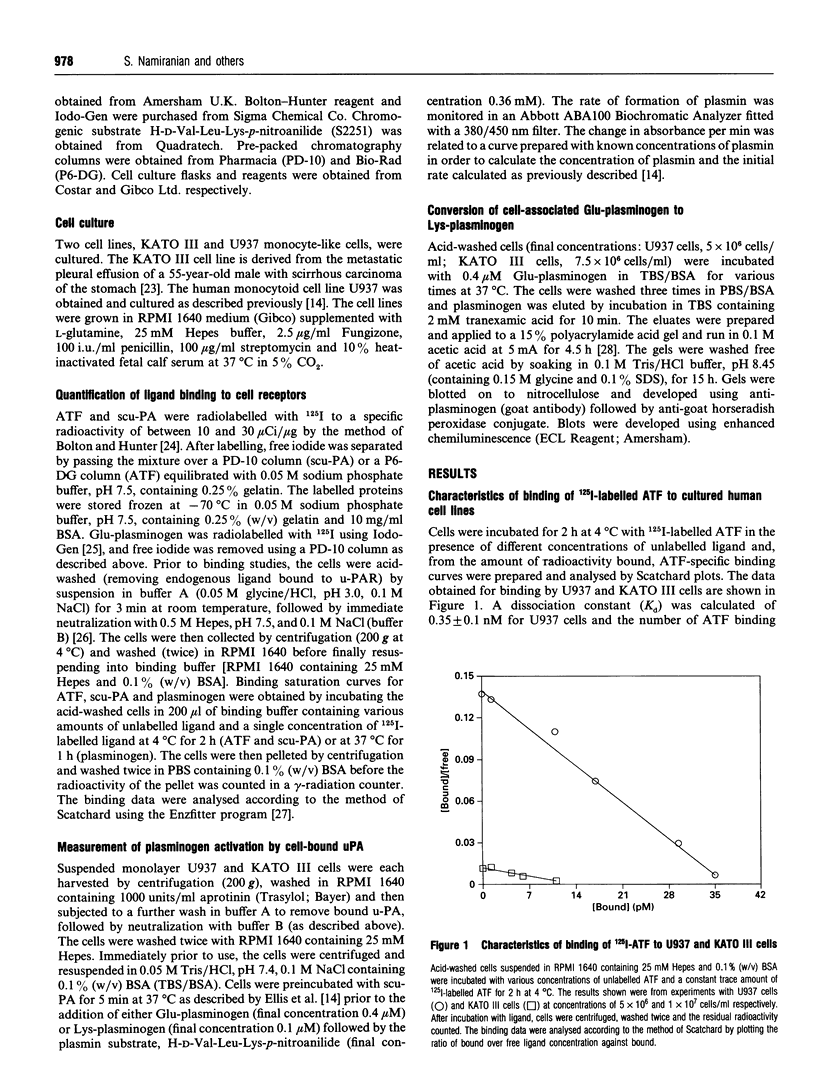
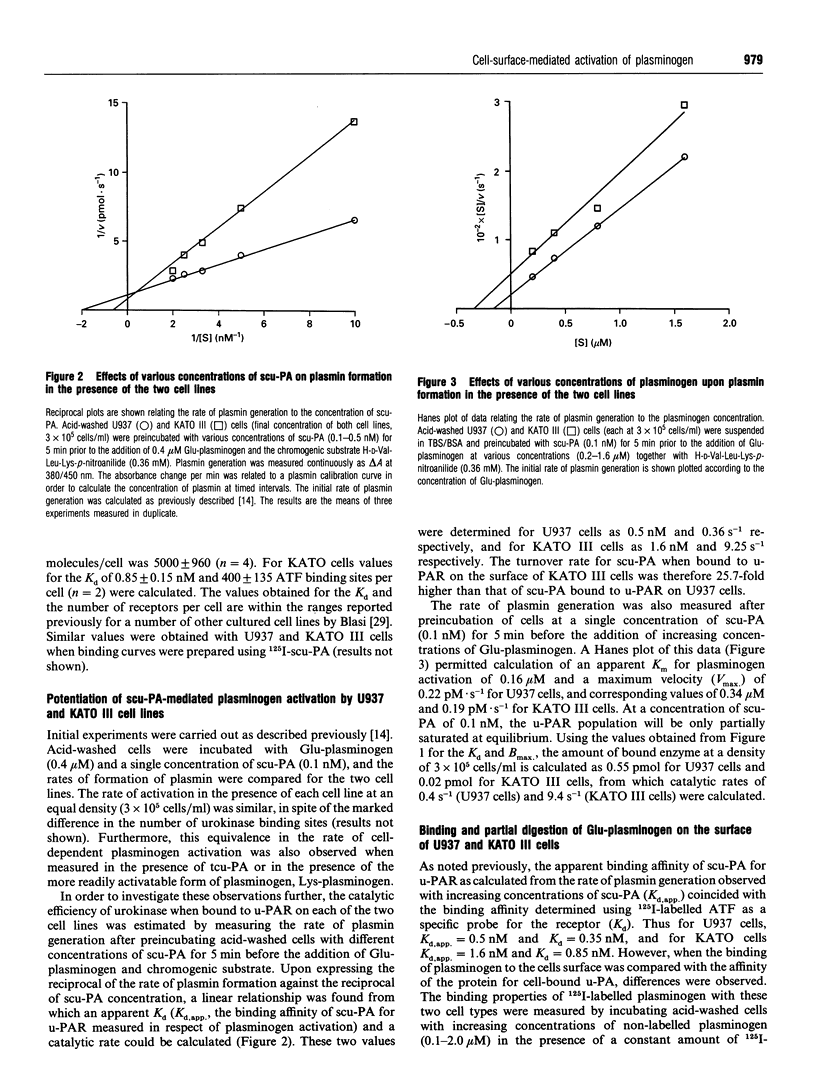
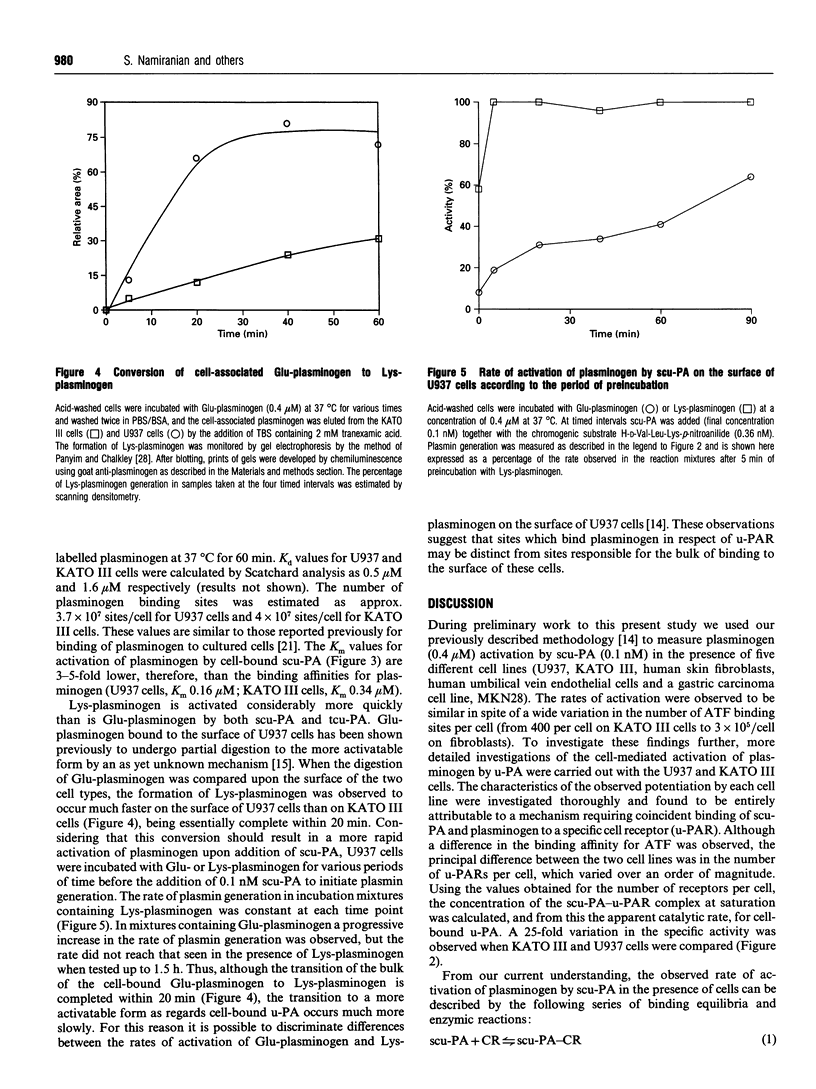
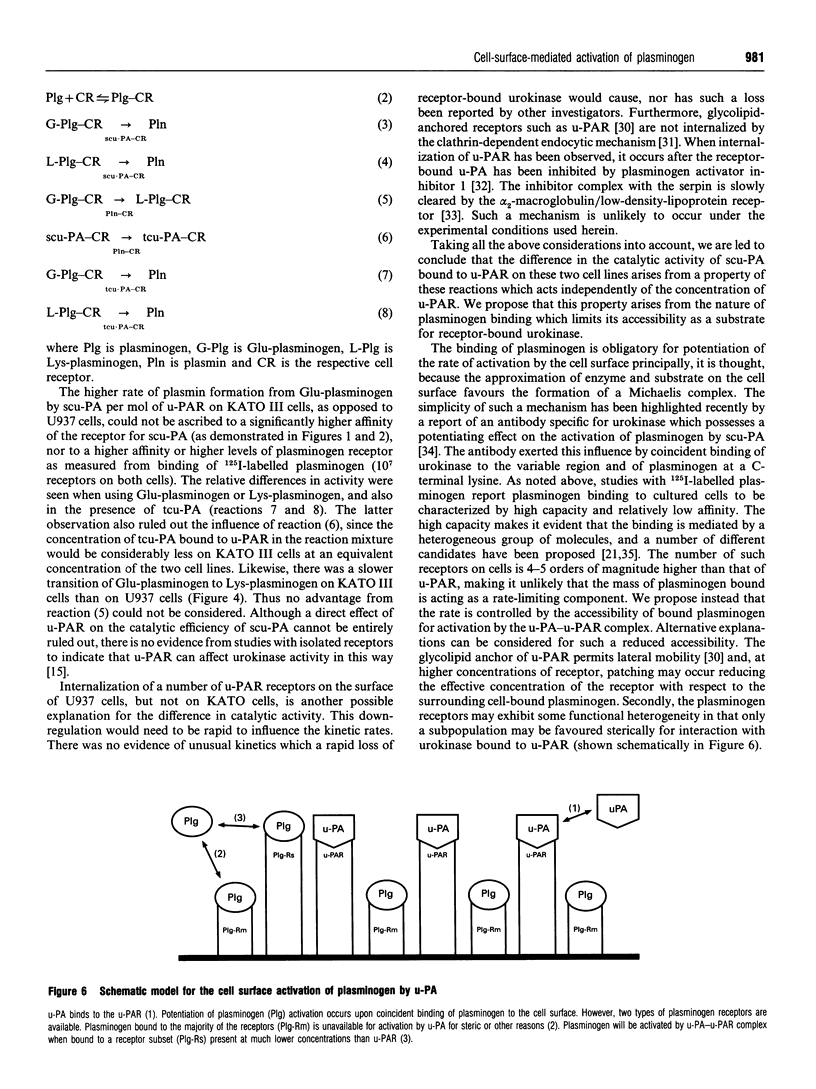
The ability of U937 monocyte-like cells and KATO III cells (a human gastric carcinoma line) to potentiate activation of plasminogen by single-chain urokinase-type plasminogen activator (scu-PA), as mediated by the cell receptor for urokinase (u-PAR), was compared. It was observed that, although the concentration of u-PAR on these cell lines differed considerably (U937 cells: 5000 receptors/cell, Kd 0.35 nM; KATO III cells: 400 receptors/cell, Kd 0.85 nM), the rate of activation of plasminogen by scu-PA in the presence of the same density of each cell line was equivalent. From data generated in the presence of increasing concentrations of scu-PA, the kcat, for plasminogen activation in the presence of each cell line was calculated and found to differ by 26-fold (0.36 s-1 on U937 cells; 9.25 s-1 on KATO III cells). However, the Km for plasminogen with respect to the rate of formation of plasmin was lower than the Kd for binding (0.2 microM compared with 0.5 microM on U937 cells; 0.34 microM compared with 1.6 microM on KATO III cells). A rapid transformation from Glu-plasminogen (native plasminogen with N-terminal Glu) to Lys-plasminogen (plasmin-degraded plasminogen with primarily N-terminal Lys-77) occurred on the surface of U937 cells (unlike KATO III cells), but this transition did not coincide with faster rates of plasminogen activation. From this evidence it is concluded that the accessibility of bound plasminogen acts to limit the rate of activation by cell-bound urokinase. The significance of this proposal is that the proteolytic