Abstract
Seagrasses are marine angiosperms that inhabit tropical and subtropical regions around the world. They play a vital role in marine biodiversity and the ecosystem by providing habitats and food for several marine organisms, stabilizing sediments, and improving water quality. Halodule uninervis from the family Cymodoceaceae has been used in traditional folk medicine for the treatment of many ailments. Additionally, several identified bioactive metabolites have been shown to contribute to its pharmacological activities, including anticancer, anti-inflammatory, and antioxidant. As such, H. uninervis could contribute to the development of novel drugs for various diseases. This review aims to compile the phytochemical composition and pharmacological activities of H. uninervis. Furthermore, details about its botanical characteristics and ecological significance are also discussed. By providing valuable insights into the role of H. uninervis in both the marine ecosystem and biomedicine, this review helps to highlight its potential as a therapeutic agent for future drug discovery and development.
Keywords: Halodule uninervis, seagrass, marine ecosystem, herbal medicine, bioactive metabolites, antioxidant, antimicrobial, anticancer, antidiabetic, green nanotechnology
1. Introduction
Since ancient civilizations, traditional practitioners relied on nature for remedies to treat various ailments and promote health. In the present times, plants and other natural products continue to be utilized in modern drug development, as 80% of the world’s population relies on these natural remedies for medicinal uses [1]. Owing to their phytochemical diversity, plant extracts and their derivatives have contributed to the development of many pharmaceuticals, including drugs such as digoxin from Digitalis lanata for the treatment of congestive heart failure [2], morphine from Papaver somniferum to treat severe pain [3], paclitaxel from Taxus brevifolia as a chemotherapeutic agent [4,5], and quinine from Cinchona officinalis for the treatment of malaria [6].
Despite terrestrial plants being the main focus in drug development for centuries, marine plants have been receiving a surge of interest in recent years, as the marine environment comprises 95% of the biosphere and offers a vast reservoir of biodiversity [7]. Having to survive in extreme and dynamic environments, marine plants have evolved unique adaptation and defense strategies, which could contribute to the production of bioactive compounds with distinctive chemical structures and pharmacological properties. However, the complexity of the marine ecosystem and limited accessibility to marine plants pose a challenge in exploring their therapeutic potential [8]. So far, several marine-derived drugs from other organisms have been approved by the US Food and Drug Administration (FDA). For instance, ziconotide, a peptide discovered from the marine cone snail Conus magus, is used for the treatment of severe chronic pain [9]. Another approved anticancer drug is trabectedin, which is derived from the Caribbean sea squirt Ecteinascidia turbinate and used in the treatment of soft tissue sarcoma [10]. Seagrasses have also been used as natural remedies for the treatment of fever, muscle and stomach pains, and wounds [11]. Moreover, the use of seagrasses extended to weaving baskets, thatching roofs, crafting paper-based materials, and preparing fertilizers [12]. There are 72 species of seagrasses divided into six families, Cymodoceaceae, Hydrocharitaceae, Posidoniaceae, Ruppiaceae, Zannichelliaceae, and Zosteraceae [13]. Of particular relevance to this review, Halodule uninervis (Forsskål) Ascherson of the family Cymodoceaceae is an abundant species found in tropical and subtropical coastal regions worldwide. As it needs to thrive in extreme environments with high-stress conditions, H. uninervis is rich in bioactive metabolites, which act as defense mechanisms and bestow the plant with many pharmacological properties, such as anticancer, antimicrobial, and antioxidant [14,15,16].
The therapeutic value of seagrasses was documented by several reviews, shedding light on their phytochemical composition as well as their antioxidant, anticancer, and anti-inflammatory properties, among others [17,18]. Indeed, interest in the pharmacology of seagrasses has been steadily increasing in recent years. Particularly, a PubMed search of “seagrass pharmacology” showed that related publications since 1985 have more than doubled in the last decade. Despite the noticeable increasing interest in the pharmacological activities of seagrasses, most of the published papers focus on their remarkable ecological role. Since there is a continuous need for the development of alternative therapeutics from natural sources with limited side effects, a thorough investigation of different seagrass species is required to discover new bioactive metabolites with potential medicinal use. Given the limited literature on the phytochemical composition and pharmacological properties of the seagrass Halodule uninervis (Figure 1), this review aims to compile all existing studies and serve as a basis for future investigations to help uncover the potential use of H. uninervis as a therapeutic agent. This updated review paper focuses on the phytochemical analysis and pharmacological implications of H. uninervis, highlighting the limitations of current studies and possible future implications. Overall, we provide (1) a description of its botanical and ecological characteristics and (2) an updated comprehensive compilation of its phytochemical composition in addition to (3) its pharmacological activities.
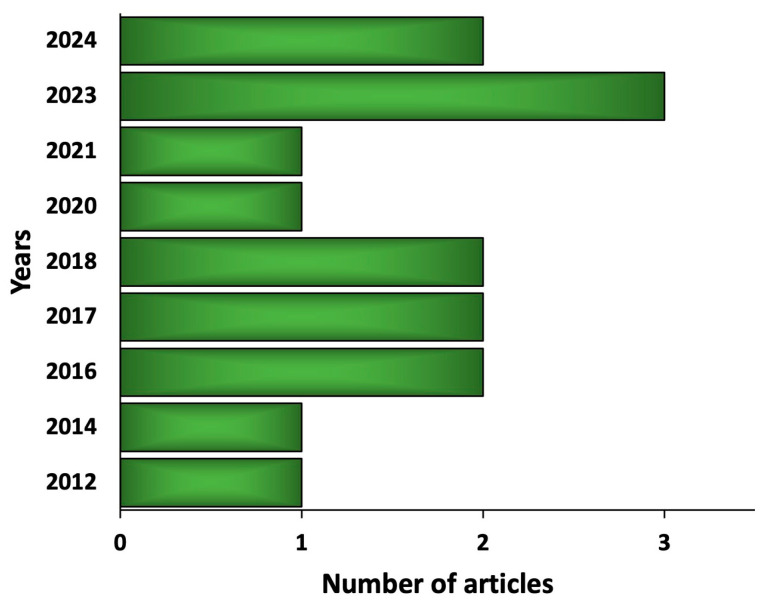
Figure 1.
A chart showing the number of published articles on the phytochemical composition and pharmacological activities of Halodule uninervis from 2012 to 2024.
2. Taxonomic Classification of Halodule uninervis (WoRMS)
Seagrasses are classified into six different families, including Cymodoceaceae, Hdyrocharitaceae, Posidoniaceae, Potamogetonaceae, Ruppiaceae, and Zosteraceae. The Halodule genus belongs to the family Cymodoceaceae, and it comprises seven different species, including Halodule beaudettei, Halodule bermudensis, Halodule ciliate, Halodule emarginata, Halodule pinifolia, Halodule uninervis, and Halodule wrightii. Table 1 showcases the taxonomic classification of Halodule uninervis as found in the World Register of Marine Species (WoRMS).
Table 1.
Taxonomic classification of Halodule uninervis.
| Kingdom | Plantae |
|---|---|
| Phylum | Tracheophyta |
| Class | Magnoliopsida |
| Order | Alismatales |
| Family | Cymodoceaceae |
| Genus | Halodule |
| Species | Halodule uninervis |
| Binomial name | Halodule uninervis (Forsskål) Ascherson |
3. Methods
Major scientific literature databases, including PubMed, ScienceDirect, Scopus, Chemical Abstracts, Henriette’s Herbal Homepage, and Medicinal and Aromatic Plants Abstracts, were utilized to search for and retrieve articles related to the review subject. General web searches were also conducted using Google and Google Scholar. The search period covered articles published between year 1968 and July 2024. The search used the keywords and MeSH terms for ‘Halodule uninervis’, AND (‘phytochemical compounds, or content, or constituents’, ‘pharmacological activities, or effects, or properties, or roles’, ‘antibacterial’, ‘anticancer’, ‘antidiabetic’, ‘antifungal’, ‘anti-inflammatory’, ‘antimicrobial’, ‘antioxidant’, or ‘clinical trials’).
4. Botanical and Ecological Characteristics of Halodule uninervis
4.1. Botanical Characteristics
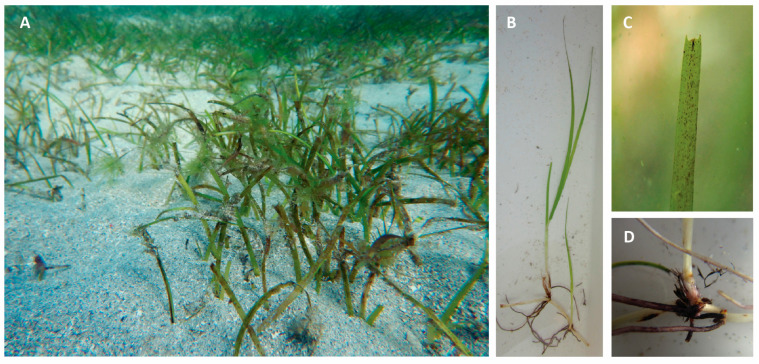
H. uninervis, commonly known as “narrowleaf seagrass” and “a’shab bahriya” in Arabic [19], is a perennial flowering plant with a vibrant green color that forms dense, large meadows under the sea resembling grassland (Figure 2A) [20].
