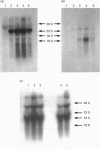
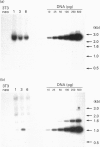
Abstract
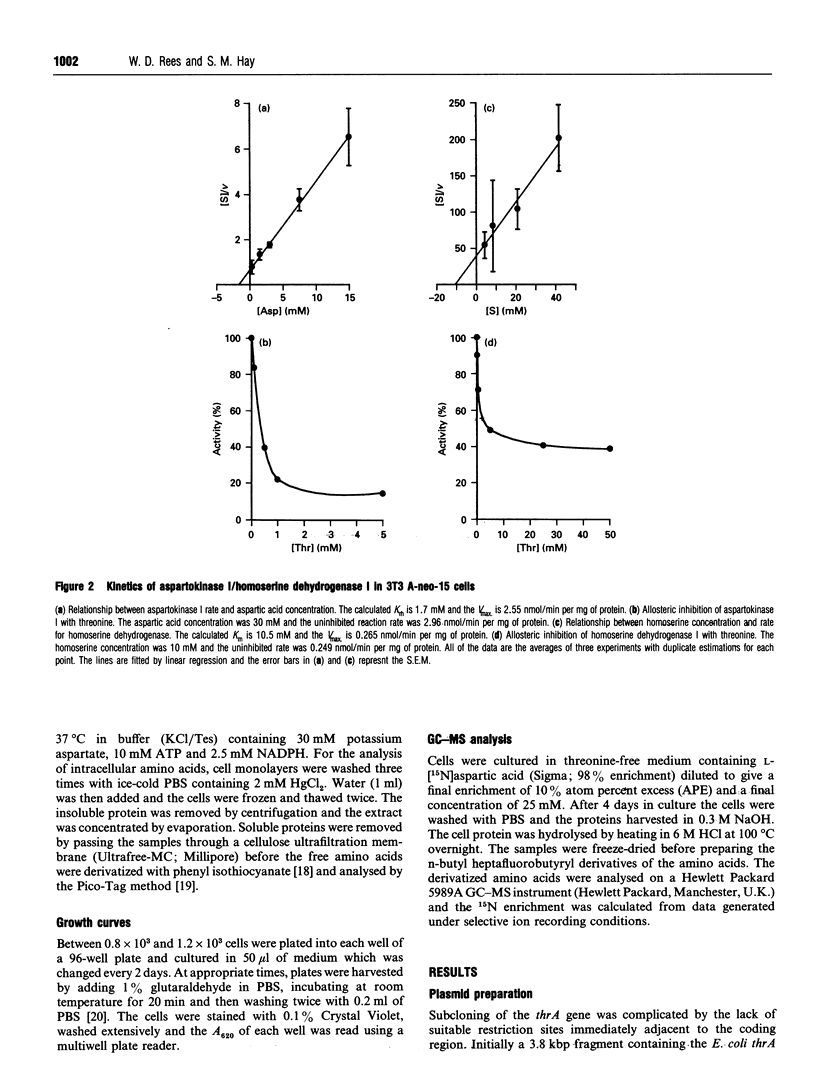
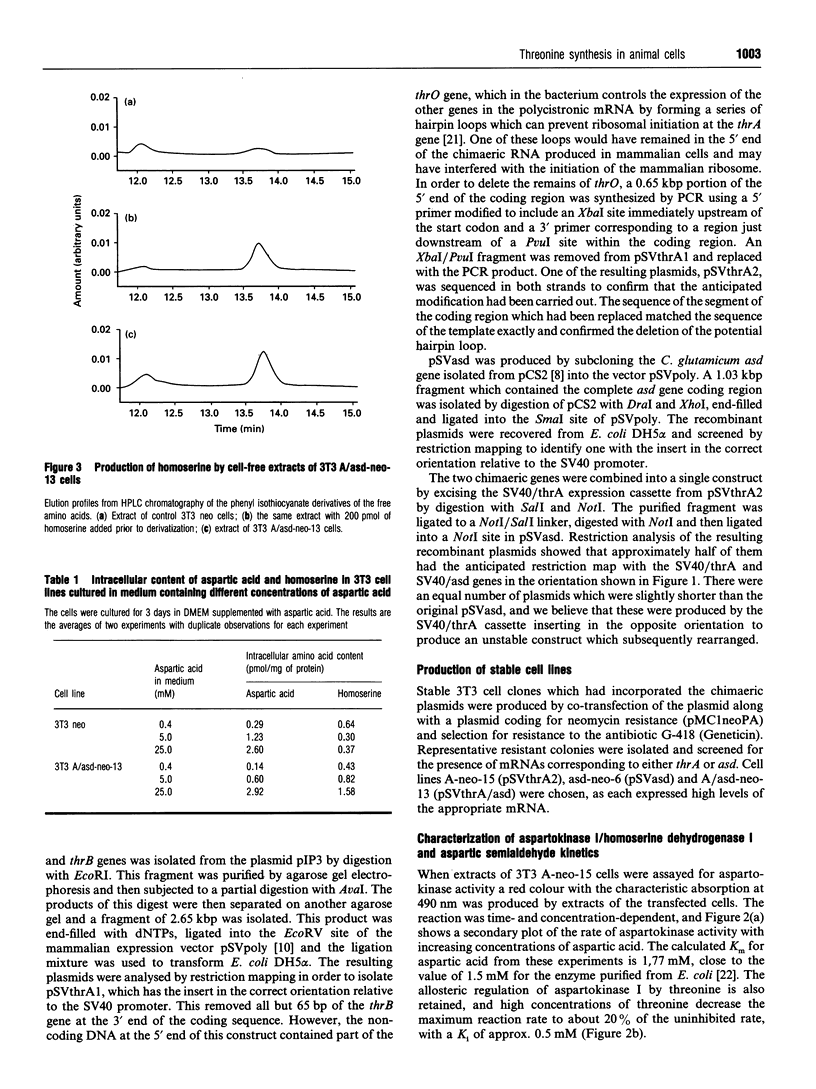
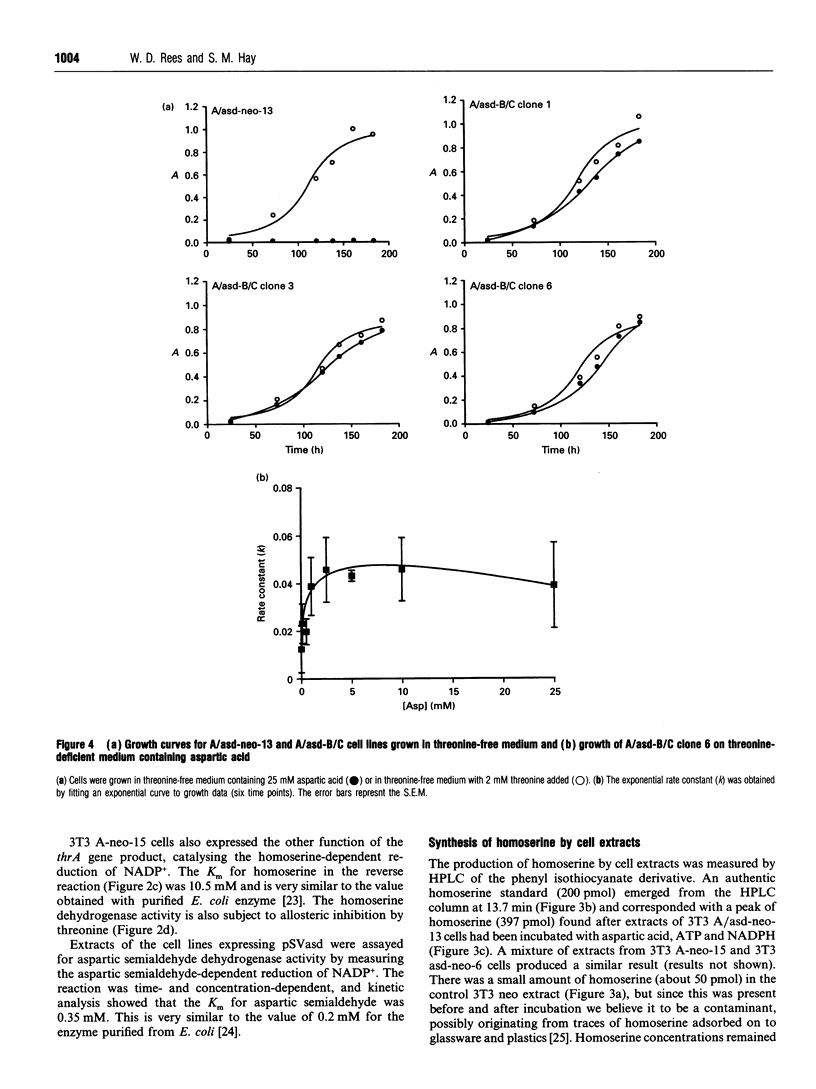
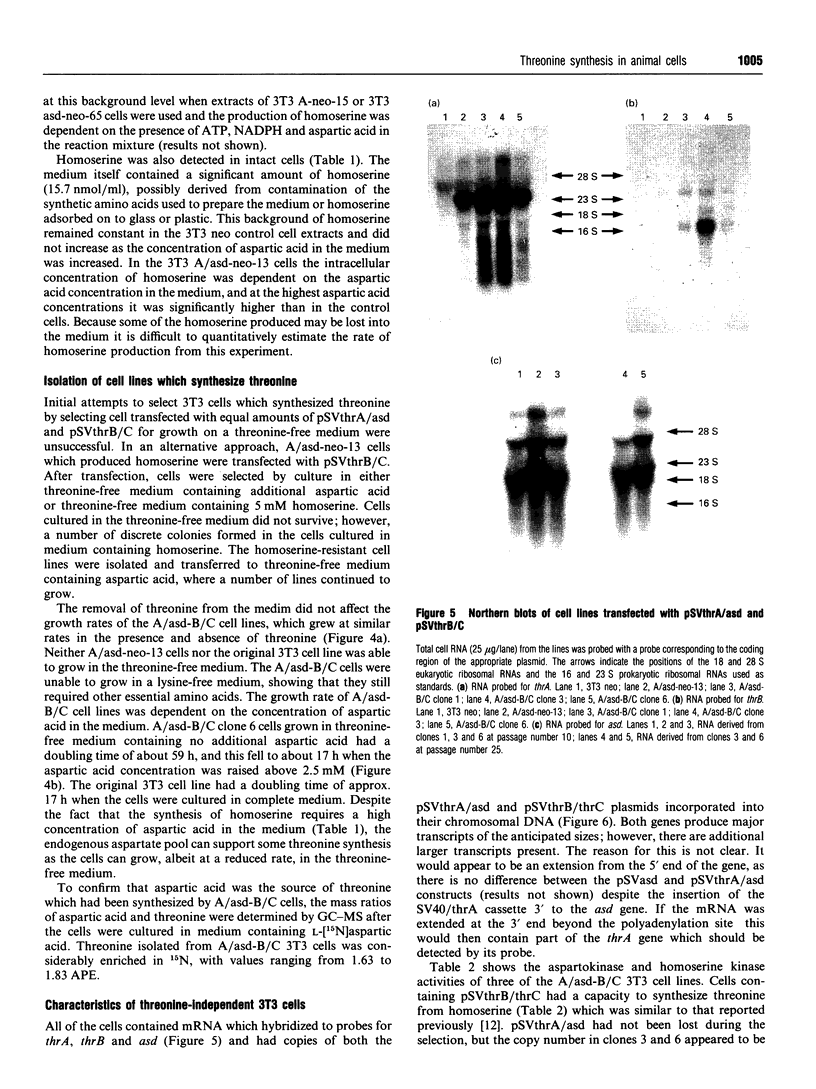
The coding regions for the Escherichia coli gene for aspartokinase I/homoserine dehydrogenase I (thrA) and the Corynebacterium glutamicum gene for aspartic semialdehyde dehydrogenase (asd) have been subcloned into a Simian Virus 40 (SV40)-based mammalian expression vector. Both enzyme activities are expressed in mouse 3T3 cells after transfer of the corresponding chimaeric gene. The kinetic parameters are similar to those of the native bacterial enzymes, and aspartokinase I/homoserine dehydrogenase I retains its allosteric regulation by threonine. An extract of the cells expressing aspartokinase I/homoserine dehydrogenase I, mixed with one from cells expressing aspartic semialdehyde dehydrogenase, produced homoserine when the mixture was incubated with aspartic acid, ATP and NADPH. The thrA and asd expression cassettes were combined into a single plasmid which, when transfected into 3T3 cells, enabled them to produce homoserine from aspartic acid. Homoserine-producing 3T3 cells were transfected with the plasmid pSVthrB/C (homoserine kinase and threonine synthase) and selected for growth on homoserine. Cell lines isolated from these cells expressed the complete bacterial threonine