Abstract
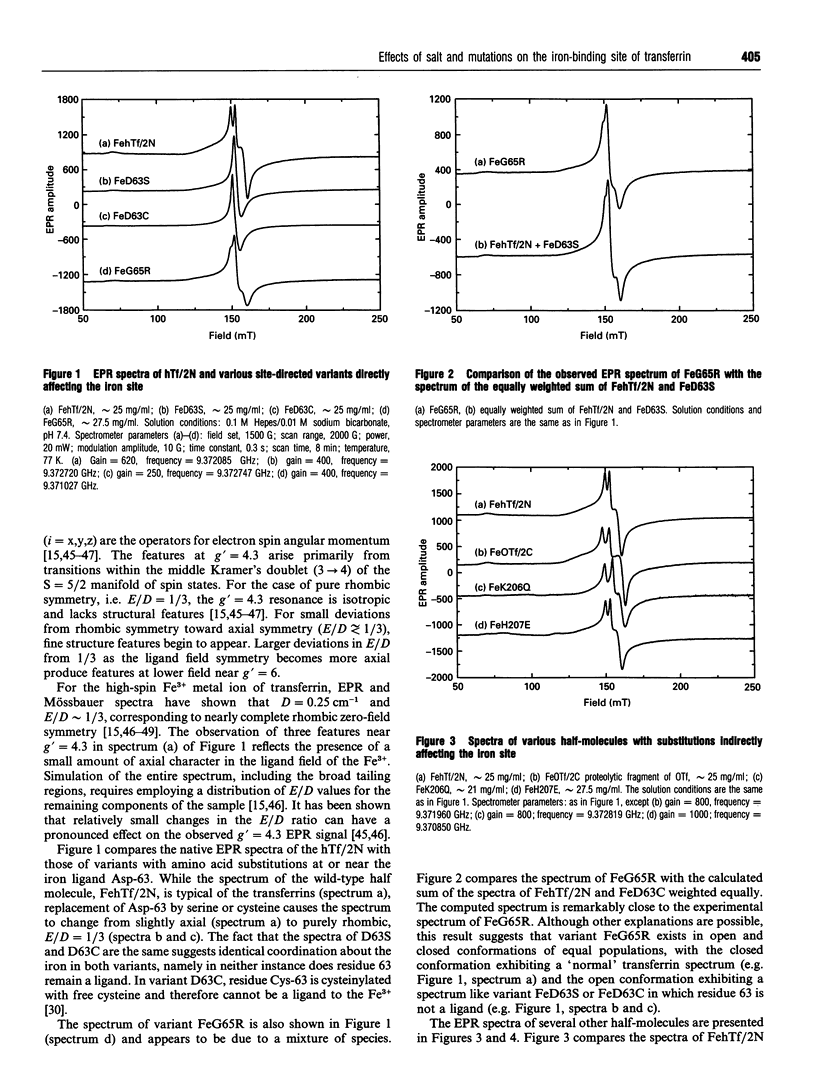
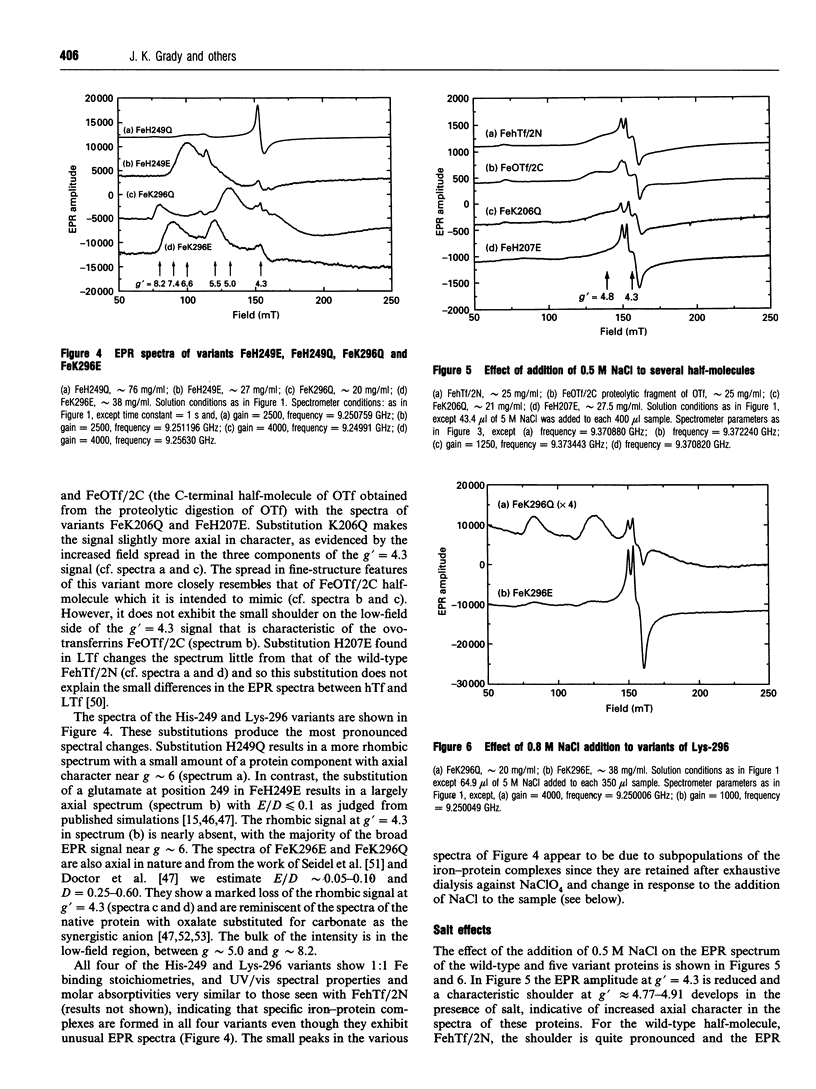
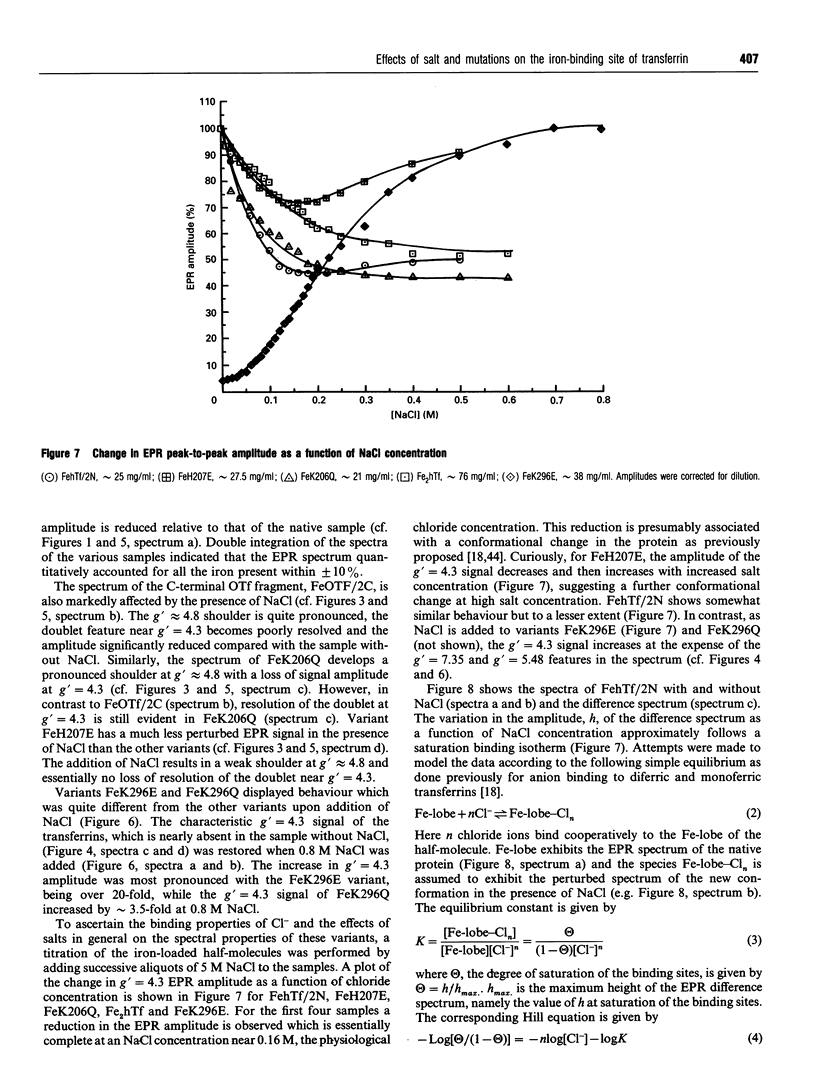
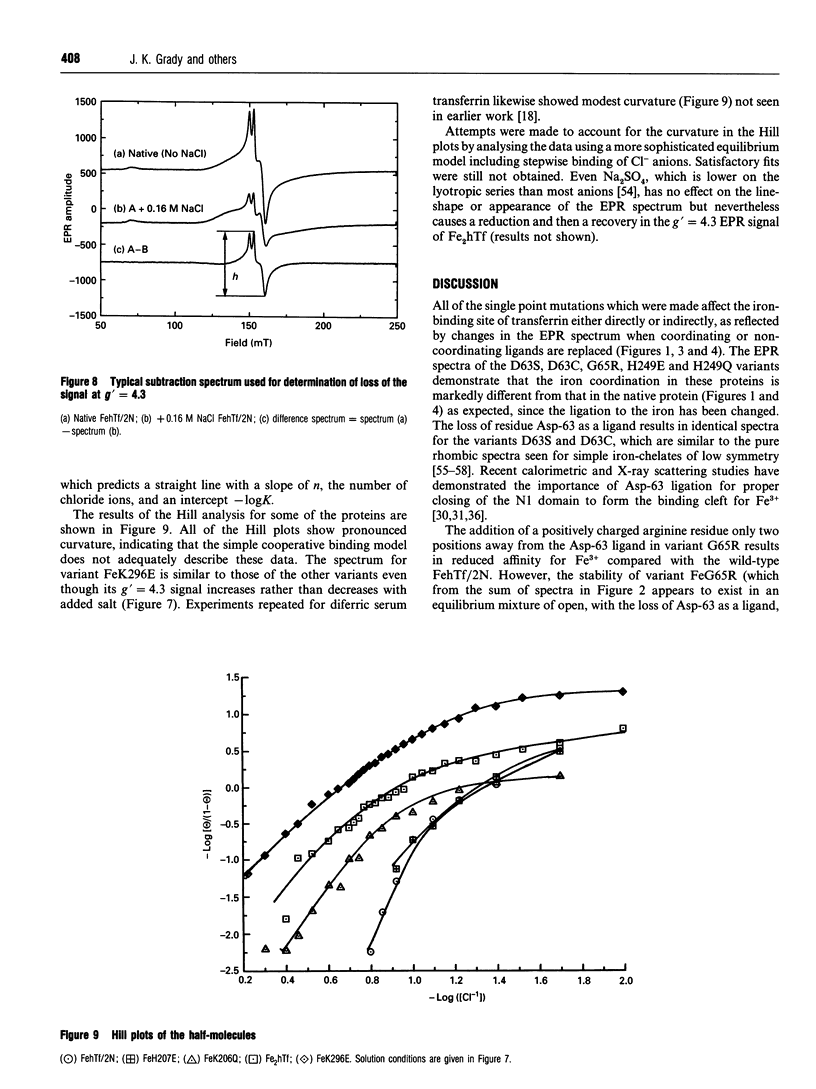
The effects of site-directed mutation and salt on the iron(III)-binding site of the recombinant half-molecule of the N-terminal lobe (hTf/2N) of human transferrin was studied by EPR spectroscopy. Changes were observed in the EPR spectra of all variants investigated (D63S, D63C, G65R, K206Q, H207E, H249E, H249Q, K296E and K296Q) compared with that of the wild-type protein. The most pronounced changes in the metal site were caused by replacement of the coordinating residues, Asp-63 and His-249, and the non-coordinating residue Lys-296, which is located in the hinge region of the iron-binding cleft. The EPR spectral changes from replacement of other non-coordinating residues were more subtle, indicating small changes in Fe3+ coordination to the protein. The EPR spectrum of variant G65R suggests that it adopts two distinct conformations in solution, one in which the two domains forming the iron-binding cleft are closed and one in which they are open; in the latter instance Asp-63 is no longer coordinated to the Fe3+. Chloride-binding studies on hTf/2N, K206Q, H207E, K296Q and K296E showed similar binding isotherms, indicating that none of the hinge region residues replaced, i.e. Lys-206, His-207 or Lys-296, are the sites of chloride binding. The results show that the coordination environment of the Fe3+ is sensitive to structural changes from site-directed mutation of both remote and coordinated residues and also to chloride-binding and ionic strength effects.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson B. F., Baker H. M., Dodson E. J., Norris G. E., Rumball S. V., Waters J. M., Baker E. N. Structure of human lactoferrin at 3.2-A resolution. Proc Natl Acad Sci U S A. 1987 Apr;84(7):1769–1773. doi: 10.1073/pnas.84.7.1769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson B. F., Baker H. M., Norris G. E., Rice D. W., Baker E. N. Structure of human lactoferrin: crystallographic structure analysis and refinement at 2.8 A resolution. J Mol Biol. 1989 Oct 20;209(4):711–734. doi: 10.1016/0022-2836(89)90602-5. [DOI] [PubMed] [Google Scholar]
