Abstract
Aging-related disorders pose significant challenges due to their complex interplay of physiological and metabolic factors, including inflammation, oxidative stress, and mitochondrial dysfunction. Curcumin, a natural compound with potent antioxidant and anti-inflammatory properties, has emerged as a promising candidate for mitigating these age-related processes. However, gaps in understanding the precise mechanisms of curcumin’s effects and the optimal dosages for different conditions necessitate further investigation. This systematic review synthesizes current evidence on curcumin’s potential in addressing age-related disorders, emphasizing its impact on cognitive function, neurodegeneration, and muscle health in older adults. By evaluating the safety, efficacy, and mechanisms of action of curcumin supplementation, this review aims to provide insights into its therapeutic potential for promoting healthy aging. A systematic search across three databases using specific keywords yielded 2256 documents, leading to the selection of 15 clinical trials for synthesis. Here, we highlight the promising potential of curcumin as a multifaceted therapeutic agent in combating age-related disorders. The findings of this review suggest that curcumin could offer a natural and effective approach to enhancing the quality of life of aging individuals. Further research and well-designed clinical trials are essential to validate these findings and optimize the use of curcumin in personalized medicine approaches for age-related conditions.
Keywords: Curcuma longa, curcumin, aging, inflammation, oxidative stress, frailty, neurodegeneration, sarcopenia, cardiovascular diseases, clinical trials
1. Introduction
Population aging is a global trend that brings medical, social, and economic challenges [1,2,3,4]. Aging is the result of a diversity of molecular and cellular damages accumulated in the body over the years. It is a natural, gradual, complex, and irreversible process, accompanied by systemic changes that cause a reduction in functionality and increase the risk of age-associated diseases that eventually result in death. Some characteristics of aging have been associated with loss of proteostasis, dysregulated nutrient sensing, increase in the oxidative processes, inflammation, telomere attrition, genomic instability, stem cell exhaustion, cellular senescence, impaired intercellular communication, and mitochondrial dysfunction [5,6,7,8,9].
As life expectancy increases, an increase in the incidence of chronic diseases is observed, including neurodegenerative diseases (NDs) such as Alzheimer’s disease (AD) and Parkinson’s disease (PD) [10], memory impairment [11], cognitive dysfunction [12,13], type 2 diabetes (DM2) [14], cardiovascular disease [15], cancer [16], and musculoskeletal disorders such as sarcopenia [4,17]. These conditions pose a substantial challenge to healthcare systems around the world due to their significant impact on the quality of life of the elderly, healthcare costs, and the global burden of disease [18,19].
Natural bioactive compounds have been used for thousands of years as adjuvants in therapeutic practice due to their efficiency, low cost, few side effects, and easy use [20,21,22]. Curcuma longa, known as turmeric or saffron, has received prominence among countless plants. It is an herbaceous and rhizomatous plant (Figure 1) from the ginger family, Zingiberaceae, and is native to Southeast Asia and India. For centuries, it has been used in cooking and as medicine [23,24,25,26,27].
Figure 1.
The main parts of the Curcuma longa plant. (A): leaves; (B): flower; (C): rhizomes; (D): sliced rhizomes; (E): rhizome powder; (F): pharmaceutical formulation, and (G): curcumin molecular structure.
Its main phenolic bioactive compounds are curcumin (~77%), demethoxycurcumin (~17–19%), and bisdemethoxycurcumin (~4%), which are extracted from the rhizomes [20,28,29], and can promote countless therapeutic actions by virtue of their anti-inflammatory and antioxidant potential, as shown in numerous clinical and preclinical studies [30,31,32,33]. It is a potential anticancer agent with the ability to modulate the main pathways involved in inflammation processes, oxidative stress (OS), carcinogenesis, autophagy, apoptosis, and cardiovascular and neurodegenerative conditions [23,34,35,36]. Furthermore, it has antidiabetic and antiobesogenic effects, provides cardiovascular protection, and can also reduce sarcopenic processes. Studies in hypertensive rats also show that it can prevent hypertension development by improving vascular remodeling and endothelial dysfunction [37,38,39,40].
Curcumin has shown positive effects in delaying the aging process and delaying age-related changes. Its potential anti-aging properties are due to its power to alter the levels of proteins associated with senescence, such as adenosine 5′-monophosphate-activated protein kinase (AMPK) and sirtuins, and preventing pro-aging proteins, such as nuclear factor-kappa-B (NF-κB) and mammalian target of rapamycin (mTOR) [41]. Numerous studies have demonstrated mechanisms by which curcumin can act on the aging process; one of them investigated the molecular mechanism of curcumin in extending the lifespan of C. elegans, and the results of the study indicated that this action potentially occurred through increased resistance to OS and negative regulation of the AMPK signaling pathway [42].
Another study demonstrated how long-time curcumin therapy can progressively reverse cognitive dysfunction in D-gal-induced senescent mice, delaying the aging process, improving locomotor activity and cognitive functions, and restoring mitochondrial enzyme complex function [43]. It was further reported that curcumin supplementation rejuvenates senescence-related changes in the thymus among senescent mice caused by D-gal through promoting proliferating cells, protecting cells from apoptosis, and elevating transcription of the autoimmune regulator [44,45]
Despite the growing body of research indicating the potential benefits of curcumin in addressing age-related disorders, there remains a gap in the understanding of its precise mechanisms of action and optimal dosages for different age-related conditions. While some studies have shown promising results in terms of curcumin’s antioxidant, anti-inflammatory, and neuroprotective properties, there is still a need for more robust clinical trials and mechanistic studies to elucidate the specific pathways through which curcumin exerts its effects on aging-related processes. Additionally, variations in bioavailability and metabolism of curcumin among individuals pose a challenge in determining the most effective dosage and formulation for therapeutic purposes. Furthermore, the lack of standardized protocols and inconsistent reporting of outcomes in existing studies hinder the ability to draw definitive conclusions regarding the efficacy of curcumin in preventing or treating age-related disorders. Addressing these gaps through well-designed clinical trials, mechanistic investigations, and standardized protocols could provide valuable insights into the full potential of curcumin as a therapeutic agent for promoting healthy aging and combating age-related conditions.
The objectives of this systematic review are to evaluate the current scientific evidence on the effects of curcumin in preventing and managing age-related disorders, to assess the safety and tolerability of curcumin supplementation in older adults, to explore the mechanisms through which curcumin may exert its beneficial effects on aging-related processes, and to identify potential gaps in the literature that warrant further research. By synthesizing data from clinical trials and studies focusing on OS, inflammation, cognition, NDs, sarcopenia, and other age-related conditions, this review aims to provide a comprehensive overview of the therapeutic potential of Curcuma longa and curcumin in promoting healthy aging and improving quality of life in the elderly population.
2. Materials and Methods
2.1. Focal Question
This systematic review was carried out with the aim of answering the following question: “Can curcumin produce beneficial effects on conditions related to the aging process?”.
2.2. Language
Only clinical studies published in English were used.
2.3. Literature Search
This systematic review included clinical trials published in MEDLINE–PubMed, COCHRANE and EMBASE. The keywords used were Curcuma longa or saffron or turmeric or curcumin or curcuminoids and OS or inflammation or mitochondrial dysfunction or sarcopenia or cognition or memory or dementia or PD or AD or aging or NDs. These descriptors guided the identification of studies related to Curcuma longa and its effects on OS and inflammatory processes related to aging. S.M.B. and Y.C.N. carried out the identification and inclusion of studies. In the case of conflicting research results, a third judge ruled (M.J.S.M.).