pathway, were independent of threonine for growth and could be maintained in medium which contained no free threonine. The threonine in the proteins of these cells became enriched in 15N when the culture medium contained [15N]aspartic acid. The production of homoserine and the growth of cells was at a maximum when there was more than 2.5 mM aspartate in the medium. Below this concentration the high Km of aspartokinase limited the flux through the pathway. In the presence of additional aspartic acid the new pathway could sustain a cell cycle time close to that of the same cells cultured in threonine-containing medium.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BLACK S., WRIGHT N. G. Homoserine dehydrogenase. J Biol Chem. 1955 Mar;213(1):51–60. [PubMed] [Google Scholar]
- BLACK S., WRIGHT N. G. beta-Aspartokinase and beta-aspartyl phosphate. J Biol Chem. 1955 Mar;213(1):27–38. [PubMed] [Google Scholar]
- Cossart P., Katinka M., Yaniv M. Nucleotide sequence of the thrB gene of E. coli, and its two adjacent regions; the thrAB and thrBC junctions. Nucleic Acids Res. 1981 Jan 24;9(2):339–347. doi: 10.1093/nar/9.2.339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falcoz-Kelly F., Janin J., Saari J. C., Véron M., Truffa-Bachi P., Cohen G. N. Revised structure of aspartokinase I-homoserine dehydrogenase I of Escherichia coli K12. Evidence for four identical subunits. Eur J Biochem. 1972 Aug 4;28(4):507–519. doi: 10.1111/j.1432-1033.1972.tb01938.x. [DOI] [PubMed] [Google Scholar]