- Anderson B. F., Baker H. M., Norris G. E., Rumball S. V., Baker E. N. Apolactoferrin structure demonstrates ligand-induced conformational change in transferrins. Nature. 1990 Apr 19;344(6268):784–787. doi: 10.1038/344784a0. [DOI] [PubMed] [Google Scholar]
- Antonini A., Leenders K. L., Meier D., Oertel W. H., Boesiger P., Anliker M. T2 relaxation time in patients with Parkinson's disease. Neurology. 1993 Apr;43(4):697–700. doi: 10.1212/wnl.43.4.697. [DOI] [PubMed] [Google Scholar]
- Bailey S., Evans R. W., Garratt R. C., Gorinsky B., Hasnain S., Horsburgh C., Jhoti H., Lindley P. F., Mydin A., Sarra R. Molecular structure of serum transferrin at 3.3-A resolution. Biochemistry. 1988 Jul 26;27(15):5804–5812. doi: 10.1021/bi00415a061. [DOI] [PubMed] [Google Scholar]
- Baker E. N., Baker H. M., Smith C. A., Stebbins M. R., Kahn M., Hellström K. E., Hellström I. Human melanotransferrin (p97) has only one functional iron-binding site. FEBS Lett. 1992 Feb 24;298(2-3):215–218. doi: 10.1016/0014-5793(92)80060-t. [DOI] [PubMed] [Google Scholar]
- Baldwin D. A., Egan T. J., Marques H. M. The effects of anions on the kinetics of reductive elimination of iron from monoferrictransferrins by thiols. Biochim Biophys Acta. 1990 Mar 29;1038(1):1–9. doi: 10.1016/0167-4838(90)90002-w. [DOI] [PubMed] [Google Scholar]
- Baldwin D. A. The kinetics of iron release from human transferrin by EDTA. Effect of salts and detergents. Biochim Biophys Acta. 1980 May 29;623(1):183–198. doi: 10.1016/0005-2795(80)90020-3. [DOI] [PubMed] [Google Scholar]
- Baldwin D. A., de Sousa D. M. The effect of salts on the kinetics of iron release from N-terminal and C terminal monoferrictransferrins. Biochem Biophys Res Commun. 1981 Apr 30;99(4):1101–1107. doi: 10.1016/0006-291x(81)90732-4. [DOI] [PubMed] [Google Scholar]