2.4. Inclusion and Exclusion Criteria
The inclusion criteria for the studies were intervention studies in humans. Exclusion criteria were studies not published in English, editorials, conferences, letters to editors, reviews, poster presentations, and case reports.
2.5. Data Extraction
There was no time limit for the search for clinical trials in this review. For data extraction, we used the PICO format (Population, Intervention, Comparison, and Outcomes).
2.6. Study Selection
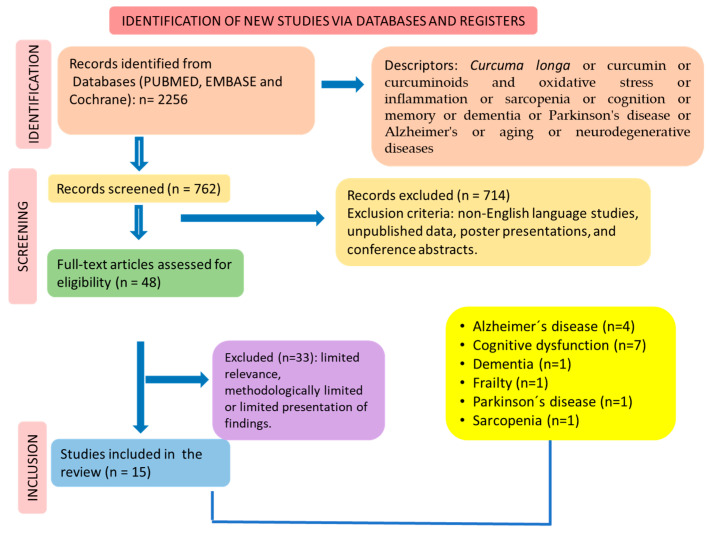
The search/selection of studies was performed according to the guidelines of PRISMA (The Preferred Reporting Items for a Systematic Review and Meta-Analysis) [46,47] (Figure 2).
Figure 2.
Flow diagram showing the study selection (according to PRISMA guidelines) [47].
2.7. Quality Assessment
The Cochrane Handbook was used to assess the risk of bias related to the studies selected for systematic reviews of interventions [48].
2.8. Registration
This study was registered by PROSPERO under the ID CR42024559316.
3. Results
Initially, 2256 documents were identified according to the search terms. After applying the inclusion/exclusion criteria, we identified 15 clinical trials that met these criteria for synthesis. Figure 2 shows the selection of the studies in accordance with PRISMA guidelines.
4. Discussion
This review explores the beneficial effects of curcumin in addressing aging-related disorders, highlighting the intricate interplay between physiological and metabolic cascades that contribute to age-related impairments. Highlighting the critical roles that inflammation, OS, and mitochondrial dysfunction play in the pathophysiology of ailments like memory loss and cognitive impairment, NDs, frailty, and sarcopenia, the review highlights curcumin’s potential as a versatile therapeutic agent. By elucidating how curcumin’s antioxidant and anti-inflammatory properties may counteract these detrimental processes, the discussion sheds light on the mechanisms through which curcumin could mitigate age-related conditions. Furthermore, the review explores the impact of curcumin on neuronal growth factors, neuroplasticity, muscle protein synthesis, and degradation, offering insights into its potential to enhance brain functions and muscle health in the aging population.
4.1. Beneficial Effects of Curcumin and Aging-Related Disorders
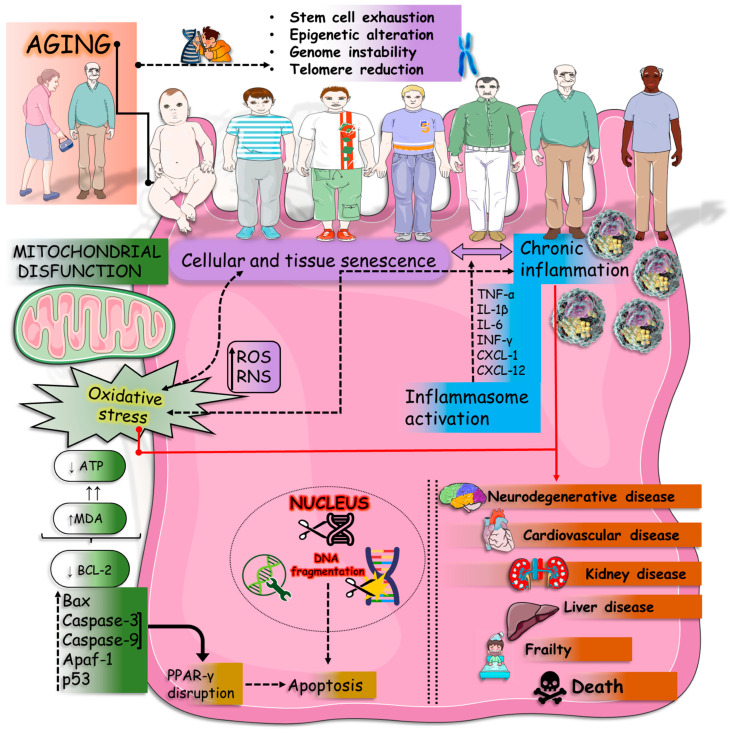
Aging-related disorders are related to impairment in several physiological and metabolic cascades (Figure 3). These processes are profoundly linked to inflammation, OS and mitochondrial dysfunction, leading to several disorders such as impaired memory and cognition, NDs (such as AD and PD), frailty, and sarcopenia.
Figure 3.
Overview of the aging process. Cellular senescence triggers inflammatory processes and the release of pro-inflammatory biomarkers such as IL-1β, IL-6, TNF-α, IFn-γ, CXCL-1, and CXCL-12. Free radicals and reactive oxygen species induce the installation of oxidative stress (OS) that aggravates inflammation. On the other hand, inflammation aggravates OS, leading to a vicious cycle. Apaf-1: apoptotic protease-activating factor-1; Bax: Bcl-2-like protein X; BCL-2: B-cell lymphoma 2; CXCL: chemokine (C-X-C motif) ligand; IL: interleukin; INF-γ: interferon-γ; MDA: malonaldehyde; TNF-α: tumor necrosis factor-α. ↓: decrease; ↑: increase.
4.2. Inflammation
Inflammatory processes are crucial in the development of disease conditions related to the aging process. Curcumin’s anti-inflammatory activity involves regulating inflammatory signaling pathways and inhibiting the production of inflammatory mediators. Curcumin can bind to Toll-like receptors (TLRs) and downregulate mitogen-activated protein kinases (MAPKs), activator protein 1 (AP-1), and NF-κB signaling pathways that play an important role in inflammatory mediator generation. Moreover, curcumin, by inhibiting the NF-κB pathway, can directly restrain the assembly or even inhibit the activation of the NOD-like receptor pyrin domain-containing 3 (NLRP3) inflammasome, a cytosolic multiprotein complex involved in the development of several inflammatory diseases [36,49,50,51,52,53,54,55]. In addition, curcumin reduces inflammation through its antioxidant properties by inhibiting nicotinamide adenine dinucleotide phosphate (NADPH) oxidase and elevating the activity of antioxidant enzymes and consequently lowering reactive oxygen species (ROS) [49,56].