Figure 2.
(A) Halodule uninervis in its local environment forming meadows. (B) Halodule uninervis whole plant showing the branching rhizome, roots at the nodes, short erect stems, and flat needle-like leaves. (C) Halodule uninervis leaf blade with 3 distinct points “teeth” at its tip. (D) Halodule uninervis branching rhizome and roots. Images were obtained from https://www.inaturalist.org/taxa/416176-Halodule-uninervis/browse_photos (accessed on 14 May 2024).
Members of the family Cymodoceaceae are dioecious, having separate male and female flowers with the reproductive parts found on the flowering stems at the base of the leaf sheath [21]. The produced seeds have a hard seed coat and are usually released directly into the sediments, preserving them until the parent plants are destroyed [22].
H. uninervis spreads via a branching rhizome that roots at the nodes (Figure 2B,D). At each node, up to six very fine roots are found with a short, erect stem [21]. About two to four alternately arranged leaves emerge on each stem, with a sturdy, persistent leaf sheath up to 3.5 cm long. The leaf blades are thin, flat needle-like structures that grow straight up to 15 cm long and 0.25–5 mm wide, with three longitudinal veins and three distinct points at the leaf tip referred to as “teeth” (Figure 2C) [23,24]. Interestingly, leaf morphology changes according to the habitat type. Long, narrow leaves are found closer to the shore, whereas short, wide leaves are found in deeper areas [25]. This could be explained by the fact that plants closer to the shore would need less surface area to absorb the sunlight needed for photosynthesis.
4.2. Ecological Characteristics
Seagrasses are the only angiosperms to recolonize the seabed. They are categorized as halophytes and are capable of growing partially or entirely submerged under water [17]. As part of the marine ecosystem, seagrasses play a critical role in serving as habitat for different marine organisms, supporting food security, stabilizing sediments, and mitigating climate change [26,27,28].
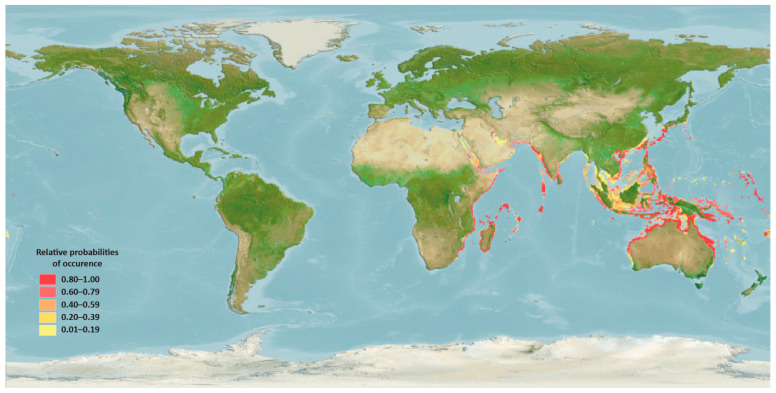
H. uninervis is a very common species that is predominantly located in tropical and subtropical regions around the world. It is widely distributed in the Indo-Pacific region [29] (Figure 3).
Figure 3.
Native distribution map for Halodule uninervis. Map retrieved from https://www.aquamaps.org.
In the Indian Ocean, it is commonly found in areas along the coastlines of the Red Sea and the Arabian Gulf, including Egypt [30], Jordan [31], Oman [32], Qatar [33], and Saudi Arabia [34]. It also spreads from the Southeast coast of South Africa [35,36] to the islands of the western Indian Ocean, including Andaman Islands [37], Mauritius [38], and Seychelles [39]. In the Pacific Ocean, it is distributed throughout Southeast Asia, including Indonesia [40], Malaysia [41], and the Philippines [42]. It is also found in the Gulf of Thailand [43] and along the coast of Vietnam [44] and southern China [45].
H. uninervis grows in a variety of habitats, where it often forms dense meadows in shallow coastal waters, lagoons, exposed or sheltered coral reefs, and sandy and muddy substrates [46]. Within these habitats, it can withstand harsh environmental conditions, such as salinity fluctuations, sedimentation, temperature extremes, and wave action. In Eastern Australia, it survives the unstable and depositional environments. In the Arabian Gulf, it withstands extreme conditions of high salinity levels by regulating the concentration of salt within its tissues and maintaining osmotic balance and high temperatures [47,48]. Several adaptation strategies have evolved to help H. uninervis survive and persist in marine environments. For instance, its flexible leaves and anchoring rhizomes help withstand wave action and reduce the risk of damage [25,49]. The modification in the dimensions of leaves and stems also helps in obtaining sufficient sunlight, with submerged plants having longer leaves and stems compared to the ones near the shore [25].
Like other seagrasses, H. uninervis plays a crucial ecological role in the marine ecosystems. By forming dense meadows undersea, it provides a habitat for a myriad of marine organisms [50,51]. H. uninervis also helps in stabilizing sediments, reducing coastal erosion, maintaining water quality, and sequestering carbon by absorbing nutrients and trapping sediments through its root system [52,53,54]. Moreover, it contributes to the complex marine food webs by providing nourishment for herbivores.
5. Phytochemical Constituents of Halodule uninervis
5.1. Bioactive Metabolites
Beyond their ecological significance, seagrasses have been used in traditional folk medicine for the treatment of various ailments, including digestive disorders, inflammatory conditions, and skin diseases, among others. As it must thrive in high-stress environments, H. uninervis is rich in phenolic compounds that confer the plant with self-defense mechanisms. Moreover, extracts from different seagrasses have been shown to possess a variety of pharmacological activities, such as anticancer, anti-inflammatory, and antimicrobial. These properties of seagrasses are attributed to the presence of a wide array of secondary metabolites.
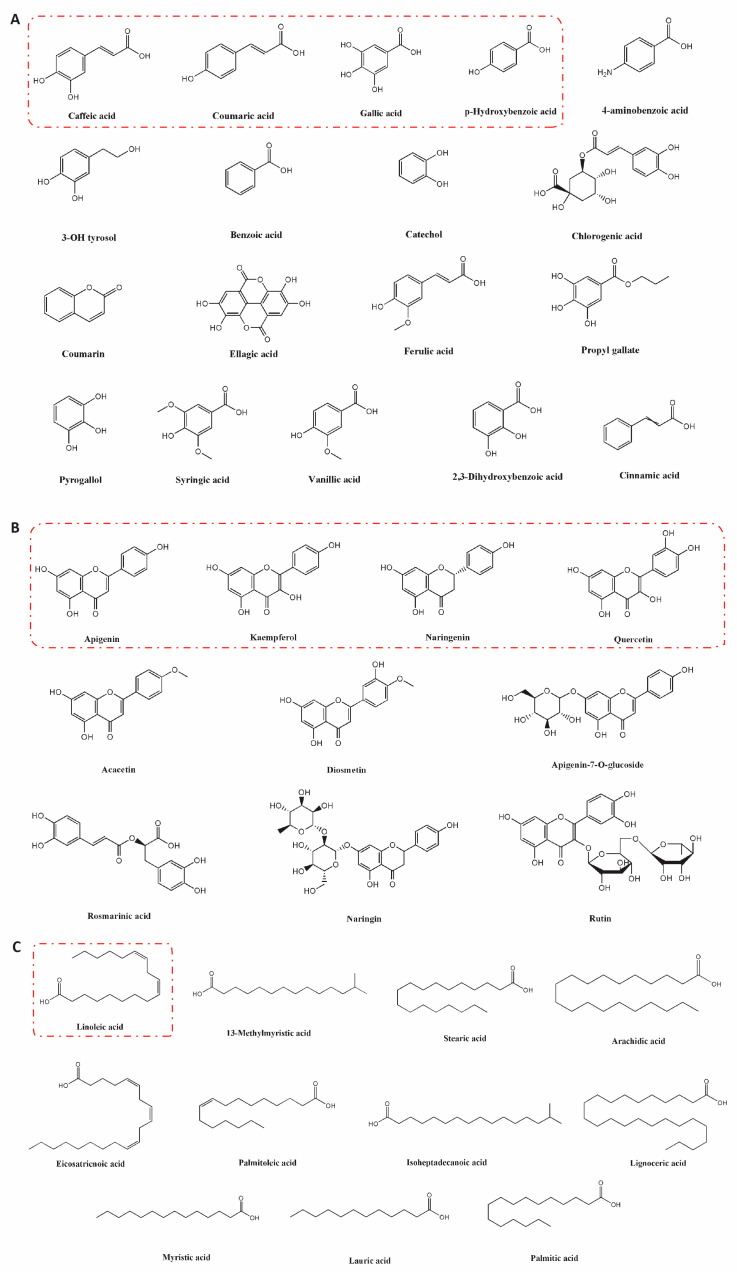
The preliminary phytochemical screening of H. uninervis revealed that it is rich in secondary metabolites, including phenols, flavonoids, quinones, tannins, terpenoids, and steroids [14,16,55,56]. However, research into the detailed phytochemical composition of H. uninervis remains limited. It is noteworthy to mention that the composition of bioactive metabolites in H. uninervis varies depending on the type of the extract. Few phenolic compounds identified in a methanolic extract of H. uninervis were caffeic acid, chlorogenic acid, gallic acid, and p-hydroxybenzoic acid [15]. In addition, this extract contained three flavonoids, including apigenin, apigenin-7-O-glucoside, and naringenin [15]. Moreover, it was rich in fatty acids, including linoleic acid (9Z,12Z-octadecadienoic), palmitic acid, and stearic acid [15]. Linoleic acid was also observed in an ethyl acetate extract of H. uninervis [16]. An investigation of an ethanolic extract of H. uninervis revealed the presence of 10 phenolic compounds and 9 flavonoids [57]. Some phenolic compounds included benzoic acid, caffeic acid, catechol, ellagic acid, and pyrogallol, whereas kaempferol-3-2-p-coumaroyl glucose, naringin, and quercetin were few of the identified flavonoids. In another study, the LC-MS/MS analysis of H. uninervis ethanolic extract revealed the presence of 4 phenolic compounds and 11 flavonoids [56]. Phenolic compounds included dihydroxybenzoic acid, hydroxybenzoic acid, vanillic acid, and coumaric acid. Identified flavonoids comprised apigenin, acacetin, diosmetin, kaempferol, quercetin glucoside, and diosmetin glucoside. A detailed list of the identified phenols and flavonoids is found in Table 2.