potential of the cell-mediated activation of plasminogen would be controlled by the accessibility of plasminogen for activation rather than by the concentration of u-PAR (the latter may act to localize proteolysis to appropriate domains on the surface of the cell).
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andreasen P. A., Nielsen L. S., Kristensen P., Grøndahl-Hansen J., Skriver L., Danø K. Plasminogen activator inhibitor from human fibrosarcoma cells binds urokinase-type plasminogen activator, but not its proenzyme. J Biol Chem. 1986 Jun 15;261(17):7644–7651. [PubMed] [Google Scholar]
- Andreasen P. A., Sottrup-Jensen L., Kjøller L., Nykjaer A., Moestrup S. K., Petersen C. M., Gliemann J. Receptor-mediated endocytosis of plasminogen activators and activator/inhibitor complexes. FEBS Lett. 1994 Feb 7;338(3):239–245. doi: 10.1016/0014-5793(94)80276-9. [DOI] [PubMed] [Google Scholar]
- Appella E., Robinson E. A., Ullrich S. J., Stoppelli M. P., Corti A., Cassani G., Blasi F. The receptor-binding sequence of urokinase. A biological function for the growth-factor module of proteases. J Biol Chem. 1987 Apr 5;262(10):4437–4440. [PubMed] [Google Scholar]
- Bajpai A., Baker J. B. Urokinase binding sites on human foreskin cells. Evidence for occupancy with endogenous urokinase. Biochem Biophys Res Commun. 1985 Dec 31;133(3):994–1000. doi: 10.1016/0006-291x(85)91234-3. [DOI] [PubMed] [Google Scholar]
- Bolton A. E., Hunter W. M. The labelling of proteins to high specific radioactivities by conjugation to a 125I-containing acylating agent. Biochem J. 1973 Jul;133(3):529–539. doi: 10.1042/bj1330529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cubellis M. V., Wun T. C., Blasi F. Receptor-mediated internalization and degradation of urokinase is caused by its specific inhibitor PAI-1. EMBO J. 1990 Apr;9(4):1079–1085. doi: 10.1002/j.1460-2075.1990.tb08213.