Another anti-inflammatory mechanism of curcumin is the nuclear factor erythroid 2–related factor 2 (Nrf2)-Kelch-like ECH-associated protein 1 (Keap1) pathway, which is strongly related to OS and inflammation by its association with NF-κB, MAPK, phosphoinositide 3-kinase (PI3K) and protein kinase C (PKC) pathways and by its role in regulating gene expression of antioxidant and detoxifying enzymes [57,58,59,60]. Furthermore, curcumin can block both the production of tumor necrosis factor (TNF)-alpha (TNF-α) and the cell signaling pathways mediated by TNF in various types of cells, decrease the release of several ILs through the downstream regulation of NF-κB by acting on peroxisome proliferator-activated receptor gamma (PPAR-γ), act as a natural free radical scavenger due to its chemical structure, and suppress pro-inflammatory pathways related to most chronic diseases [49,58].
Curcumin can also regulate immune cells such as dendritic cells, T regulatory cells, and T helper 17 (Th17) cells, which produce interleukin (IL)-17, IL-22, and IL-23, inducing an inflammatory response. Curcumin mainly inhibits Th17 differentiation and regulates Treg, which are anti-inflammatory cells, and induces Th17 balance by inhibiting the IL-23/Th17 pathway, maintaining immune homeostasis [35,36,49,61,62]. Also, it can induce the polarization of macrophages into an anti-inflammatory M2 phenotype. Furthermore, levels of pro-inflammatory mediators such as IL-1, IL-1β, IL-6, IL-8, IL-17, IL-27, nitric oxide (NO), inducible nitric oxide synthase (iNOS), C-X-C motif chemokine ligand 8 (CXCL8), C-C motif chemokine ligand 2 (CCL2), cyclooxygenase 2 (COX-2), granulocyte colony-stimulating factor (G-CSF), and monocyte chemotactic protein-1 (MCP-1) can be decreased by curcumin [20,32,49,63].
Curcumin can also work as an anti-inflammatory mediator by modulating the Janus kinase/signal transducer and activator of transcription (JAK/STAT) signaling pathways [64,65] and reducing macrophage infiltration and mRNA levels of macrophage M1 [66]. Moreover, the protective role of curcumin on gastric mucosal inflammation in mice induced by cisplatin occurred by decreasing IL-1β, IL-17, IL-23, TNF-α, and myeloperoxidase, enhancing levels of IL-10, and downregulating activation of NF-κB [67].
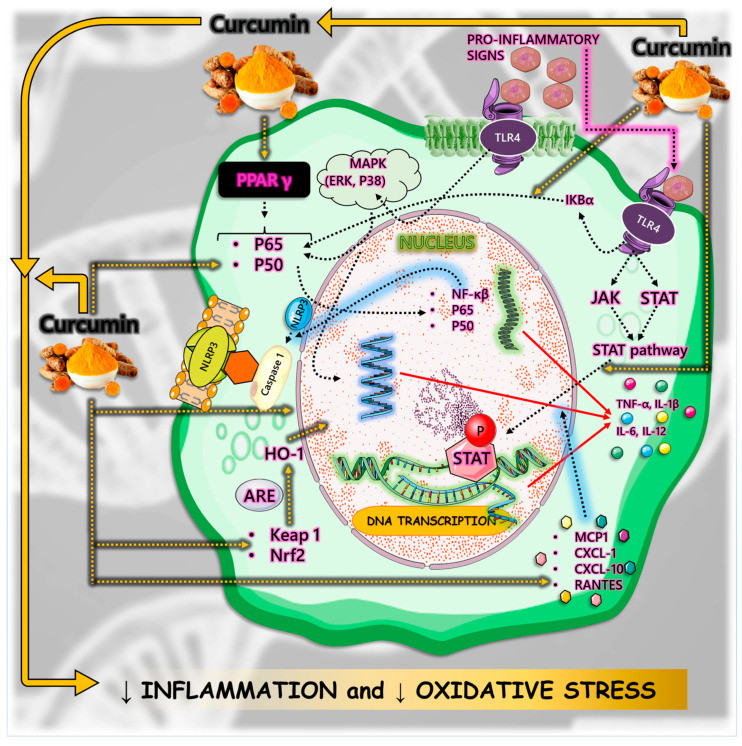
Another point to bring to light is that inflammation is closely related to OS. The significant accumulation of free radicals such as ROS or reactive nitrogen species (RNS) leads to OS, which aggravates inflammation by stimulating the transcription of factors related to inflammation. Curcumin can decrease ROS production through NADPH oxidase and augment antioxidant molecules (such as catalase, superoxide dismutase, and glutathione peroxidase enzymes) activities, and is associated with Nrf2-Keap1 pathways [68,69]. Figure 4 summarizes the anti-inflammatory pathways modulated by curcumin.
Figure 4.
The effects of curcumin against inflammatory pathways. Curcumin inhibits the MAPK, ERK, p38, p65, p50, and NFkB pathways and the consequent release of pro-inflammatory cytokines such as interleukin (IL)-1; IL-12, and tumor necrosis factor-α (TNF-α). Besides that, curcumin also inhibits the Janus kinase/signal transducer and active factor of transcription (JAK-STAT) cascade. There is stimulation of Kelch-like ECH-associated protein 1 (Keap1) and nuclear factor erythroid-2 related factor 2 (Nrf2) that also interfere with pro-inflammatory pathways. ARE: antioxidant responsive elements;CXCL: chemokine (C-X-C motif) ligand; ERK: protein kinase RNA-like endoplasmic reticulum kinase; HO-1: Heme-oxygenase-1; IKB: IkappaB kinase; MAPK: mitogen-activated protein kinase; MCP1: monocyte chemoattractant protein-1; NF-κβ: nuclear factor-kappa beta,; NRLP34: NLR family pyrin domain containing; PPAR: peroxisome proliferator-activated receptor; RANTES: IL-8 superfamily cytokines; TLR4: Toll-like receptor. ↓: decrease.
Among the “pillars” of aging, inflammaging should be mentioned. This term is applied to define the close relationship observed between low-grade chronic inflammation and the aging process with no infectious conditions. This scenario is related to several impairments, including brain conditions. As already pointed out, the immune system has a critical role in the aging process or the “biological age”, which can be evaluated by metabolomics and genomics. Biological age is different from chronological age. Biological age may be influenced by genetics, lifestyle, and the environment. However, the immune system can be impaired substantially in the normal aging process, leading to great consequences for the body [70]. Curcumin can inhibit the formation of free radicals and other pro-inflammatory biomarkers related to age-related diseases [71,72].
4.3. Oxidative Stress
Aging is a gradual combination of important tissue and cellular changes, integrating structural, functional, and physiological changes, leading to functional disorders and increasing susceptibility to death. Named “hallmarks of aging”, this process is linked to molecular events such as dysregulated nutrient sensing, telomere wear, genomic instability, epigenetic changes, cellular senescence, loss of proteostasis, altered intercellular communication, and stem cell exhaustion [73,74,75,76,77,78,79,80]. OS and inflammatory processes are associated with the aging process. Various circumstances, such as stress, infections, exposure to inflammation, smoke, and radiation, produce ROS due to metabolism [81]. These molecules can lead to irreversible cellular damage when the endogenous antioxidant system or the intake of exogenous antioxidants is insufficient [82,83,84]. OS is linked to the genesis of numerous health conditions, both in the aging phase and even in the younger stages of life. These include obesity, hypertension, diabetes, cardiovascular diseases (CVDs), NDs, cataracts, and cancer [85,86,87,88].