Table 2.
Bioactive metabolites in Halodule uninervis.
| Extract | Analytical Methods | Main Results | Compounds | Reference |
|---|---|---|---|---|
| Methanolic extract of the leaves | HPLC | 8 phenolic compounds, 3 flavonoids, and 12 fatty acids |
|
[15] |
| Ethanolic extract of the root and shoot parts | HPLC | 10 phenolic compounds and 9 flavonoids |
|
[57] |
| Ethanolic extract of the leaves | LC-MS/MS | 4 phenolic compounds and 12 flavonoids |
|
[56] |
Caffeic acid, coumaric acid, gallic acid, and hydroxybenzoic acid are the major identified phenols in different extracts of H. uninervis (Figure 4A).
Figure 4.
The structures of the identified (A) phenolic acids, (B) flavonoids, and (C) fatty acids in different extracts of Halodule uninervis. Red boxes highlight the major compounds in each group.
Apigenin, kaempferol, naringenin, quercetin, and their derivatives are the major identified flavonoids (Figure 4B). As evident by the aforementioned studies, compositional studies of H. uninervis are insufficient. Therefore, future investigations should focus on performing a detailed analysis of phenolic compounds and flavonoids found in H. uninervis extracts as well as other groups of bioactive metabolites.
5.2. Macro- and Micronutrients
Apart from the bioactive metabolites with medicinal benefits, studies have demonstrated that H. uninervis also contains compounds with nutritional value, including both macro- and micronutrients. Macronutrients, such as carbohydrates, lipids, and proteins, play an important role in the adaptive mechanisms against stress conditions. An analysis of an aqueous extract of H. uninervis showed glucose, galactose, and xylose as the major monosaccharides [15]. This extract also had high protein content, whereas an ethanolic extract from H. uninervis showed high protein content but had low levels of carbohydrates [57].
Other compounds with nutritional value include micronutrients, such as minerals. An analysis of essential minerals in H. uninervis revealed the presence of nitrogen, potassium, calcium, magnesium, and phosphorus, whereas the identified nonessential minerals were iron, zinc, manganese, and copper [58,59]. The presence of macro- and micronutrients in Halodule uninervis make it a valuable nutritive source for marine organisms that feed on them. Moreover, the calorific value in H. uninervis leaves and rhizomes was comparable to that of beetroots, carrots, ladies’ fingers, and cauliflowers [60]. Taken together, these results indicate that H. uninervis could contribute to human nutritional needs by being utilized in dietary and pharmaceutical supplements.
6. Pharmacological Activities of Halodule uninervis
6.1. Antioxidant Activities
Oxidative stress results from an imbalance between free radicals and antioxidants in the body. It is implicated in the onset and progression of many diseases, such as atherosclerosis, cancer, inflammation, and neurodegenerative disorders. Synthetic antioxidants have been extensively employed to reduce oxidative stress. However, due to concerns over the safety of these antioxidants, natural sources, such as plants, have been utilized instead, owing to the presence of bioactive metabolites that possess strong antioxidant activity. Table 3 summarizes the antioxidant potential of different H. uninervis extracts.
Table 3.
The antioxidant effects of Halodule uninervis.
| Extract | Dose | Methods | Observations | References |
|---|---|---|---|---|
| Methanolic extract | 500, 1000, 1500, and 2000 ppm | DPPH radical scavenging assay and Ferric reducing power (FRAP) |
|
[61] |
| Methanolic extract | N/A | FRAP and Trolox equivalent antioxidant capacity (TEAC) assays |
|
[62] |
| Methanol/chloroform extract, USM 1 content, phenolic extract | 100–1000 mg/mL | DPPH radical scavenging assay |
|
[15] |
| Ethanolic extract of the leaves | 5, 10, 25, 50, 100, 200, and 400 μg/mL | DPPH radical scavenging assay |
|
[56] |
1 Unsaponifiable matter.
The methanolic extract of H. uninervis showed robust scavenging activities against the 1,1-diphenyl-2-picrylhydrazyl (DPPH) radical, indicating the presence of antioxidants [61]. Similarly, significant antioxidant activity was observed in the methanolic extract through the trolox equivalent antioxidant capacity assay, where it effectively scavenged the 2,2′-azino-bis-(3-ethylbenzothiazoline-6-sulfonic acid) radical (ABTS) [62]. The antioxidant activity of the methanol/chloroform extract, unsaponifiable matter (USM) content, and phenolic extract of H. uninervis was evaluated using the DPPH radical scavenging assay. The results showed that the USM content exhibited the strongest radical scavenging activity, which was attributed to the presence of dibuylhydroxy-toluene (BHT) antioxidant, a widely used preservative in food and cosmetics to prevent oxidation of free radicals [15]. An ethanolic extract of the leaves of H. uninervis was evaluated for its antioxidant capacity using the DPPH radical scavenging assay [56]. The findings revealed a significant antioxidant activity in a dose-dependent manner with an IC50 of 301.31 μg/mL.
The observed antioxidant activity of H. uninervis was associated with the presence of bioactive metabolites, including phenols and flavonoids, which are known antioxidant compounds that play an important role in plant defense mechanisms as well as a protective role in human health. However, the chemical assays used in the aforementioned studies are non-specific and prone to interference. Therefore, they are not enough to validate the antioxidant potential of H. uninervis and its associated pharmacological properties. Future research should focus on implementing in vivo methods for assessing antioxidant activities.
6.2. Antimicrobial Activities
Infectious diseases persist as a serious threat and a major cause of death due to the existence of antibiotic-resistant pathogens. This has urged the need to search for and develop new antimicrobial agents by utilizing natural compounds. Indeed, medicinal plants have been extensively studied as potential antimicrobial agents due to their production of bioactive compounds with known therapeutic properties. Several studies evaluated the effectiveness of H. uninervis extracts as antibacterial agents as illustrated in Table 4.
Table 4.
The antimicrobial activities of Halodule uninervis.
| Extract | Dose | Experimental Model | Main Results | Reference |
|---|---|---|---|---|
| Methanolic extract of the leaves | 500, 1000, 1500, and 2000 ppm |
|
|
[14] |
| Aqueous extract of the leaves | N/A |
|
|
[63] |
| Extract of the leaves in organic solvents (chloroform, ethanol, ethyl acetate, petroleum ether) | N/A |
|
|
[63] |
| Ethanolic extract | 10 mg/mL |
|
|
[55] |
| Extract of the leaves or roots in methanol, dichloromethane, and hexane | 100 mg/mL |
|
|
[64] |
| H. uninervis-synthesized AgNPs 1 | 25, 50, 100 ppm |
|
|
[65] |
1 Silver nanoparticles.
The results have shown that a methanolic extract from the leaves of H. uninervis exhibits stronger antibacterial activity against Gram-positive bacteria, such as Bacillus subtilis and Listeria monocytogenes, compared to Gram-negative bacteria, such as Aeromonas hydrophila and Vibrio harveyi [14]. Gumgumjee et al. evaluated the antimicrobial activity of H. uninervis leaf extracts in aqueous solution and other organic solvents [63]. The results showed that the ethanolic extract exhibited the highest level of inhibition among all solvents, whereas the aqueous extract showed no activity, with the exception of Pseudomonas aeruginosa. An ethanolic extract of H. uninervis exhibited inhibitory effects on the growth of Bacillus cereus, a Gram-positive bacteria, and on Proteus vulgaris, a Gram-negative bacteria. It also inhibited the growth of the fungus Cryptococcus neoformas [55]. A recent study demonstrated a potent antibacterial effect of H. uninervis crude extracts from leaves or roots in hexane, dichloromethane, and methanol solvents against Salmonella typhi [64]. The hexane extracts from both the leaves and roots had the strongest inhibitory activity.
These studies support the potential use of H. uninervis extracts as antibacterial agents. Further research should explore the mechanism for the observed antibacterial activity. Moreover, the effect of H. uninervis extracts against other pathogenic microorganisms, such as fungi, pests, and yeast, should also be investigated.