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danø K., Andreasen P. A., Grøndahl-Hansen J., Kristensen P., Nielsen L. S., Skriver L. Plasminogen activators, tissue degradation, and cancer. Adv Cancer Res. 1985;44:139–266. doi: 10.1016/s0065-230x(08)60028-7. [DOI] [PubMed] [Google Scholar]
- Eaton D. L., Scott R. W., Baker J. B. Purification of human fibroblast urokinase proenzyme and analysis of its regulation by proteases and protease nexin. J Biol Chem. 1984 May 25;259(10):6241–6247. [PubMed] [Google Scholar]
- Ellis V., Behrendt N., Danø K. Plasminogen activation by receptor-bound urokinase. A kinetic study with both cell-associated and isolated receptor. J Biol Chem. 1991 Jul 5;266(19):12752–12758. [PubMed] [Google Scholar]
- Ellis V., Danø K. Potentiation of plasminogen activation by an anti-urokinase monoclonal antibody due to ternary complex formation. A mechanistic model for receptor-mediated plasminogen activation. J Biol Chem. 1993 Mar 5;268(7):4806–4813. [PubMed] [Google Scholar]
- Ellis V., Scully M. F., Kakkar V. V. Plasminogen activation initiated by single-chain urokinase-type plasminogen activator. Potentiation by U937 monocytes. J Biol Chem. 1989 Feb 5;264(4):2185–2188. [PubMed] [Google Scholar]
- Ellis V., Wun T. C., Behrendt N., Rønne E., Danø K. Inhibition of receptor-bound urokinase by plasminogen-activator inhibitors. J Biol Chem. 1990 Jun 15;265(17):9904–9908. [PubMed] [Google Scholar]