- Brown-Mason A., Brown S. A., Butcher N. D., Woodworth R. C. Reversible association of half-molecules of ovotransferrin in solution. Basis of co-operative binding to reticulocytes. Biochem J. 1987 Jul 1;245(1):103–109. doi: 10.1042/bj2450103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown J. P., Woodbury R. G., Hart C. E., Hellström I., Hellström K. E. Quantitative analysis of melanoma-associated antigen p97 in normal and neoplastic tissues. Proc Natl Acad Sci U S A. 1981 Jan;78(1):539–543. doi: 10.1073/pnas.78.1.539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day C. L., Anderson B. F., Tweedie J. W., Baker E. N. Structure of the recombinant N-terminal lobe of human lactoferrin at 2.0 A resolution. J Mol Biol. 1993 Aug 20;232(4):1084–1100. doi: 10.1006/jmbi.1993.1462. [DOI] [PubMed] [Google Scholar]
- Dewan J. C., Mikami B., Hirose M., Sacchettini J. C. Structural evidence for a pH-sensitive dilysine trigger in the hen ovotransferrin N-lobe: implications for transferrin iron release. Biochemistry. 1993 Nov 16;32(45):11963–11968. doi: 10.1021/bi00096a004. [DOI] [PubMed] [Google Scholar]
- Dubach J., Gaffney B. J., More K., Eaton G. R., Eaton S. S. Effect of the synergistic anion on electron paramagnetic resonance spectra of iron-transferrin anion complexes is consistent with bidentate binding of the anion. Biophys J. 1991 May;59(5):1091–1100. doi: 10.1016/S0006-3495(91)82324-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ecker D. J., Lancaster J. R., Jr, Emery T. Siderophore iron transport followed by electron paramagnetic resonance spectroscopy. J Biol Chem. 1982 Aug 10;257(15):8623–8626. [PubMed] [Google Scholar]
- Egan T. J., Zak O., Aisen P. The anion requirement for iron release from transferrin is preserved in the receptor-transferrin complex. Biochemistry. 1993 Aug 17;32(32):8162–8167. doi: 10.1021/bi00083a016. [DOI] [PubMed] [Google Scholar]
- Evans R. W., Williams J., Moreton K. A variant of human transferrin with abnormal properties. Biochem J. 1982 Jan 1;201(1):19–26. doi: 10.1042/bj2010019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funk W. D., MacGillivray R. T., Mason A. B., Brown S. A., Woodworth R. C. Expression of the amino-terminal half-molecule of human serum transferrin in cultured cells and characterization of the recombinant protein. Biochemistry. 1990 Feb 13;29(6):1654–1660. doi: 10.1021/bi00458a043. [DOI] [PubMed] [Google Scholar]