Numerous antioxidants can contribute to preventing the effects of the aging process. Produced by exogenous and endogenous pathways, ROS can be attenuated by enzymatic and non-enzymatic antioxidants. There are several defense systems, including peroxidase, glutathione, catalase, superoxide dismutase, thioredoxin, cytochrome c oxidase (complex IV), representing endogenous antioxidants and vitamin E, coenzyme Q, carotenoids and ascorbic acid, representing some possibilities of exogenous antioxidants [89,90,91].
Cells constantly strive to maintain the level of ROS essential for their normal functioning. However, excessive production of ROS reduces the activity of the antioxidant enzymatic defense system and the content of non-enzymatic proteins (GSH), which compromises the general defense system and prevents it from eliminating excess free radicals [92,93]. ROS, produced in hyperoxia and inflammatory conditions, combined with a low and damaged antioxidant defense system, change the homeostasis of the biological system as a whole. In excess, they cause oxidative damage to deoxyribonucleic acid (DNA). They can react with them and attack nitrogenous bases and the sugar–phosphate skeleton, instigating single- and double-stranded DNA breaks, which are also linked to premature aging. Considering all these consequences, OS can stimulate various pathologies (chronic and acute), cause acute diseases (trauma and stroke), and accelerate aging processes [94,95].
As a dietary phenolic compound, curcumin is useful for longevity through declining OS, modulating signal transduction, and gene expression. Curcumin can extend shelf life by inhibiting lipid peroxidation and also increasing antioxidant activities [96]. It has enormous potential to minimize age-related cellular damage caused by the generation of ROS. It can stabilize Nrf2 and enhance the expression of heme oxygenase-1 (HO-1), in addition to stimulating the Nrf2 pathway, which is essential in the activation of antioxidant enzymes, such as thioredoxin reductase, heme oxygenase, sirtuins, and Hsp70 [97,98,99]. Curcumin can promote significant neuroprotective actions by modulating neuroinflammatory signaling pathways, scavenging ROS, and inhibiting or reducing the production of pro-inflammatory mediators [100].
An interesting study showed that, on sepsis-induced cardiac dysfunction, curcumin can activate sirtuin 1 (SIRT1), elevate the expression of mitochondrial biogenesis-related genes Nrf2, Pgc1α, and Tfam, reduce dynamin-related protein 1 transport to mitochondria, and restore mitochondrial morphology and function in heart cells [101].
Tetrahydro-curcumin, one of the most important metabolites of curcumin, was used in a study to remove ROS from hyperglycemia and increase the concentration of reduced glutathione (γ-glutamylcysteinyl glycine) in cultured rat lenses [102]. In another study, microsomal lipid peroxidation inhibition was reported in male rats’ liver supplemented with 1% turmeric [103]. Regarding the effects of curcumin on several target molecules that are directly or indirectly related to different metabolic functions [104], a study reported an increase in cellular antioxidant defenses in rats subjected to treatment with cyclophosphamide to stimulate lung injury, fed with curcumin for seven days before receiving the treatment [105]. Curcumin functions as a biochemical antioxidant; it can extend lifespan by inhibiting lipid peroxidation and increasing antioxidant activities, and as a dietary phenolic compound, it can promote longevity by reducing OS and modulating gene expression [106,107].
4.4. Mitochondrial Dysfunction and Apoptosis
Mitochondrial dysfunction is related to several aging conditions and disorders. Deregulated levels of ROS can potentially cause oxidative damage in the mitochondrial DNA (mtDNA), affecting the organelle’s function and inducing redox signaling to the other cell organelle [59,108]. Then, mitochondrial dysfunction is characterized by higher NO synthesis, OS, and lower ATP production and oxygen consumption. However, mitochondria have ROS scavenging systems where superoxide dismutase turns superoxide radical into hydrogen peroxide. It suffers the action of catalysts such as glutathione peroxidase, which breaks hydrogen peroxide into water.
Curcumin can improve antioxidant activity and reduce oxidative damage in mitochondria by increasing the effect of superoxide dismutase, glutathione, and catalase and also inhibiting ROS-generated enzymes such as cyclooxygenase, lipoxygenase, and xanthine hydrogenase/oxidase [109,110].
In mice with chronic kidney disease-induced mitochondrial dysfunction, curcumin could improve mitochondrial biogenesis and mitochondrial function and suppress OS, probably by inhibiting glycogen synthase kinase-3β (GSK-3β) activity. Curcumin modulated levels of mitochondrial ATP and the basal mitochondrial oxygen consumption rate; it also attenuated mitochondrial superoxide production. Furthermore, curcumin could inhibit the deleterious alterations in mitochondria morphology and enhance the expression levels of mitochondrial transcription factor A (TFAM), nuclear respiratory factor 1 (NRF-1), and PPAR-γ coactivator 1-α (PGC-1α), which were decreased in mouse muscle [111,112].
Furthermore, by improving mitochondrial function, curcumin pretreatment protected rat bone marrow mesenchymal stem cells against hypoxia and reoxygenation injury. The pretreatment improved ATP production and reduced ROS formation and changes in mitochondrial membrane potential, which are caused by the excessive levels of ROS that alter mitochondrial membrane permeability and can lead to apoptosis. In addition, curcumin also prevented hypoxia and reoxygenation-induced cell viability reduction and improved nuclei morphology [113].
Curcumin has been shown effective in inducing apoptosis in different cancer cells, mainly due to its potential to inhibit the phosphatidylinositol 3-kinase/protein kinase B (PI3K/AKT) pathway, whose activation hinders apoptosis by upregulating anti-apoptotic genes such as B-cell lymphoma 2 (Bcl-2) and downregulating pro-apoptotic genes like Bcl-2 associated protein X (Bax) [114,115]. The PI3K/AKT pathway inhibition by curcumin can be explained by the upregulation of phosphatase and tensin homolog (PTEN). This tumor suppressor downregulates PI3K/AKT signaling and gene expression in AKT activation [115]. Also, curcumin inhibits the tyrosine kinase epidermal growth factor receptor (EGFR), which activates the PI3K/AKT pathway and induces apoptosis in neoplastic cells [116].
The JAK/STAT pathway is also a signaling pathway that regulates apoptosis and is affected by curcumin in a myeloproliferative neoplasm model. In this experiment, curcumin inhibited JAK2/STAT and mammalian target of rapamycin complex 1 (mTORC1) pathways in JAK2 V617F-mutated cells, inducing apoptosis [117]. Furthermore, curcumin presented the ability to sensitize neoplastic cells to suffer apoptosis mediated by death receptor pathways, which are unlocked with death ligands, like TNF and Fas ligand, culminating in caspase-8 activation and consequent apoptosis. Notwithstanding, curcumin can induce endoplasmic reticulum stress-induced apoptosis pathway in tumor cells [115].