- Gardner J. F. Regulation of the threonine operon: tandem threonine and isoleucine codons in the control region and translational control of transcription termination. Proc Natl Acad Sci U S A. 1979 Apr;76(4):1706–1710. doi: 10.1073/pnas.76.4.1706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillies R. J., Didier N., Denton M. Determination of cell number in monolayer cultures. Anal Biochem. 1986 Nov 15;159(1):109–113. doi: 10.1016/0003-2697(86)90314-3. [DOI] [PubMed] [Google Scholar]
- Hama H., Kayahara T., Tsuda M., Tsuchiya T. Inhibition of homoserine dehydrogenase I by L-serine in Escherichia coli. J Biochem. 1991 Apr;109(4):604–608. doi: 10.1093/oxfordjournals.jbchem.a123427. [DOI] [PubMed] [Google Scholar]
- Heinrikson R. L., Meredith S. C. Amino acid analysis by reverse-phase high-performance liquid chromatography: precolumn derivatization with phenylisothiocyanate. Anal Biochem. 1984 Jan;136(1):65–74. doi: 10.1016/0003-2697(84)90307-5. [DOI] [PubMed] [Google Scholar]
- Kalinowski J., Bachmann B., Thierbach G., Pühler A. Aspartokinase genes lysC alpha and lysC beta overlap and are adjacent to the aspartate beta-semialdehyde dehydrogenase gene asd in Corynebacterium glutamicum. Mol Gen Genet. 1990 Dec;224(3):317–324. doi: 10.1007/BF00262424. [DOI] [PubMed] [Google Scholar]