- Grossmann J. G., Mason A. B., Woodworth R. C., Neu M., Lindley P. F., Hasnain S. S. Asp ligand provides the trigger for closure of transferrin molecules. Direct evidence from X-ray scattering studies of site-specific mutants of the N-terminal half-molecule of human transferrin. J Mol Biol. 1993 Jun 5;231(3):554–558. doi: 10.1006/jmbi.1993.1308. [DOI] [PubMed] [Google Scholar]
- Harris W. R. Equilibrium constants for the complexation of metal ions by serum transferrin. Adv Exp Med Biol. 1989;249:67–93. doi: 10.1007/978-1-4684-9111-1_6. [DOI] [PubMed] [Google Scholar]
- Kretchmar S. A., Teixeira M., Huynh B. H., Raymond K. N. Mössbauer studies of electrophoretically purified monoferric and diferric human transferrin. Biol Met. 1988;1(1):26–32. doi: 10.1007/BF01128014. [DOI] [PubMed] [Google Scholar]
- Lin L. N., Mason A. B., Woodworth R. C., Brandts J. F. Calorimetric studies of the N-terminal half-molecule of transferrin and mutant forms modified near the Fe(3+)-binding site. Biochem J. 1993 Jul 15;293(Pt 2):517–522. doi: 10.1042/bj2930517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindley P. F., Bajaj M., Evans R. W., Garratt R. C., Hasnain S. S., Jhoti H., Kuser P., Neu M., Patel K., Sarra R. The mechanism of iron uptake by transferrins: the structure of an 18 kDa NII-domain fragment from duck ovotransferrin at 2.3 A resolution. Acta Crystallogr D Biol Crystallogr. 1993 Mar 1;49(Pt 2):292–304. doi: 10.1107/S0907444992012101. [DOI] [PubMed] [Google Scholar]
- Mansour A. N., Thompson C., Theil E. C., Chasteen N. D., Sayers D. E. Fe(III).ATP complexes. Models for ferritin and other polynuclear iron complexes with phosphate. J Biol Chem. 1985 Jul 5;260(13):7975–7979. [PubMed] [Google Scholar]
- Mason A. B., Funk W. D., MacGillivray R. T., Woodworth R. C. Efficient production and isolation of recombinant amino-terminal half-molecule of human serum transferrin from baby hamster kidney cells. Protein Expr Purif. 1991 Apr-Jun;2(2-3):214–220. doi: 10.1016/1046-5928(91)90074-s. [DOI] [PubMed] [Google Scholar]
- Najarian R. C., Harris D. C. Oxalate and spin-labeled oxalate as probes of the anion binding site of human transferrin. Metal to anion distance. J Biol Chem. 1978 Jan 10;253(1):38–42. [PubMed] [Google Scholar]