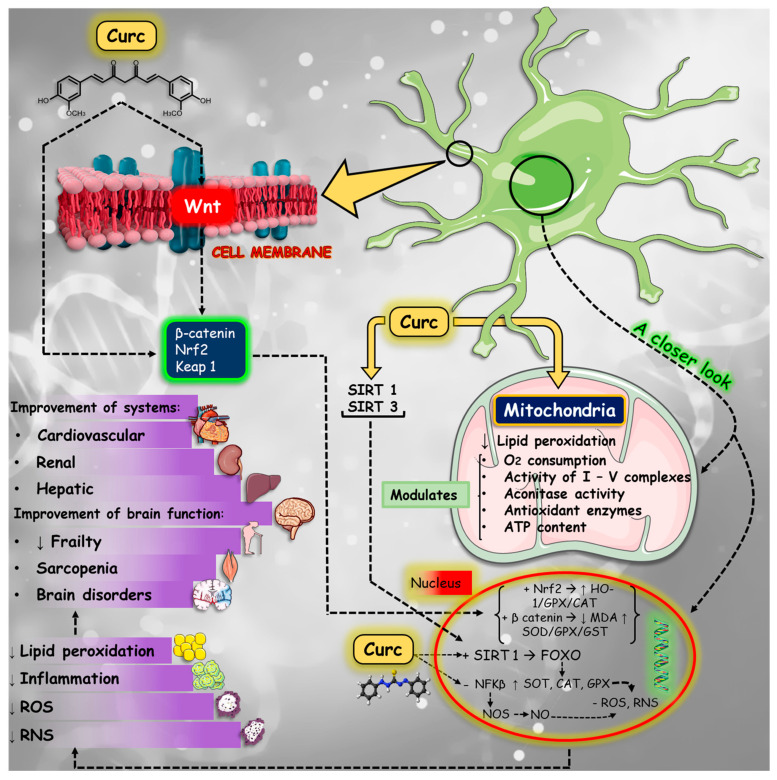
Curcumin also showed inhibitory effects on apoptosis in non-cancerous diseases, which enhances its therapeutic applications. In diabetic cardiomyopathy, it reduced ROS and activated PI3K-AKT signaling pathways, resulting in the downregulation of Bax and caspase-3 expression and consequent apoptosis inhibition [118]. In a septic acute kidney injury mouse model, curcumin showed anti-inflammatory capacity and attenuation of apoptosis by JAK2/STAT3 and NF-κB signaling pathway inhibition, resulting in increased levels of Bcl-2 and decreased levels of Bax and caspase-3 [119]. Figure 5 summarizes some curcumin effects related to mitochondrial function and reduction of OS.
Figure 5.
Effects of curcumin on mitochondrial dysfunction and oxidative stress in different signaling pathways. Curcumin can upregulate sirtuin (SIRT), Ketch-like ECH-associated protein 1, nuclear factor erythroid-2 related factor 2 (Keap1-Nrf2), and Wnt/β catenin pathways, and inhibit nuclear factor kappa beta (NF-κβ). The results of the stimulation and inhibition of these pathways is the modulation of lipid peroxidation, oxygen consumption, aconitase and antioxidant enzyme modulation, and ATP production in mitochondria. Moreover, curcumin is related to the upregulation of the synthesis of glutathione peroxidase (GPX), superoxide dismutase (SOD), reduction of malonaldehyde (MDA), reactive oxygen species (ROS), and reactive nitrogen species (RNS). The results of curcumin effects are improvements in cardiovascular, renal, and hepatic diseases. Furthermore, there is a reduction in frailty, sarcopenia, and brain disorders. ↓: decrease; ↑: increase.
4.5. Neurodegenerative Diseases
NDs include a heterogeneous group of neurologic conditions that covers dementia predominantly, multiple sclerosis, amyotrophy lateral sclerosis, AD, and PD, and can lead to neural cell death [120,121,122,123,124].
Usually, NDs are irreversible, progressive, and related to loss of function; as the structures degenerate, a gradual and progressive loss of motor skills and/or cognitive skills can lead to loss of function, debilitation, and mental impairment. Neuroinflammation and OS are pathophysiologies common to all forms of ND. Among the most common NDs are AD (the most frequent), PD, and amyotrophic lateral sclerosis; in AD, the symptoms include progressive and irreversible cognitive deficits that may present with changes in behavior and mood, and memory loss is also common as the disease progresses; it corresponds to 60% to 80% of dementia cases, and more than 30 million people have this condition. These diseases affect most elderly individuals but can also occur at other ages and are characterized by having a constant progressive course due to the increasing decrease in specific neurons in the brain [125,126,127,128,129,130,131,132].
Therapeutic resources that only treat the symptoms and prevent the progression of the disease are used at the moment, such as drugs including cholinesterase inhibitors used for AD that do not change the course of the disease and only provide improvement in symptoms of behavioral deficits. Still, several patients exhibit little effectiveness in the therapeutic response due to the difficulty of adherence to treatment that has a high cost, physiological variability, and adverse effects, which include nausea, vomiting, dizziness, and diarrhea [133,134,135,136].
These reasons led to the need for other therapeutic approaches, and Curcuma longa, due to its antioxidant, immunomodulatory, and anti-inflammatory properties, may be an option. Mechanisms such as glucose metabolism and endothelial function are closely linked to processes of neurogenesis, neuroinflammation, and synaptic plasticity, and improvement of the function and structure of synapses, regulation of proteins, and delay of the neural dysfunction process has been associated with the consumption of curcumin [137,138,139]. Figure 6 shows some effects of curcumin on the prevention of ND.
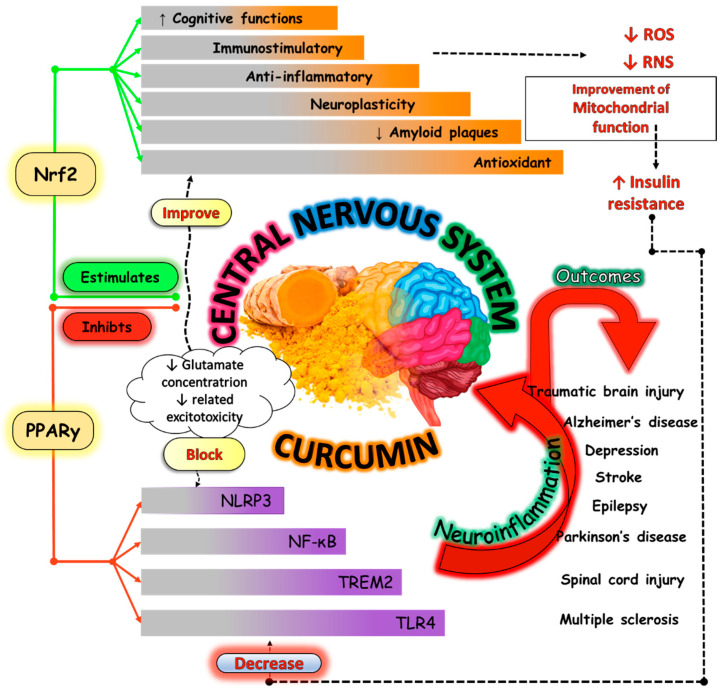
Figure 6.