6.3. Larvicidal Effect
Mosquito-transmitted diseases continue to be a major cause of death worldwide. Mosquitoes act as vectors of many infectious pathogens, causing serious diseases, including dengue fever, lymphatic filariasis, malaria, Zika virus, among others. Though synthetic pesticides have been widely used to control mosquito larvae, their toxicity to non-targeted organisms and the environment necessitates the use of alternative approaches. Indeed, plant products have been utilized as natural insecticides due to their ecofriendly nature and immense potential for the control of mosquito larvae.
A study was conducted to assess the mosquito larvicidal activity of H. uninervis ethyl acetate extract against Culex pipiens, the main vector of lymphatic filariasis in Egypt. The results revealed effective inhibitory activity with a lethal concentration LC50 of 3.97 ppm [66]. A methanolic extract of H. uninervis from the Saudi Red Sea exhibited moderate toxicity against the fourth stage larvae of Aedes aegypti. The mosquito larvicidal properties of H. uninervis are summarized in Table 5. Overall, H. uninervis proves to be a promising ecofriendly agent for the control of mosquito vectors.
Table 5.
The larvicidal effect of Halodule uninervis.
| Extract | Dose | Methods | Main Results | Reference |
|---|---|---|---|---|
| Ethyl acetate extract | 0–8 ppm |
|
|
[66] |
| Methanolic extract | 50, 100, 300, 500, 700 ppm |
|
|
[65] |
| H. uninervis-synthesized AgNPs 1 | 5, 10, 15, 20, 25 ppm |
|
|
[65] |
1 Silver nanoparticles.
6.4. Anticancer Activities
Cancer is a major public health problem and a leading cause of death worldwide. Conventional treatment regimens for cancer are non-selective, result in adverse side effects, and grow tumor resistance. This mandated resorting to medicinal plants for alternative treatment approaches [67,68].
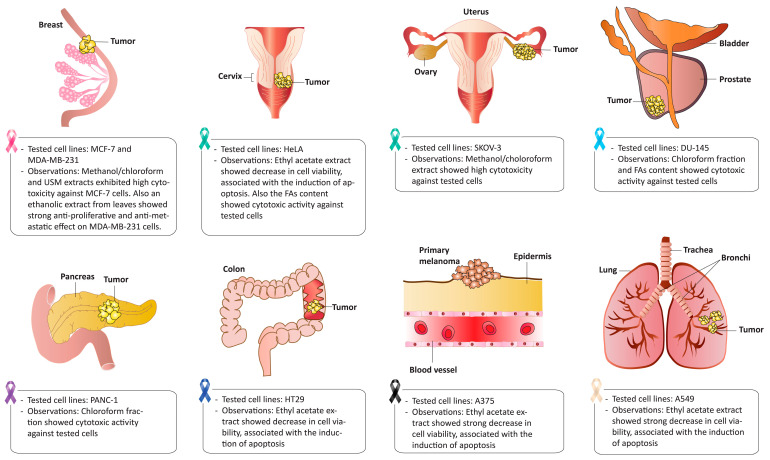
The anticancer activity of H. uninervis was evaluated using an ethyl acetate extract against several cancer cell lines in vitro, including malignant melanoma A375, lung carcinoma A549, cervix adenocarcinoma HeLa, and colorectal adenocarcinoma HT29 cells. the findings indicated that the extract exhibited the highest cytotoxic activity against A549 lung cancer cells by inducing apoptosis [16]. In another study, different seagrasses collected from the Saudi Red Sea were screened for their cytotoxic activity against several cancer cell lines, including breast cancer MCF-7, prostate cancer DU-145, HeLa, ovarian cancer SKOV-3, and pancreatic cancer PANC-1 [15]. The crude extract of H. uninervis showed a potent cytotoxicity against SKOV-3 cells and demonstrated the most significant inhibitory effects on MCF-7 cells compared to other treatments. The chloroform fraction of the extract inhibited the viability of DU-145 and PANC-1 cells. Additionally, the extracted free fatty acids (FAs) showed anti-proliferative activity against and HeLa cells, while unsaponifiable matter (USM) exhibited cytotoxic effects only against MCF-7 cells. The anticancer activity of a crude ethanolic extract of H. uninervis leaves was investigated against triple-negative breast cancer MDA-MB-231 cells [56]. Results demonstrated a dose- and time-dependent decrease in cell proliferation through the induction of G0/G1 cell cycle arrest and the activation of the intrinsic apoptosis pathway. Moreover, the extract mitigated cancer metastasis by inhibiting cell adhesion, migration, invasion, and in ovo angiogenesis. These effects of H. uninervis were potentially mediated by the downregulation of the proto-oncogenic STAT3 signaling pathway. The promising anticancer activities of H. uninervis are illustrated in Figure 5 and summarized in Table 6.
Figure 5.
Halodule uninervis exhibits potent anticancer activity against several cancers.
Table 6.
The anticancer activities of Halodule uninervis.
| Extract | Dose | Experimental Model | Observations | References |
|---|---|---|---|---|
| Ethyl acetate extract | 25, 50, and 100 mg/mL |
|
|
[16] |
| Methanol/chloroform extract | 100–1000 mg/mL |
|
|
[15] |
| Chloroform fraction | 100–1000 mg/mL |
|
|
[15] |
| FAs content | 100–1000 mg/mL |
|
|
[15] |
| USM 3 content | 100–1000 mg/mL |
|
|
[15] |
| Ethanolic extract of the leaves | 100 and 200 μg/mL |
|
|
[56] |
1 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide; 2 Sulforhodamine B; 3 Unsaponifiable matter.
However, most of the cited studies used cell viability assays to evaluate the anticancer activity of different H. uninervis extracts. This assay works best for screening purposes; therefore, additional research is required to confirm the anticancer potential of H. uninervis and to investigate the underlying molecular mechanisms.
6.5. Antidiabetic Effect
Diabetes is a chronic metabolic disorder characterized by hyperglycemia, elevated blood glucose concentration, due to insulin deficiency or malfunction. Uncontrolled diabetes leads to serious complications in many organs, including a loss of vision and kidney function, heart attacks, and strokes. The use of herbal medicine for the treatment of diabetes has been favored over synthetic drugs due to its low cost and low incidence of side effects.
The antidiabetic potential of H. uninervis extracts using different extraction solvents was tested in vitro and in vivo (Table 7).
Table 7.
The antidiabetic effects of Halodule uninervis.
| Extract | Dose | Experimental Model | Observations | References |
|---|---|---|---|---|
| Ethanolic extract | 125, 250, 500, 1000, and 2000 ppm | In vitro inhibition of α-glucosidase enzyme assay |
|
[69] |
| Ethyl acetate extract | 125, 250, 500, 1000, and 2000 ppm | In vitro inhibition of α-glucosidase enzyme assay |
|
[69] |
| Methanolic extract | 150 and 250 mg/kg | Streptozotocin-induced diabetic mouse model |
|
[70] |
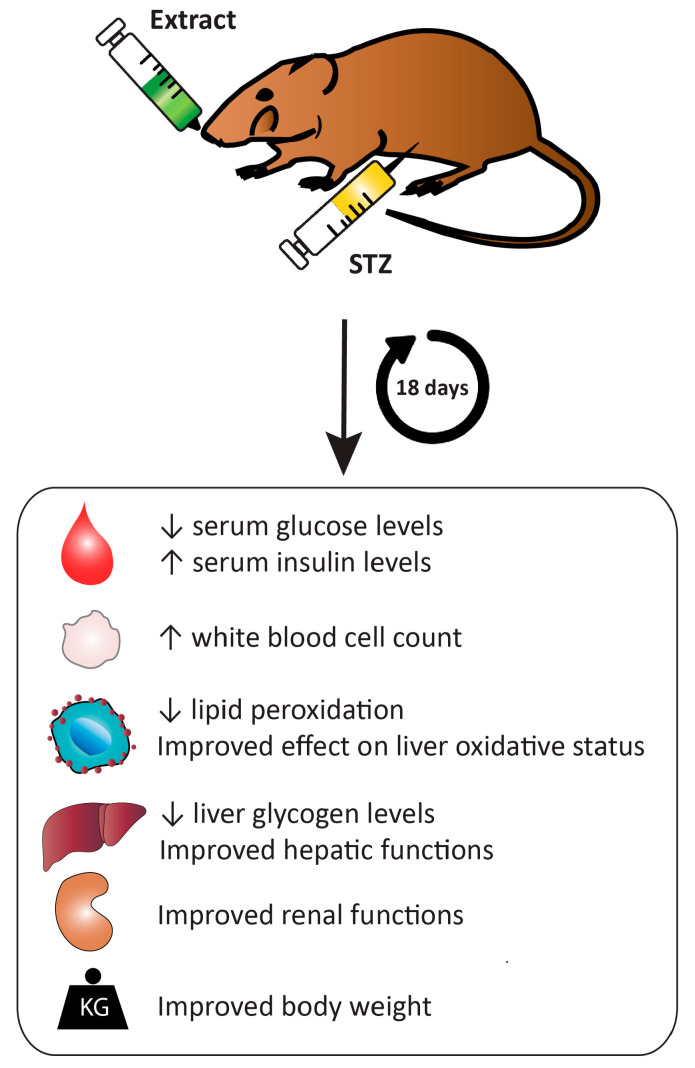
A recently conducted study evaluated the antidiabetic activity of H. uninervis extract with ethanol and ethyl acetate solvents [69]. The in vitro inhibition of the α-glucosidase enzyme assay revealed that the ethanolic extract possesses better antidiabetic activity compared to ethyl acetate extract. In a Streptozotocin-induced diabetic mouse model, the oral administration of methanolic extract from H. uninervis significantly reduced serum glucose levels [70]. Additionally, the extract recovered weight loss observed in diabetic mice, improved low white blood cell count, and exhibited a protective effect on liver oxidative status. This is presented in Figure 6 and Table 7. These results verify the potential use of Halodule uninervis as a potential anti-hyperglycemic agent for the treatment of diabetes.