- Fraker P. J., Speck J. C., Jr Protein and cell membrane iodinations with a sparingly soluble chloroamide, 1,3,4,6-tetrachloro-3a,6a-diphrenylglycoluril. Biochem Biophys Res Commun. 1978 Feb 28;80(4):849–857. doi: 10.1016/0006-291x(78)91322-0. [DOI] [PubMed] [Google Scholar]
- Hall S. W., Humphries J. E., Gonias S. L. Inhibition of cell surface receptor-bound plasmin by alpha 2-antiplasmin and alpha 2-macroglobulin. J Biol Chem. 1991 Jul 5;266(19):12329–12336. [PubMed] [Google Scholar]
- Miles L. A., Dahlberg C. M., Plescia J., Felez J., Kato K., Plow E. F. Role of cell-surface lysines in plasminogen binding to cells: identification of alpha-enolase as a candidate plasminogen receptor. Biochemistry. 1991 Feb 12;30(6):1682–1691. doi: 10.1021/bi00220a034. [DOI] [PubMed] [Google Scholar]
- Miles L. A., Plow E. F. Binding and activation of plasminogen on the platelet surface. J Biol Chem. 1985 Apr 10;260(7):4303–4311. [PubMed] [Google Scholar]
- Miles L. A., Plow E. F. Receptor mediated binding of the fibrinolytic components, plasminogen and urokinase, to peripheral blood cells. Thromb Haemost. 1987 Oct 28;58(3):936–942. [PubMed] [Google Scholar]
- Nielsen L. S., Hansen J. G., Skriver L., Wilson E. L., Kaltoft K., Zeuthen J., Danø K. Purification of zymogen to plasminogen activator from human glioblastoma cells by affinity chromatography with monoclonal antibody. Biochemistry. 1982 Dec 7;21(25):6410–6415. doi: 10.1021/bi00268a014. [DOI] [PubMed] [Google Scholar]
- Panyim S., Chalkley R. High resolution acrylamide gel electrophoresis of histones. Arch Biochem Biophys. 1969 Mar;130(1):337–346. doi: 10.1016/0003-9861(69)90042-3. [DOI] [PubMed] [Google Scholar]
- Ploug M., Rønne E., Behrendt N., Jensen A. L., Blasi F., Danø K. Cellular receptor for urokinase plasminogen activator. Carboxyl-terminal processing and membrane anchoring by glycosyl-phosphatidylinositol. J Biol Chem. 1991 Jan 25;266(3):1926–1933. [PubMed] [Google Scholar]