Curcumin and its effects on nervous system disorders. Activation of NOD-like receptor pyrin domain-containing 3 (NLRP3), Toll-like receptor 4 (TLR4), nuclear factor-kappa beta (NF-κβ), and triggering receptor expressed on myeloid cell 2 (TREM2) is associated with neuroinflammation and the risk of developing conditions such as AD and PD, brain injury, depression, and multiple sclerosis. However, curcumin can block PPAR-γ, which is an important mediator for the expression of these inflammatory factors. In addition, curcumin can stimulate nuclear factor erythroid-2 related factor 2 (Nrf2) and leads to improvement of inflammation, OS, cognitive functions, neuroplasticity, and memory. This activity can result in a decrease in ROS and RNS, improving mitochondrial function, and a decrease in insulin resistance, which reduces the activity of the inflammatory factors mentioned. Furthermore, it can reduce β amyloid plaque accumulation to avoid future inflammation of the nervous system. ↓: decrease; ↑: increase.
4.5.1. Cognition
Although crystallized intelligence remains unchanged, neural aging leads to important changes in fluid intelligence as age advances, resulting in impairments in attention, memory, processing speed, and visuospatial and psychomotor abilities [140,141]. The pharmacological class most used in these cases is acetylcholinesterase (AChE) enzyme inhibitors, as therapeutic options are limited. However, numerous studies have shown that curcumin has relevant effects on cognitive function [142,143].
Zhi et al. [44] investigated male C57BL/6 mice with trigeminal neuralgia administered 100 mg/kg/day curcumin twice a day for 14 days to observe its effects on orofacial allodynia and cognitive impairment and showed an increase in the density of dendritic spines, and in the regulation and proportion of dendritic spines, relieving the synaptic damage neurons in the hippocampus, in addition to increased mechanical and cold pain thresholds and improved spatial learning and memory deficits. As also presented in male Otsuka Long-Evans Tokushima Fatty rats as a model of spontaneous DM2, they were subjected to physical exercise or physical exercise in combination with 5 g/kg of curcumin to investigate their cognitive responses; then, it was evidenced that both groups showed a significantly lower escape latency and a longer swimming time spent in the target quadrant; however, the best results were seen in the group in combination with curcumin, indicating a decrease in learning and memory deficits [144].
The improvement in cognitive function may be partially linked to an increase in neurogenesis, as analyzed in male Sprague Dawley rats in a model of Gulf War illness treated with 30 mg/kg curcumin for 30 days and exposed to the object localization test to evaluate cognitive function, as well as the new object test to analyze recognition memory and measurement of hippocampal neurogenesis. The results observed were a greater exploration time of both an object moved to a different location and a new object over a familiar object, as well as an increase in hippocampal neurogenesis; in addition, the improvement in cognitive function may be partially linked to greater neurogenesis [145].
Behind all cognitive impairment, there is an exacerbated OS mechanism due to mitochondrial changes caused by aging, which trigger a theory known as “free radical aging”. It is explained by the reduction in ATP production, culminating in lower consumption of oxygen, followed by a higher concentration of free molecules (O2) to bind with NO, generating peroxynitrite (ONOO−) or hydroxyl radicals (•OH). This exacerbated production of ROS unbalances antioxidant activities mediated by the enzymatic action of superoxide dismutase and glutathione peroxidase, increasing lipid peroxidation and the oxidation of DNA and proteins, thereby, in addition to stimulating the release of pro-inflammatory and pro-apoptotic factors, causing apoptosis and autophagy of neural cells [146,147].
Using 100 mg/kg of curcumin one hour before treatment with cisplatin (an important chemoattractant that can lead to cognitive impairment) in C57BL/6 mice showed that the autophagy caused by cisplatin was induced through the transcription factor 4/protein kinase B/the mammalian target of the rapamycin (ATF4-Akt-mTOR) signaling pathway by endoplasmic reticulum stress. The analysis also showed that curcumin increased the activation of the AMPK/c-Jun N-terminal kinase (JNK), culminating in the inhibition of Akt and mTOR and upregulation of the Bcl-2 protein (anti-apoptotic), suppressing apoptosis, being associated with increased neurogenesis and synaptogenesis in the hippocampus, and clarifying the improvement and recovery of cognitive function, as seen in the MWM test (improved spatial learning and memory) and NORT (increased recognition function). In addition, it increased the amount of cells positive for the marker of neurogenesis doublecortin in the hippocampus and the density of the dendritic spine, and inhibited the levels of Bax- and Bcl-2-interacting mediator of cell death (Bim) proteins (pro-apoptotic members); the opposite is seen when cisplatin is administered alone, which despite presenting a considerable level of autophagy also induces an exaggerated increase in apoptosis, attenuating neurogenesis and synaptogenesis, explaining cognitive impairment [148].
Rueda et al. investigated the actions of 300 mg/kg of curcumin in prenatal or early postnatal stages on geomorphology and cognition in pregnant female mice with Ts65Dn Down syndrome [149]. The use of curcumin acted to increase brain weight, the density of positive bromodeoxyuridine and 4’6-diamidino-2-phenylindole in the hippocampus of those on short-term prenatal and short-term postnatal treatment, and the levels of postsynaptic density protein 95 and synaptophysin. There was a decrease in escape latency, and in the probe test, there was an increase in the number of crossings in the platform position, indicating an improvement in cognitive status and neuromorphology.
4.5.2. Memory
As noted above, using curcumin as a drug therapy adjuvant shows several neurological benefits, including preserving and improving memory and learning, as seen in research using rodents with neurological deficits [150,151,152,153,154,155].
The study by Changleck et al. investigated the effects of curcumin on lead-induced inflammation and cholinergic dysfunction in male ICR mice [156]. It was possible to demonstrate that the groups receiving treatment associated with curcumin showed a significant increase in AChE levels in the brain, decreased TNF-α, COX-2, phosphorylation of inhibitory kappa B kinase beta (IKKβ), extracellular signal-regulated kinase (ERK), and JNK. Moreover, an improvement in spatial memory was observed, especially in those undergoing treatment with curcumin 200 mg/kg. Another study analyzed the effects of 50 mg/kg/day curcumin supplementation for 4 weeks on memory deficits, lactate content, and monocarboxylate transporter 2 (MCT2) in a model of sixteen amyloid precursor protein (APP)/presenilin 1 (PS1) transgenic male and female mice [157]. The analyses demonstrated that the group treated with curcumin showed an increase in escape length and platform passage time, indicating an improvement in memory deficit, as well as lactate levels and protein MCT2, which were significantly increased in the cerebral cortex and hippocampus.
The effects of consuming 5, 15, and 45 mg/kg of curcumin associated with cholinergic drugs on the cholinergic system of male Wistar mice, compared to the isolated administration of agonists (nicotine, pilocarpine) and cholinergic antagonists (succinylcholine and scopolamine), demonstrated a significant improvement in memory retention in those undergoing treatment with agonists [158].
Ikram et al. investigated the neuroprotective mechanisms of dietary supplementation of 50 mg/kg of curcumin for 6 weeks on male C57BL/6N mice and HT22 cells from the hippocampus of mice with neurodegeneration [159]. The authors observed that ethanol increased ROS, lipoperoxidation, Toll-like receptor-4 (TLR4) expression, and receptors for advanced glycation end products (RAGEs); concomitantly, there was also an increase in phosphorylated (p)-JNK, p-NF-κB, apoptotic markers (Bax, cleaved caspase-3, and PARP-1), and decreased anti-apoptotic markers; these were responsible for neuroinflammation, neurodegeneration, and synaptic dysfunction. When the curcumin was administered, there was both in vitro and in vivo a decrease in ROS and an increase in the expression of Nrf2/HO-1, an important cytoprotective and detoxifying agent. Furthermore, chronic use of curcumin was associated with an inhibition of apoptotic markers and an increase in Bcl-2, indicating a rescue against neurodegeneration and memory impairment that was confirmed through Nissl staining and FJB of neuronal cells.