Figure 6.
Halodule uninervis has an antidiabetic effect. The oral administration of a methanolic extract of H. uninervis in streptozotocin-induced diabetic mouse model improved the glycemic profile.
6.6. Green Nanotechnology
Nanotechnology is a multidisciplinary field that involves the design, production, and characterization of materials at the nanoscale. At this scale, materials exhibit distinctive properties, rendering them highly valuable for implications across several sectors, including agriculture, cosmetology, electronics, food industry, and medicine [71]. Nanomaterials can be broadly classified into carbon-based, composite, organic, and inorganic based on their structural composition [72]. Silver nanoparticles (AgNPs), a type of metal-based inorganic nanomaterials, exhibit a high surface area-to-volume ratio and the ability to release silver ions, making them potent antimicrobial agents [73]. They have been integrated into dental products, medical implants, surgical tools, wound dressings, and textiles to prevent infections and promote healing. Conventional methods for synthesizing AgNPs include chemical processes through reduction reactions [74,75], physical methods such as laser ablation [76,77], and green synthesis routes via the use of biomaterials such as bacteria [78], fungi [79], and plant extracts [80]. The green synthesis of AgNPs ensures the production of ecofriendly and nontoxic nanoparticles, with enhanced stability, biocompatibility, and biological activities.
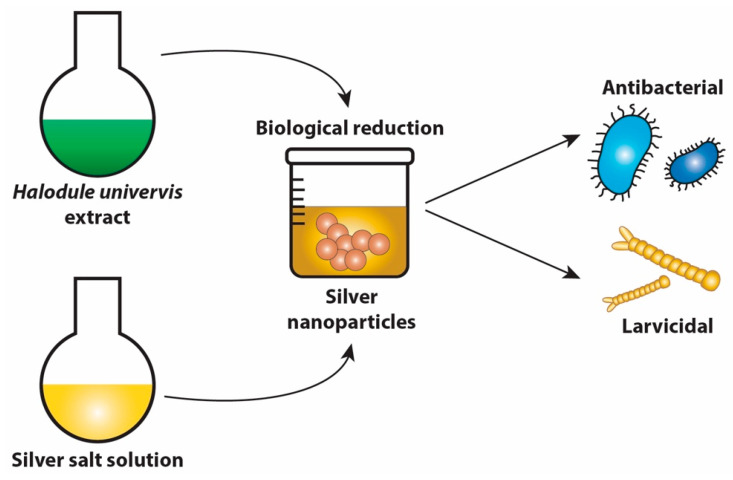
H. uninervis aqueous extract has been utilized as a reducing and capping agent for the green synthesis of silver nanoparticles (AgNPs). The antibacterial activity of H. uninervis-synthesized AgNPs was evaluated against Bacillus subtilis, Klebsiella pneumoniae, and Salmonella typhi, with the strongest inhibitory activity observed against B. subtilis [65] (Table 4). Additionally, the synthesized AgNPs showed significant mosquito larvicidal activity (Table 5). Their small size allows them to pass through the insect cuticle and induce changes in cell morphology and physiological processes [65]. Figure 7 illustrates the antibacterial and larvicidal activities of H. uninervis-synthesized AgNPs. Overall, these results underscore the potential of H. uninervis in the green synthesis of nanoparticles, warranting further investigation, particularly due to their potent activity even at low doses.
Figure 7.
Halodule uninervis is used in the green synthesis of silver nanoparticles (AgNPs) with antibacterial and larvicidal activities.
7. Safety Profile
As the use of herbal medicinal products is globally increasing, major concerns surrounding their safety profile are emerging. Since most of these products are classified as foods or dietary supplements in most countries, the evaluation of their safety or toxicity is not mandatory prior to market release [81,82,83]. However, to avoid potential adverse effects to human health, toxicological studies are essential when developing herbal medicine to ensure its efficacy, quality, and safety. Many studies evaluated the safety profile of extracts from different seagrasses, and they were found nontoxic with a potential use in pre-clinical trials [64,84,85,86,87].
Toxicological studies for H. uninervis extracts are still limited. Acute toxicity studies of the methanolic H. uninervis extract showed no mortality or toxic effects in mice administered intraperitoneally at a dose range of 50 to 450 mg/kg. Animals were kept under observation to identify symptoms of toxicity for two weeks following treatment [70]. The safety of different extracts of H. uninervis leaves and roots was determined by the brine shrimp lethality assay. Brine shrimp eggs were treated with a range of 24 to 240 mg/mL of H. uninervis extracts for 24 h. The results revealed that both hexane and dichloromethane extracts were nontoxic with no significant difference between them or between the leaves and roots [64]. Overall, further toxicological evaluation is necessary to validate the safety profile of H. uninervis for therapeutic purposes.
8. Conclusions and Future Implications
Plant-derived natural products have been extensively used in drug discovery and development for centuries, providing a diverse array of bioactive metabolites with therapeutic potentials. Compared to terrestrial plants, research on the medicinal properties of marine plants is relatively limited. Halodule uninervis is a marine angiosperm that plays a crucial role in coastal marine ecosystems, yet scientific research on its therapeutic potential is still scarce. Based on its available phytochemical profile, H. uninervis contains various bioactive metabolites known for their antioxidant, anticancer, antimicrobial, and anti-inflammatory properties. Therefore, as the demand for natural and alternative therapeutics grows, the use of H. uninervis in novel drug development and therapy is potentially promising. Nonetheless, further investigation is required to thoroughly analyze the phytochemical composition of H. uninervis and evaluate the variation of bioactive metabolites found in extracts from different parts of the seagrass. Future studies should also aim to identify the bioactive metabolites mediating the biological activities of H. uninervis. Isolating and characterizing new metabolites from H. uninervis could reveal novel molecular mechanisms of action that could be utilized for the development of drugs targeting many diseases. These bioactive metabolites could also be used in nutraceuticals and the food industry. Furthermore, after identifying and isolating the bioactive metabolites, the safety profile and efficacy of these compounds should be evaluated in vivo models before being tested in clinical trials and used for drug development. It is important to note that there is not enough evidence in the literature highlighting the anti-inflammatory activity of H. uninervis. As such, future studies should focus on investigating its anti-inflammatory potential since chronic inflammation is the onset of various diseases, including atherosclerosis, cardiovascular, diabetes, and cancer. Despite the limited knowledge present about its phytochemical profile and pharmacological activities, H. uninervis holds a promising potential in the pharmaceutical industry since its use, among other seagrasses, as natural remedies in traditional medicine has been well documented. Unfortunately, H. uninervis faces various threats, including habitat loss and degradation due to coastal development, pollution, sedimentation, and climate changes [88]. Conservation efforts, such as strengthening scientific research on seagrass meadows, managing pollutant emissions, and launching awareness campaigns, should be implemented to preserve this valuable marine species [89]. Overall, compounds derived from H. uninervis and other seagrasses may provide useful leads for the development of novel pharmaceutical drugs.
Author Contributions
Conceptualization, N.W. and E.B.; methodology, N.W., J.E.M., A.B. and M.B.; writing—original draft preparation, N.W.; writing—review and editing, N.W., J.E.M., M.B., A.A.-S. and E.B.; supervision, E.B. and M.B.; funding acquisition, M.B. All authors have read and agreed to the published version of the manuscript.
Conflicts of Interest
The authors declare no conflicts of interest.
Funding Statement
This work was supported by URB grant from the American University of Beirut to E.B. (104391) and a University of Petra grant to A.B. (1/4/2024).