- Plow E. F., Felez J., Miles L. A. Cellular regulation of fibrinolysis. Thromb Haemost. 1991 Jul 12;66(1):32–36. [PubMed] [Google Scholar]
- Plow E. F., Freaney D. E., Plescia J., Miles L. A. The plasminogen system and cell surfaces: evidence for plasminogen and urokinase receptors on the same cell type. J Cell Biol. 1986 Dec;103(6 Pt 1):2411–2420. doi: 10.1083/jcb.103.6.2411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pöllänen J., Hedman K., Nielsen L. S., Danø K., Vaheri A. Ultrastructural localization of plasma membrane-associated urokinase-type plasminogen activator at focal contacts. J Cell Biol. 1988 Jan;106(1):87–95. doi: 10.1083/jcb.106.1.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pöllänen J., Saksela O., Salonen E. M., Andreasen P., Nielsen L., Danø K., Vaheri A. Distinct localizations of urokinase-type plasminogen activator and its type 1 inhibitor under cultured human fibroblasts and sarcoma cells. J Cell Biol. 1987 Apr;104(4):1085–1096. doi: 10.1083/jcb.104.4.1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saksela O., Rifkin D. B. Cell-associated plasminogen activation: regulation and physiological functions. Annu Rev Cell Biol. 1988;4:93–126. doi: 10.1146/annurev.cb.04.110188.000521. [DOI] [PubMed] [Google Scholar]
- Scully M. F., Ellis V., Watahiki Y., Kakkar V. V. Activation of pro-urokinase by plasmin: non-Michaelian kinetics indicates a mechanism of negative cooperativity. Arch Biochem Biophys. 1989 Feb 1;268(2):438–446. doi: 10.1016/0003-9861(89)90311-1. [DOI] [PubMed] [Google Scholar]