- Kalinowski J., Cremer J., Bachmann B., Eggeling L., Sahm H., Pühler A. Genetic and biochemical analysis of the aspartokinase from Corynebacterium glutamicum. Mol Microbiol. 1991 May;5(5):1197–1204. doi: 10.1111/j.1365-2958.1991.tb01893.x. [DOI] [PubMed] [Google Scholar]
- Parsot C., Cossart P., Saint-Girons I., Cohen G. N. Nucleotide sequence of thrC and of the transcription termination region of the threonine operon in Escherichia coli K12. Nucleic Acids Res. 1983 Nov 11;11(21):7331–7345. doi: 10.1093/nar/11.21.7331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rees W. D., Flint H. J., Fuller M. F. A molecular biological approach to reducing dietary amino acid needs. Biotechnology (N Y) 1990 Jul;8(7):629–633. doi: 10.1038/nbt0790-629. [DOI] [PubMed] [Google Scholar]
- Rees W. D., Grant S. D., Hay S. M., Saqib K. M. Threonine synthesis from homoserine as a selectable marker in mammalian cells. Biochem J. 1994 May 1;299(Pt 3):637–644. doi: 10.1042/bj2990637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rees W. D., Hay S. M., Flint H. J. Expression of Escherichia coli homoserine kinase in mouse 3T3 cells. Biochem J. 1992 Feb 1;281(Pt 3):865–870. doi: 10.1042/bj2810865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rees W. D., Hay S. M. The expression of Escherichia coli threonine synthase and the production of threonine from homoserine in mouse 3T3 cells. Biochem J. 1993 Apr 1;291(Pt 1):315–322. doi: 10.1042/bj2910315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaul O., Galili G. Threonine Overproduction in Transgenic Tobacco Plants Expressing a Mutant Desensitized Aspartate Kinase of Escherichia coli. Plant Physiol. 