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.World Health Organization . WHO Global Report on Traditional and Complementary Medicine 2019. World Health Organization; Geneva, Switzerland: 2019. [(accessed on 30 June 2024)]. Available online: https://iris.who.int/handle/10665/312342. [Google Scholar]
- 2.Rathore S.S., Curtis J.P., Wang Y., Bristow M.R., Krumholz H.M. Association of serum digoxin concentration and outcomes in patients with heart failure. JAMA. 2003;289:871–878. doi: 10.1001/jama.289.7.871. [DOI] [PubMed] [Google Scholar]
- 3.Behar M., Olshwang D., Magora F., Davidson J.T. Epidural morphine in treatment of pain. Lancet. 1979;313:527–529. doi: 10.1016/S0140-6736(79)90947-4. [DOI] [PubMed] [Google Scholar]
- 4.Weaver B.A. How Taxol/paclitaxel kills cancer cells. Mol. Biol. Cell. 2014;25:2677–2681. doi: 10.1091/mbc.e14-04-0916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Markman M., Mekhail T.M. Paclitaxel in cancer therapy. Expert Opin. Pharmacother. 2002;3:755–766. doi: 10.1517/14656566.3.6.755. [DOI] [PubMed] [Google Scholar]
- 6.Achan J., Talisuna A.O., Erhart A., Yeka A., Tibenderana J.K., Baliraine F.N., Rosenthal P.J., D’Alessandro U. Quinine, an old anti-malarial drug in a modern world: Role in the treatment of malaria. Malar. J. 2011;10:144. doi: 10.1186/1475-2875-10-144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Molinski T.F., Dalisay D.S., Lievens S.L., Saludes J.P. Drug development from marine natural products. Nat. Rev. Drug Discov. 2009;8:69–85. doi: 10.1038/nrd2487. [DOI] [PubMed] [Google Scholar]
- 8.Ahmed I., Asgher M., Sher F., Hussain S.M., Nazish N., Joshi N., Sharma A., Parra-Saldívar R., Bilal M., Iqbal H.M. Exploring marine as a rich source of bioactive peptides: Challenges and opportunities from marine pharmacology. Mar. Drugs. 2022;20:208. doi: 10.3390/md20030208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schmidtko A., Lötsch J., Freynhagen R., Geisslinger G. Ziconotide for treatment of severe chronic pain. Lancet. 2010;375:1569–1577. doi: 10.1016/S0140-6736(10)60354-6. [DOI] [PubMed] [Google Scholar]
- 10.Gordon E.M., Sankhala K.K., Chawla N., Chawla S.P. Trabectedin for soft tissue sarcoma: Current status and future perspectives. Adv. Ther. 2016;33:1055–1071. doi: 10.1007/s12325-016-0344-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.de la Torre-Castro M., Rönnbäck P. Links between humans and seagrasses—An example from tropical East Africa. Ocean Coast. Manag. 2004;47:361–387. doi: 10.1016/j.ocecoaman.2004.07.005. [DOI] [Google Scholar]
- 12.Milchakova N. Ecosystem Services of Seagrasses. In: Grigore M.-N., editor. Handbook of Halophytes: From Molecules to Ecosystems towards Biosaline Agriculture. Springer International Publishing; Cham, Switzerland: 2021. pp. 837–856. [Google Scholar]
- 13.Short F.T., Short C.A., Novak A.B. Seagrasses. In: Finlayson C.M., Milton G.R., Prentice R.C., Davidson N.C., editors. The Wetland Book: II: Distribution, Description and Conservation. Springer; Dordrecht, The Netherlands: 2016. pp. 1–19. [Google Scholar]
- 14.Supriadi A., Baehaki A., Pratama M.C. Antibacterial activity of methanol extract from seagrass of Halodule uninervis in the coastal of Lampung. [(accessed on 20 June 2024)];Pharm. Lett. 2016 8:77–79. Available online: https://www.researchgate.net/profile/Ace-Baehaki/publication/306499588_Antibacterial_activity_of_methanol_extract_from_seagrass_of_Halodule_Uninervis_in_the_coastal_of_Lampung/links/5976cf78aca2728d02706f14/Antibacterial-activity-of-methanol-extract-from-seagrass-of-Halodule-Uninervis-in-the-coastal-of-Lampung.pdf. [Google Scholar]
- 15.Ghandourah M., Hawas U.W., Abou El-Kassem L.T., Shaher F.M. Fatty Acids and Other Chemical Compositions of Some Seagrasses Collected from the Saudi Red Sea with Potential of Antioxidant and Anticancer Agents. Thalass. Int. J. Mar. Sci. 2021;37:13–22. doi: 10.1007/s41208-020-00258-0. [DOI] [Google Scholar]
- 16.Parthasarathi P., Umamaheswari A., Banupriya R., Elumalai S. Phytochemical screening and in-vitro anticancer activity of ethyl acetate fraction of Seagrass Halodule uninervis from Mandapam Coastal Region Rameswaram Gulf of Mannar India. Int. J. Pharm. Sci. Drug Res. 2021;13:677–684. doi: 10.25004/IJPSDR.2021.130611. [DOI] [Google Scholar]
- 17.Gono C.M.P., Ahmadi P., Hertiani T., Septiana E., Putra M.Y., Chianese G. A comprehensive update on the bioactive compounds from seagrasses. Mar. Drugs. 2022;20:406. doi: 10.3390/md20070406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kim D.H., Mahomoodally M.F., Sadeer N.B., Seok P.G., Zengin G., Palaniveloo K., Khalil A.A., Rauf A., Rengasamy K.R. Nutritional and bioactive potential of seagrasses: A review. S. Afr. J. Bot. 2021;137:216–227. doi: 10.1016/j.sajb.2020.10.018. [DOI] [Google Scholar]
- 19.Guiry M., Guiry G. AlgaeBase. World-Wide Electronic Publication. National University of Ireland; Galway, Ireland: 2013. [(accessed on 30 April 2024)]. Available online: https://www.algaebase.org. [Google Scholar]
- 20.Jawad L.A. The Arabian Seas: Biodiversity, Environmental Challenges and Conservation Measures. Springer Nature; Berlin/Heidelberg, Germany: 2021. [Google Scholar]
- 21.Isaac F.M. Marine botany of the Kenya coast: 4 Angiosperms. J. East Afr. Nat. Hist. 1968;1968:29–47. [Google Scholar]
- 22.Kendrick G.A., Waycott M., Carruthers T.J., Cambridge M.L., Hovey R., Krauss S.L., Lavery P.S., Les D.H., Lowe R.J., Vidal O.M.I. The central role of dispersal in the maintenance and persistence of seagrass populations. Bioscience. 2012;62:56–65. doi: 10.1525/bio.2012.62.1.10. [DOI] [Google Scholar]
- 23.Kuo J., Den Hartog C. Seagrass taxonomy and identification key. Glob. Seagrass Res. Methods. 2001;33:31–58. doi: 10.1016/B978-044450891-1/50003-7. [DOI] [Google Scholar]
- 24.Lanyon J. Guide to the Identification of Seagrasses in the Great Barrier Reef Region. Great Barrier Reef Marine Park Authority; Townsville, Australia: 1986. [Google Scholar]
- 25.Bujang J.S., Nazri N.A., Zakaria M.H., Arshad A., Ogawa H. Morphological plasticity of Halodule species in response to different environments. Mar. Res. Indones. 2008;33:2–16. doi: 10.14203/mri.v33i1.457. [DOI] [Google Scholar]
- 26.Nagelkerken I., Van der Velde G., Gorissen M., Meijer G., Van’t Hof T., Den Hartog C. Importance of mangroves, seagrass beds and the shallow coral reef as a nursery for important coral reef fishes, using a visual census technique. Estuar. Coast. Shelf Sci. 2000;51:31–44. doi: 10.1006/ecss.2000.0617. [DOI] [Google Scholar]
- 27.Ondiviela B., Losada I.J., Lara J.L., Maza M., Galván C., Bouma T.J., van Belzen J. The role of seagrasses in coastal protection in a changing climate. Coast. Eng. 2014;87:158–168. doi: 10.1016/j.coastaleng.2013.11.005. [DOI] [Google Scholar]
- 28.Potouroglou M., Bull J.C., Krauss K.W., Kennedy H.A., Fusi M., Daffonchio D., Mangora M.M., Githaiga M.N., Diele K., Huxham M. Measuring the role of seagrasses in regulating sediment surface elevation. Sci. Rep. 2017;7:11917. doi: 10.1038/s41598-017-12354-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Short F., Carruthers T., Waycott M., Kendrick G., Fourqurean J., Callabine A., Kenworthy W., Dennison W. Halodule Uninervis. Elsevier; Amsterdam, The Netherlands: 2010. [DOI] [Google Scholar]
- 30.Mahdy A., Ghallab A., Madkour H., Osman A. Status of Seagrass community in Northern Protected Islands, Hurghada, Red Sea, Egypt. Aquat. Sci. Fish Resour. (ASFR) 2021;2:1–8. doi: 10.21608/asfr.2021.55440.1011. [DOI] [Google Scholar]