- Sekiguchi M., Sakakibara K., Fujii G. Establishment of cultured cell lines derived from a human gastric carcinoma. Jpn J Exp Med. 1978 Feb;48(1):61–68. [PubMed] [Google Scholar]
- Stoppelli M. P., Corti A., Soffientini A., Cassani G., Blasi F., Assoian R. K. Differentiation-enhanced binding of the amino-terminal fragment of human urokinase plasminogen activator to a specific receptor on U937 monocytes. Proc Natl Acad Sci U S A. 1985 Aug;82(15):4939–4943. doi: 10.1073/pnas.82.15.4939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoppelli M. P., Tacchetti C., Cubellis M. V., Corti A., Hearing V. J., Cassani G., Appella E., Blasi F. Autocrine saturation of pro-urokinase receptors on human A431 cells. Cell. 1986 Jun 6;45(5):675–684. doi: 10.1016/0092-8674(86)90782-8. [DOI] [PubMed] [Google Scholar]
- Turner A. J. PIG-tailed membrane proteins. Essays Biochem. 1994;28:113–127. [PubMed] [Google Scholar]
- Vassalli J. D., Baccino D., Belin D. A cellular binding site for the Mr 55,000 form of the human plasminogen activator, urokinase. J Cell Biol. 1985 Jan;100(1):86–92. doi: 10.1083/jcb.100.1.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vassalli J. D., Dayer J. M., Wohlwend A., Belin D. Concomitant secretion of prourokinase and of a plasminogen activator-specific inhibitor by cultured human monocytes-macrophages. J Exp Med. 1984 Jun 1;159(6):1653–1668. doi: 10.1084/jem.159.6.1653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vassalli J. D., Sappino A. P., Belin D. The plasminogen activator/plasmin system. J Clin Invest. 1991 Oct;88(4):1067–1072. doi: 10.1172/JCI115405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wun T. C., Ossowski L., Reich E. A proenzyme form of human urokinase. J Biol Chem. 1982 Jun 25;257(12):7262–7268. [PubMed] [Google Scholar]