1992 Nov;100(3):1157–1163. doi: 10.1104/pp.100.3.1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stacey A., Schnieke A. SVpoly: a versatile mammalian expression vector. Nucleic Acids Res. 1990 May 11;18(9):2829–2829. doi: 10.1093/nar/18.9.2829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szczesiul M., Wampler D. E. Regulation of a metabolic system in vitro: synthesis of threonine from aspartic acid. Biochemistry. 1976 May 18;15(10):2236–2244. doi: 10.1021/bi00655a033. [DOI] [PubMed] [Google Scholar]
- Théze J., Kleidman L., St Girons I. Homoserine kinase from Escherichia coli K-12: properties, inhibition by L-threonine, and regulation of biosynthesis. J Bacteriol. 1974 May;118(2):577–581. doi: 10.1128/jb.118.2.577-581.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wampler D. E., Westhead E. W. Two aspartokinases from Escherichia coli. Nature of the inhibition and molecular changes accompanying reversible inactivation. Biochemistry. 1968 May;7(5):1661–1671. doi: 10.1021/bi00845a007. [DOI] [PubMed] [Google Scholar]
- Wedler F. C., Ley B. W. Homoserine dehydrogenase-I (Escherichia coli): action of monovalent ions on catalysis and substrate association-dissociation. Arch Biochem Biophys. 1993 Mar;301(2):416–423. doi: 10.1006/abbi.1993.1165. [DOI] [PubMed] [Google Scholar]