- 31.Al-Rousan S., Al-Horani F., Eid E., Khalaf M. Assessment of seagrass communities along the Jordanian coast of the Gulf of Aqaba, Red Sea. Mar. Biol. Res. 2011;7:93–99. doi: 10.1080/17451001003660319. [DOI] [Google Scholar]
- 32.Jupp B., Durako M.J., Kenworthy W., Thayer G., Schillak L. Distribution, abundance, and species composition of seagrasses at several sites in Oman. Aquat. Bot. 1996;53:199–213. doi: 10.1016/0304-3770(96)01023-6. [DOI] [Google Scholar]
- 33.Abdelbary E.M., Al Ashwal A.A. The Arabian Seas: Biodiversity, Environmental Challenges and Conservation Measures. Springer; Berlin/Heidelberg, Germany: 2021. Distribution and Abundance of Seagrasses in Qatar Marine Zone; pp. 327–362. [DOI] [Google Scholar]
- 34.Qurban M.A.B., Karuppasamy M., Krishnakumar P.K., Garcias-Bonet N., Duarte C.M. Oceanographic and Biological Aspects of the Red Sea. Springer; Berlin/Heidelberg, Germany: 2019. Seagrass distribution, composition and abundance along the Saudi Arabian coast of Red Sea; pp. 367–385. [DOI] [Google Scholar]
- 35.Bandeira S. Diversity and distribution of seagrasses around Inhaca Island, southern Mozambique. S. Afr. J. Bot. 2002;68:191–198. doi: 10.1016/S0254-6299(15)30419-1. [DOI] [Google Scholar]
- 36.Mcmillan C. Flowering under controlled conditions by Cymodocea serrulata, Halophila stipulacea, Syringodium isoetifolium, Zostera capensis and Thalassia hemprichii from Kenya. Aquat. Bot. 1980;8:323–336. doi: 10.1016/0304-3770(80)90062-5. [DOI] [Google Scholar]
- 37.Ragavan P., Jayaraj R., Muruganantham M., Jeeva C., Ubare V.V., Saxena A., Mohan P. Species composition and Distribution of Seagrasses of the Andaman and Nicobar Islands. [(accessed on 24 June 2024)];Vegetos. 2016 29:78–87. Available online: https://www.researchgate.net/profile/Sivaperuman-Chandrakasan/publication/377267675_Species_Abundance_and_Distribution_of_Coastal_and_Marine_Bird_of_India/links/659e03e12468df72d3063d30/Species-Abundance-and-Distribution-of-Coastal-and-Marine-Bird-of-India.pdf#page=83. [Google Scholar]
- 38.Daby D. Some quantitative aspects of seagrass ecology in a coastal lagoon of Mauritius. Mar. Biol. 2003;142:193–203. doi: 10.1007/s00227-002-0924-4. [DOI] [Google Scholar]
- 39.Kalugina-Gutnik A., Perestenko L., Titlyanova T. Species composition, distribution and abundance of algae and seagrasses of the Seychelles Islands. Atoll Res. Bull. 1992;369:1–67. doi: 10.5479/si.00775630.369.1. [DOI] [Google Scholar]
- 40.Adharini R.I., Yuniarga T.R., Prasetya N.L., Rachman F. Community Structure of Seagrass in Harapan Island, Seribu Islands, Indonesia. Ilmu Kelaut. Indones. J. Mar. Sci. 2022;27:20–28. doi: 10.14710/ik.ijms.27.1.20-28. [DOI] [Google Scholar]
- 41.Sidik B.J., Harah Z.M., Pauzi A.M., Madhavan S. Halodule species from Malaysia—Distribution and morphological variation. Aquat. Bot. 1999;65:33–45. doi: 10.1016/S0304-3770(99)00029-7. [DOI] [Google Scholar]
- 42.Castillejos J., Collantes C.A., Trinchera H., Morata-Fuentes J. Community Structure of Seagrasses in Cuatro Islas, Philippines. Int. Proc. Chem. Biol. Environ. Eng. 2018;103:55. doi: 10.7763/IPCBEE. [DOI] [Google Scholar]
- 43.Prathep A., Rattanachot E., Tuntiprapas P. Seasonal variations in seagrass percentage cover and biomass at Koh Tha Rai, Nakhon Si Thammarat Province, Gulf of Thailand. [(accessed on 24 June 2024)];Sonklanakarin J. Sci. Technol. 2010 32:497. Available online: https://www.thaiscience.info/journals/Article/SONG/10660119.pdf. [Google Scholar]
- 44.Nguyen H.D., Nguyen X.H., Pham H.T., Nguyen T.L. Seagrass beds along the southern coast of Vietnam and their significance for associated flora and fauna. Collect. Mar. Res. Work. 2000;10:149–190. [Google Scholar]
- 45.Huang X., Huang L., Li Y., Xu Z., Fong C., Huang D., Han Q., Huang H., Tan Y., Liu S. Main seagrass beds and threats to their habitats in the coastal sea of South China. Chin. Sci. Bull. 2006;51:136–142. doi: 10.1007/s11434-006-9136-5. [DOI] [Google Scholar]
- 46.McKenzie L.J., Yoshida R.L., Aini J.W., Andréfouet S., Colin P.L., Cullen-Unsworth L.C., Hughes A.T., Payri C.E., Rota M., Shaw C. Seagrass ecosystems of the Pacific Island Countries and Territories: A global bright spot. Mar. Pollut. Bull. 2021;167:112308. doi: 10.1016/j.marpolbul.2021.112308. [DOI] [PubMed] [Google Scholar]
- 47.Khalafallah A.A., Geneid Y.A., Shaetaey S.A., Shaaban B. Responses of the seagrass Halodule uninervis (Forssk.) Aschers. to hypersaline conditions. Egypt. J. Aquat. Res. 2013;39:167–176. doi: 10.1016/j.ejar.2013.10.003. [DOI] [Google Scholar]
- 48.Short F.T. World Atlas of Seagrasses. Univ of California Press; Berkeley, CA, USA: 2003. [Google Scholar]
- 49.Den Hartog C. The Sea-Grasses of the World. North-Holland; Amsterdam, The Netherlands: 1970. [(accessed on 30 June 2024)]. Available online: https://pdf.usaid.gov/pdf_docs/PNAAM467.pdf. [Google Scholar]
- 50.Lefcheck J.S., Hughes B.B., Johnson A.J., Pfirrmann B.W., Rasher D.B., Smyth A.R., Williams B.L., Beck M.W., Orth R.J. Are coastal habitats important nurseries? A meta-analysis. Conserv. Lett. 2019;12:e12645. doi: 10.1111/conl.12645. [DOI] [Google Scholar]
- 51.Heck Jr K., Hays G., Orth R.J. Critical evaluation of the nursery role hypothesis for seagrass meadows. Mar. Ecol. Prog. Ser. 2003;253:123–136. doi: 10.3354/meps253123. [DOI] [Google Scholar]
- 52.Singh S., Lal M.M., Southgate P.C., Wairiu M., Singh A. Trace metal content in sediment cores and seagrass biomass from a tropical southwest Pacific Island. Mar. Pollut. Bull. 2021;171:112745. doi: 10.1016/j.marpolbul.2021.112745. [DOI] [PubMed] [Google Scholar]
- 53.Fourqurean J.W., Duarte C.M., Kennedy H., Marbà N., Holmer M., Mateo M.A., Apostolaki E.T., Kendrick G.A., Krause-Jensen D., McGlathery K.J. Seagrass ecosystems as a globally significant carbon stock. Nat. Geosci. 2012;5:505–509. doi: 10.1038/ngeo1477. [DOI] [Google Scholar]
- 54.De Boer W. Seagrass–sediment interactions, positive feedbacks and critical thresholds for occurrence: A review. Hydrobiologia. 2007;591:5–24. doi: 10.1007/s10750-007-0780-9. [DOI] [Google Scholar]
- 55.Ahmed F.S., Mahmoud A.-B.S.E.-D., EL-Swaify Z.A., Salah El-Din R.A. A comparative Evaluation of Phytochemical and Antimicrobial Properties of Selected Aquatic and Terrestrial Halophyte Plants Growing in Egypt. Int. J. Theor. Appl. Res. 2023;2:169–182. doi: 10.21608/ijtar.2023.220991.1070. [DOI] [Google Scholar]
- 56.Wehbe N., Badran A., Baydoun S., Al-Sawalmih A., Maresca M., Baydoun E., Mesmar J.E. The Antioxidant Potential and Anticancer Activity of Halodule uninervis Ethanolic Extract against Triple-Negative Breast Cancer Cells. Antioxidants. 2024;13:726. doi: 10.3390/antiox13060726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ahmed F.S., El-Saied A.-B.S.E.-D., Salah El Din R.A.E.-l., El Swaify Z.A. Phytochemical screening and anticancer activities of some terrestrial and aquatic plants growing in saline habitat. Al-Azhar J. Agric. Res. 2023;48:89–106. doi: 10.21608/ajar.2024.220188.1184. [DOI] [Google Scholar]
- 58.Wan Hazma W., Muta Harah Z., Japar Sidik B., Natrah F. Macro and micro nutrients of tropical seagrasses, Halophila ovalis, H. spinulosa and Halodule uninervis in Johore, Malaysia. Iran. J. Fish. Sci. 2015;14:246–261. [Google Scholar]
- 59.Immaculate J., Lilly T., Patterson J. Macro and micro nutrients of seagrass species from Gulf of Mannar, India. MOJ Food Process Technol. 2018;6:391–398. doi: 10.15406/mojfpt.2018.06.00193. [DOI] [Google Scholar]
- 60.Pradheeba M., Dilipan E., Nobi E., Thangaradjou T., Sivakumar K. Evaluation of Seagrasses for Their Nutritional Value. 2011. [(accessed on 25 June 2024)]. Available online: https://api.semanticscholar.org/CorpusID:56039382.