- Oe H., Doi E., Hirose M. Amino-terminal and carboxyl-terminal half-molecules of ovotransferrin: preparation by a novel procedure and their interactions. J Biochem. 1988 Jun;103(6):1066–1072. doi: 10.1093/oxfordjournals.jbchem.a122381. [DOI] [PubMed] [Google Scholar]
- Price E. M., Gibson J. F. Electron paramagnetic resonance evidence for a distinction between the two iron-binding sites in transferrin and in conalbumin. J Biol Chem. 1972 Dec 25;247(24):8031–8035. [PubMed] [Google Scholar]
- Record M. T., Jr, Anderson C. F., Lohman T. M. Thermodynamic analysis of ion effects on the binding and conformational equilibria of proteins and nucleic acids: the roles of ion association or release, screening, and ion effects on water activity. Q Rev Biophys. 1978 May;11(2):103–178. doi: 10.1017/s003358350000202x. [DOI] [PubMed] [Google Scholar]
- Richardson D. R., Baker E. The uptake of iron and transferrin by the human malignant melanoma cell. Biochim Biophys Acta. 1990 Jun 12;1053(1):1–12. doi: 10.1016/0167-4889(90)90018-9. [DOI] [PubMed] [Google Scholar]
- Rose T. M., Plowman G. D., Teplow D. B., Dreyer W. J., Hellström K. E., Brown J. P. Primary structure of the human melanoma-associated antigen p97 (melanotransferrin) deduced from the mRNA sequence. Proc Natl Acad Sci U S A. 1986 Mar;83(5):1261–1265. doi: 10.1073/pnas.83.5.1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlabach M. R., Bates G. W. The synergistic binding of anions and Fe3+ by transferrin. Implications for the interlocking sites hypothesis. J Biol Chem. 1975 Mar 25;250(6):2182–2188. [PubMed] [Google Scholar]
- Seidel A., Bill E., Häggström L., Nordblad P., Kilár F. Complementary Mössbauer and EPR studies of iron(III) in diferric human serum transferrin with oxalate or bicarbonate as synergistic anions. Arch Biochem Biophys. 1994 Jan;308(1):52–63. doi: 10.1006/abbi.1994.1008. [DOI] [PubMed] [Google Scholar]
- Shongwe M. S., Smith C. A., Ainscough E. W., Baker H. M., Brodie A. M., Baker E. N. Anion binding by human lactoferrin: results from crystallographic and physicochemical studies. Biochemistry. 1992 May 12;31(18):4451–4458. doi: 10.1021/bi00133a010. [DOI] [PubMed] [Google Scholar]