Zhang et al. demonstrated that the acute use (single dose) of 50, 100, and 200 mg of curcumin showed no benefit on the spatial memory of male Sprague Dawley rats with memory deficit induced by a ventricular injection of β-amyloid peptide (1–42); the opposite was seen in those on chronic treatment with the same dosages of curcumin, with a significant decrease being observed in escape latency, and an increase in the frequency of crossing the platform location and in spatial preference for the target quadrant, especially when using doses of 100 mg or 200 mg of curcumin [160].
4.5.3. Alzheimer’s Disease
AD is marked mainly by accumulations of extracellular amyloid plaques, composed of β-amyloid peptides (Aβ) responsible for directly inducing tau hyperphosphorylation and neurite degeneration and intracellular neurofibrillary tangles, composed by hyperphosphorylated microtubule-associated protein (MAPT) tau protein, which disrupts microtubules and impairs axonal transport in the brain [161,162,163]
Besides that, both aging and the accumulation of Aβ deposits pathologically stimulate microglia and astrocytes, inducing inflammatory processes with the intense release of pro-inflammatory biomarkers, such as IL-8, TNF-α, IL-1β, and IL-γ, with an increase in the enzymatic activity of beta-secretase and gamma-secretase due to decreased action of the AMPK pathway, which is dependent on JNK, cleaving the beta-APP and leading to the formation of more Aβ aggregates that bind to the RAGE, increasing the concentrations of ROS and RNS, stimulating the activation of NF-κB, which will trigger the production of NO. OS leads to a decrease in the activity of antioxidants, including glutathione, catalase, and glutathione-S-transferase, with a consequent increase in lipid peroxidation and nitrite levels. This inflammatory cascade is responsible for neuroinflammation and neurodegeneration [164,165,166,167,168,169].
Fortunately, in addition to AChE inhibitor medications, studies have shown that curcumin therapy acts protectively in the pathogenesis of AD, reducing OS and inflammation, in addition to inhibiting the formation of Aβ fibrils from Aβ40 (1–40) and Aβ42(1–42) and amyloid plaques [170,171,172,173,174,175].
The blood–brain barrier, which prevents most drugs from reaching their targets, makes the central nervous system the final frontier in drug delivery [176,177]. Yang et al. showed that in oral ingestion or peripheral injection of curcumin in Swedish Mutant (APPsw) Tg2576 transgenic mice, there was a blood–brain barrier crossing of curcumin that binds to amyloid plaques in vivo and in vitro [178]. With increasing doses of curcumin, it was possible to see a significant inhibition of Aβ aggregation and an induction of disaggregation of pre-aggregated Aβ40 in vivo. Furthermore, curcumin’s aggregation-inhibiting effects were superior to those demonstrated by non-steroidal anti-inflammatory drugs such as naproxen and ibuprofen. A second analysis carried out in APPsw AD Tg2576 transgenic mice treated with a low dose of curcumin (160 ppm) or a high dose (5000 ppm) showed declines in the levels of IL-1β and oxidized proteins, a 16.5% decrease in an astrocyte marker—glial fibrillary acidic protein (generally elevated in inflammatory conditions)—and 39.2% × 43% of insoluble and soluble Aβ levels in the entorhinal cortex and hippocampus. To conclude, a reduction in amyloid load by 43.6% and 32.6% in the average number of plaques was observed [171].
In a mouse model of AD, supplementation with 200 mg/kg of curcumin promoted a decrease in neurofibrillary degeneration and loss of hippocampal neurons, as well as a decline in Bax levels and an increase in Bcl-2 rates (decrease in apoptosis with a blockade of cytochrome c release from mitochondria) [179].
Under curcumin treatment, Kunming mice with irreversible brain lesions presented an increase in superoxide dismutase levels and a decline in brain malonaldehyde (MDA), as well as upregulation of Nrf2, NAD(P)H quinine oxidoreductase 1 (NQO1), HO-1, and γ-glutamyl cysteine synthetase (γ-GCS) in brain cells. These actions led to a significant improvement in OS, and a positive evolution in memory and spatial learning [180]. Similar results were shown in a study on synaptosomes obtained from the cerebral cortex of rats with neurodegenerative damage induced by Aβ1-42 in combined treatment with boric acid and curcumin. There were significant reductions in MDA and AChE and an increase in synaptophysin [181].
Aβ aggregation and tau hyperphosphorylation can also be induced by the interaction between p25 with glycogen synthase kinase 3 (GSK3β), and cyclin-dependent kinase 5 (Cdk5) [182,183]. A mouse model of AD induced by scopolamine or Aβ1-42 treated with curcumin showed a significant decline in lipid peroxidation and increased superoxide dismutase levels, in addition to a reduction in Aβ aggregation and tau hyperphosphorylation through the regulation of GSK3β, Cdk5, p35, and p25 [184,185].
Curcumin also has an inhibitory role on the thioredoxin-interacting protein (TXNIP)/NLRP3 inflammasome pathway and the JNK-NF-κB signaling cascade through the controlled regulation of AMPK, causing a decrease in neuronal apoptosis due to lower levels of pro-caspase 1 and consequently lower levels of IL-1β and IL-18 [186,187], since the activation of AMPK provides the suppression of inflammation and OS [188]. As analyzed in a group of male C57BL/6 J mice in an AD model, the use of 150 mg/kg of curcumin improved spatial learning and spatial working memory and led to a decrease in lesions and apoptosis of neural cells; in addition, the deposition of Aβ1–42 and neuroinflammation were significantly reduced, reducing the levels of TNF-α, IL-6, IL-1β, and MDA, and augmenting the levels of superoxide dismutase and activation of the AMPK pathway [189].
The use of curcumin nanomaterials has also been widely used in therapeutic environments by increasing the aqueous solubility and bioavailability of curcumin in target tissues [190,191]. Therefore, nanomaterial composed of curcumin led to improvement in spatial learning and memory retention. Besides that, it promoted neurogenesis through increasing brain-derived neurotrophic factor (BDNF) levels and improved neuroinflammation by inhibiting the NLRP3 inflammasome activation pathway induced by Aβ deposition, through decreasing IL-18, CD68, and NLRP3 both in the hippocampus and the cortical area. A second nanomaterial with curcumin also reduced/inhibited neuronal death, as NLRP3 promotes the synthesis of a speck-like protein associated with apoptosis containing a CARD (ASC), a protein that recruits and activates pro-caspase-1 and IL-18 contributing to the activation of neuronal death [192]. Another nano curcumin administered once a week for three months in AD Tg2576 transgenic mice also provided a lower density of amyloid plaques in the hippocampal region through thioflavin T (ThT) staining and an improvement in working memory and signaling, observed through contextual fear conditioning tests and RAM [193].
4.5.4. Parkinson’s Disease
The second most common ND, PD, is also progressive and irreversible, and around 1% of individuals over 50 years old are affected [194,195,196,197]. The loss of dopaminergic fibers of the brain and the progressive worsening of motor symptoms characterizes the disease that, as it progresses, leads to the loss of 50 to 70% of all dopaminergic neurons of the patient in the substantia nigra [131,196,198,199].