- 61.Baehaki A., Supriadi A., Pratama M.C. Antioxidant Activity of Methanol Extract of Halodule uninervis Seagrass from the Coastal of Lampung, Indonesia. [(accessed on 24 June 2024)];Res. J. Pharm. Biol. Chem. Sci. 2016 7:1173–1177. Available online: http://repository.unsri.ac.id/id/eprint/105203. [Google Scholar]
- 62.Ramah S., Etwarysing L., Auckloo N., Gopeechund A., Bhagooli R., Bahorun T. Prophylactic antioxidants and phenolics of seagrass and seaweed species: A seasonal variation study in a Southern Indian Ocean Island, Mauritius. [(accessed on 30 June 2024)];Internet J. Med. Update-EJOURNAL. 2014 9:27–37. Available online: https://www.ajol.info/index.php/ijmu/article/view/101361. [Google Scholar]
- 63.Gumgumjee N.M., Bukhari D.A., Alshehri W.A., Hajar A. Antibacterial activity of Halodule uninervis leaves extracts against some bacterial pathogens strains. [(accessed on 24 June 2024)];Pharmacophore. 2018 9:52–59. Available online: https://pharmacophorejournal.com/lujsxTa. [Google Scholar]
- 64.Hamisi M.I., Mbusi L.D., Lyimo T.J. Antibacterial activity against Salmonella typhi and phytochemical screening of seven seagrass species from the coast of Tanzania. [(accessed on 24 June 2024)];West. Indian Ocean J. Mar. Sci. 2023 22:83–93. Available online: https://www.ajol.info/index.php/wiojms/article/view/241179. [Google Scholar]
- 65.Mahyoub J.A., Aziz A.T., Panneerselvam C., Murugan K., Roni M., Trivedi S., Nicoletti M., Hawas U.W., Shaher F.M., Bamakhrama M.A. Seagrasses as sources of mosquito nano-larvicides? Toxicity and uptake of Halodule uninervis-biofabricated silver nanoparticles in dengue and Zika virus vector Aedes aegypti. J. Clust. Sci. 2017;28:565–580. doi: 10.1007/s10876-016-1127-3. [DOI] [Google Scholar]
- 66.Khattab R., Gaballa A., Zakaria S., Sallam I., Ali A. Larvicidal effect of crude extracts of some marine plants (mangrove and seagrasses) on mosquitoes of Culex pipiens. Egypt. J. Aquat. Biol. Fish. 2012;16:99–105. doi: 10.21608/ejabf.2012.2128. [DOI] [Google Scholar]
- 67.Khan T., Ali M., Khan A., Nisar P., Jan S.A., Afridi S., Shinwari Z.K. Anticancer plants: A review of the active phytochemicals, applications in animal models, and regulatory aspects. Biomolecules. 2019;10:47. doi: 10.3390/biom10010047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Greenwell M., Rahman P. Medicinal plants: Their use in anticancer treatment. Int. J. Pharm. Sci. Res. 2015;6:4103. doi: 10.13040/IJPSR.0975-8232.6(10).4103-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Baehaki A., Lestari S., Hendri M., Ariska F. Antidiabetic Activity with N-Hexane, Ethyl-Acetate and Ethanol Extract of Halodule uninervis Seagrass. Pharmacogn. J. 2020;12:805–808. doi: 10.5530/pj.2020.12.115. [DOI] [Google Scholar]
- 70.Karthikeyan R., Sundarapandian M. Antidiabetic activity of methanolic extract of Halodule uninervis in streptozotocin-induced diabetic mice. [(accessed on 24 June 2024)];J. Pharm. Sci. Res. 2017 9:1864–1868. Available online: https://www.proquest.com/scholarly-journals/antidiabetic-activity-methanolic-extract-halodule/docview/1967755443/se-2?accountid=8555. [Google Scholar]
- 71.Mazari S.A., Ali E., Abro R., Khan F.S.A., Ahmed I., Ahmed M., Nizamuddin S., Siddiqui T.H., Hossain N., Mubarak N.M. Nanomaterials: Applications, waste-handling, environmental toxicities, and future challenges–A review. J. Environ. Chem. Eng. 2021;9:105028. doi: 10.1016/j.jece.2021.105028. [DOI] [Google Scholar]
- 72.Mekuye B., Abera B. Nanomaterials: An overview of synthesis, classification, characterization, and applications. Nano Sel. 2023;4:486–501. doi: 10.1002/nano.202300038. [DOI] [Google Scholar]
- 73.Bruna T., Maldonado-Bravo F., Jara P., Caro N. Silver nanoparticles and their antibacterial applications. Int. J. Mol. Sci. 2021;22:7202. doi: 10.3390/ijms22137202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Gudikandula K., Charya Maringanti S. Synthesis of silver nanoparticles by chemical and biological methods and their antimicrobial properties. J. Exp. Nanosci. 2016;11:714–721. doi: 10.1080/17458080.2016.1139196. [DOI] [Google Scholar]
- 75.Wang H., Qiao X., Chen J., Ding S. Preparation of silver nanoparticles by chemical reduction method. Colloids Surf. A Physicochem. Eng. Asp. 2005;256:111–115. doi: 10.1016/j.colsurfa.2004.12.058. [DOI] [Google Scholar]
- 76.Alhamid M.Z., Hadi B.S., Khumaeni A. Synthesis of silver nanoparticles using laser ablation method utilizing Nd: YAG laser; Proceedings of the AIP Conference Proceedings; Surakarta, Indonesia. 20 July 2019. [Google Scholar]
- 77.Mwenze N.M., Juma M., Maaza M., Birech Z., Dhlamini M. Materials Today: Proceedings. Elsevier; Amsterdam, The Netherlands: 2023. Laser liquid ablation for silver nanoparticles synthesis and conjugation with hydroxychloroquine for COVID-19 treatment. [DOI] [Google Scholar]
- 78.Ali I., Qiang T.Y., Ilahi N., Adnan M., Sajjad W. Green synthesis of silver nanoparticles by using bacterial extract and its antimicrobial activity against pathogens. Int. J. Biosci. 2018;13:1–5. doi: 10.12692/ijb/13.5.1-15. [DOI] [Google Scholar]
- 79.Feroze N., Arshad B., Younas M., Afridi M.I., Saqib S., Ayaz A. Fungal mediated synthesis of silver nanoparticles and evaluation of antibacterial activity. Microsc. Res. Tech. 2020;83:72–80. doi: 10.1002/jemt.23390. [DOI] [PubMed] [Google Scholar]
- 80.Logeswari P., Silambarasan S., Abraham J. Synthesis of silver nanoparticles using plants extract and analysis of their antimicrobial property. J. Saudi Chem. Soc. 2015;19:311–317. doi: 10.1016/j.jscs.2012.04.007. [DOI] [Google Scholar]
- 81.Saggar S., Mir P.A., Kumar N., Chawla A., Uppal J., Kaur A. Traditional and herbal medicines: Opportunities and challenges. Pharmacogn. Res. 2022;14:107–114. doi: 10.5530/pres.14.2.15. [DOI] [Google Scholar]
- 82.Hossain C.M., Gera M., Ali K.A. Current status and challenges of herbal drug development and regulatory aspect: A global perspective. Asian J. Pharm. Clin. Res. 2022;15:31–41. doi: 10.22159/ajpcr.2022.v15i12.46134. [DOI] [Google Scholar]
- 83.Ekor M. The growing use of herbal medicines: Issues relating to adverse reactions and challenges in monitoring safety. Front. Pharmacol. 2014;4:177. doi: 10.3389/fphar.2013.00177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Amutha V., Aiswarya D., Deepak P., Selvaraj R., Tamilselvan C., Perumal P., Balasubramani G. Toxicity potential evaluation of ethyl acetate extract of Cymodocea serrulata against the mosquito vectors vis-a-vis zebrafish embryos and Artemia salina cysts. S. Afr. J. Bot. 2023;152:230–239. doi: 10.1016/j.sajb.2022.12.005. [DOI] [Google Scholar]
- 85.Kavitha D., Padmini R., Alekhya V., Chandravadivelu G., Dhanaraju M.D. Comparative acute toxicity study of Syringodium isoetifolium on aquatic and rodent experimental animals. Hacet. Univ. J. Fac. Pharm. 2023;43:221–231. doi: 10.52794/hujpharm.1140865. [DOI] [Google Scholar]
- 86.Orno T.G., Rantesalu A. Invitro citotoxicity assays of seagrass (Enhalus acoroides) methanol extract from Soropia Coastal waters Southeast Sulawesi Regency. Indones. J. Med. Lab. Sci. Technol. 2020;2:27–33. doi: 10.33086/ijmlst.v2i1.1463. [DOI] [Google Scholar]
- 87.Amudha P., Vanitha V. Toxicological, biochemical and histopathological evaluation of the ethanolic extract of seagrass-Enhalus acoroides in albino wistar rats. Biocatal. Agric. Biotechnol. 2019;18:101082. doi: 10.1016/j.bcab.2019.101082. [DOI] [Google Scholar]
- 88.Unsworth R.K., McKenzie L.J., Collier C.J., Cullen-Unsworth L.C., Duarte C.M., Eklöf J.S., Jarvis J.C., Jones B.L., Nordlund L.M. Global challenges for seagrass conservation. Ambio. 2019;48:801–815. doi: 10.1007/s13280-018-1115-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Duarte C.M. The future of seagrass meadows. Environ. Conserv. 2002;29:192–206. doi: 10.1017/S0376892902000127. [DOI] [Google Scholar]