- Smith C. A., Anderson B. F., Baker H. M., Baker E. N. Metal substitution in transferrins: the crystal structure of human copper-lactoferrin at 2.1-A resolution. Biochemistry. 1992 May 12;31(18):4527–4533. doi: 10.1021/bi00133a020. [DOI] [PubMed] [Google Scholar]
- Spartalian K., Lang G., Woodworth R. C. Mössbauer spectroscopy of iron-ovotransferrin: a crystal field interpretation. Biochemistry. 1991 Jan 29;30(4):1004–1009. doi: 10.1021/bi00218a017. [DOI] [PubMed] [Google Scholar]
- Thompson C. P., McCarty B. M., Chasteen N. D. The effects of salts and amino group modification on the iron binding domains of transferrin. Biochim Biophys Acta. 1986 Apr 22;870(3):530–537. doi: 10.1016/0167-4838(86)90262-1. [DOI] [PubMed] [Google Scholar]
- Williams J., Chasteen N. D., Moreton K. The effect of salt concentration on the iron-binding properties of human transferrin. Biochem J. 1982 Mar 1;201(3):527–532. doi: 10.1042/bj2010527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodworth R. C., Mason A. B., Funk W. D., MacGillivray R. T. Expression and initial characterization of five site-directed mutants of the N-terminal half-molecule of human transferrin. Biochemistry. 1991 Nov 12;30(45):10824–10829. doi: 10.1021/bi00109a002. [DOI] [PubMed] [Google Scholar]
- Yang A. S., Gaffney B. J. Determination of relative spin concentration in some high-spin ferric proteins using E/D-distribution in electron paramagnetic resonance simulations. Biophys J. 1987 Jan;51(1):55–67. doi: 10.1016/S0006-3495(87)83311-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zak O., Aisen P. Evidence for functional differences between the two sites of rabbit transferrin: effects of serum and carbon dioxide. Biochim Biophys Acta. 1990 Apr 9;1052(1):24–28. doi: 10.1016/0167-4889(90)90052-f. [DOI] [PubMed] [Google Scholar]
- Zak O., Aisen P. Spectroscopic and thermodynamic studies on the binding of gadolinium(III) to human serum transferrin. Biochemistry. 1988 Feb 9;27(3):1075–1080. doi: 10.1021/bi00403a033. [DOI] [PubMed] [Google Scholar]