There is an increased amount of evidence that implicates OS and immunological alterations in the pathogenesis of PD. The accumulation of excessive ROS and other free radicals overloads the decaying dopaminergic neurons and creates an environment conducive to this disease [200]. Possible contributors to OS have been associated with the process of PD such as mitochondria, endoplasmic reticulum, dopamine, and α-Sinuclein, and it seems that their interactions and not their actions collaborate for progressive neurodegeneration [201]. Neuroinflammation can be very important for the pathogenesis of PD since it has destructive repercussions on the nigrostriatal dopaminergic pathways, which activate brain glial cells (especially microglia and astrocytes) to release several soluble factors that may be neurotoxic and/or pro-inflammatory [199,202,203,204].
For its anti-inflammatory and antioxidant properties, curcumin has been widely used as a food additive and as an adjuvant therapy to prevent or treat ND [205,206]. One study showed that it is possible to reduce deficits related to PD by raising the level of antioxidant enzymes only with curcumin [207]. It can also protect black matter from cells, inhibit apoptotic signaling pathways with NF-κB, decrease lipid peroxidation and protein aggregates, and also OS damage to the mitochondrial membrane [125,208].
Curcumin can promote neuroprotection and inhibit the α-Synuclein aggregation in a PD model. Its use inhibited NFκB activity protein stimulated by lipopolysaccharide. It can reduce the production and aggregation of α-synuclein. The authors of this study suggested that curcumin can be an option as adjuvant therapy for the management of PD and other synucleopathies [209].
Other authors investigated the effects of iron oxide nanoparticles capped with curcumin (FeONPs-Cur) in motor imbalance and neurochemical modifications in a PD model (reserpine induction). These animals showed a significant reduction in motor activity associated with a decrease in 5-hydroxytryptamine, norepinephrine, and dopamine and increased levels of malonaldehyde, nitric oxide, and monoamine oxidase. Glutathione, Na+/K+/ATPase, and AchE significantly decreased in both brain areas. Using iron oxide nanoparticles capped with curcumin (FeONPs-Cur) restored homeostasis and prevented motor deficits, suggesting that FeONPs-Cur could be an antiparkinsonian candidate [210].
4.6. Fragility
Fragility is characterized by excessive vulnerability of the individual to endogenous and exogenous stressors, which in general leads to a high risk of developing negative situations related to health and disability, accentuating the prevalence of chronic diseases [74,211,212,213,214]. Besides the existence of several fragility evaluation instruments, there is still no agreement on a standard instrument identifying fragility [215]. In one of them, the methodology conceptualizes frailty as an energy imbalance syndrome that culminates in slowness, fatigue, decreased muscle mass, physical activity, and strength [216]. It detected older adults with a higher risk of falls, mortality, and adverse events after surgery [217,218,219,220]. Studies with geographic coverage and methods of selection of diverse samples have shown estimates of frailty prevalence among older people [221,222,223].
A study in the United States showed that 15% of the non-basilar elderly population is fragile and 45% pre-fragile. There is a prevalence of frailty among women, older people, and racial and ethnic minorities. Despite these characteristics, there was substantial variability in the prevalence of frailty among demographic regions. There is a marked increase in the prevalence of chronic diseases and disabilities with frailty [211,214,224].
Curcumin, at a dose of 2.5 g 2 times daily and 150 mg of lipid curcumin nanoparticles (Theracurmin) 2 times daily, may have beneficial effects on muscle recovery, reducing the expression of muscle damage moved by exercise, reducing the loss of maximum voluntary contraction and minimizing the increase in blood levels of creatine kinase [225,226] which shows that the use of curcumin can be an important adjuvant in the control of pain caused by loss of muscle mass [227].
A pilot study (phase IIb clinical trial) showed that the use of curcumin C3 Complex® can improve muscle strength and physical function in older people (sedentary and >65 years with c-reactive protein > 1 mg/dL) at risk for mobility disability [228].
4.7. Sarcopenia
Sarcopenia is characterized by loss of muscle mass, functionality, and strength and is an important factor in loss of mobility and frailty in the elderly [229,230,231,232,233,234]. It is estimated that by 2050, there will be 426 million people aged 80 years or more and 2.1 billion people aged 60 years or more. Sarcopenia is linked to several comorbidities besides disability, such as osteoporosis, DM2, and obesity [235,236,237]. It is a multifactorial pathogenesis that encompasses various mechanisms such as insulin resistance, anabolic resistance, malnutrition with decreased availability of amino acids, and chronic inflammation [238,239,240]. In 2019, an estimate suggested that the economic expenses related to sarcopenia in the United States were USD 40.4 billion, an average of USD 260 per person [241].
One study demonstrated that low-grade persistent inflammation impacts muscle protein degradation and synthesis by several signaling pathways impacting sarcopenia; low-grade inflammation is a symptom of cells that begin the senescence phase and exit the cell cycle. During aging, pro-inflammatory TNF-α, C-reactive protein, and IL-6 are somewhat elevated in circulation [242]. It has also been reported that older people with sarcopenia show significantly higher levels of circulating IL-6 and TNF-α [243] and that high levels of IL-6 and CRP increase the risk of loss of muscle strength [244]. A longitudinal study of 10 years also demonstrated that plasma levels of TNF-α, IL-6, and IL-1 were reliable biomarkers of morbidity and mortality in elderly participants [245].
Due to the relevance of inflammation and OS in the origin of sarcopenia, compounds with antioxidant and anti-inflammatory effects have the potential to act as complementary to current treatments for this disease. Curcumin is one of the potential compounds with these characteristics. Curcumin has also been reported to target a class of signaling molecules that alter cellular functions and exert their therapeutic effects; it has been linked to numerous health benefits in several studies, including muscle health [246,247,248,249].
The preservation of muscle mass in the course of aging is paramount for the prevention of sarcopenia. Studies reported that curcumin increased muscle mass without altering body mass in F344XBN rats at 32 months of age, supplemented with a 0.2% diet for 4 months. These results were similar to those reported in previous studies [250,251,252,253]. Another study with 12-month-old male Sprague Dawley rats that had LPS-induced sarcopenia and received treatment with 150 mg/kg of curcumin for two months showed improvement in muscle endurance, pressure strength, and fat/lean ratio [254]. The supplementation of curcumin (40 and 80 mg/kg) 30 min before forced exercise in 10-month-old ICR rats for 28 days could complement exercise-based therapy to prevent muscle problems such as sarcopenia systematizing the expression of genes associated with protein synthesis, inflammation, and apoptosis in chronic forced exercise [255].
The protective effects of curcumin in muscle atrophy induced by dexamethasone using differentiated C2C12 cells were evaluated and showed that treatment with curcumin reduced the expression of Murf-1 and Atrogin-1, preventing protein degradation. It also increased the level of Akt phosphorylation, an essential protein in the mTOR signaling pathway that stimulates protein synthesis and prevents protein degradation [256]. The significant increase, impairment, and recruitment of satellite cells to delay the onset of pre-sarcopenia and sarcopenia were demonstrated in a study in 18-month-old C57BL6J and C57BL10ScSN mice, which received 120 µg/kg of curcumin for six months. The increase in the proportion of positive satellite cells for MyoD isolated from muscles of the aged posterior limbs and the development of sustained myofibers in the aged soleus muscle showed this [234,247].
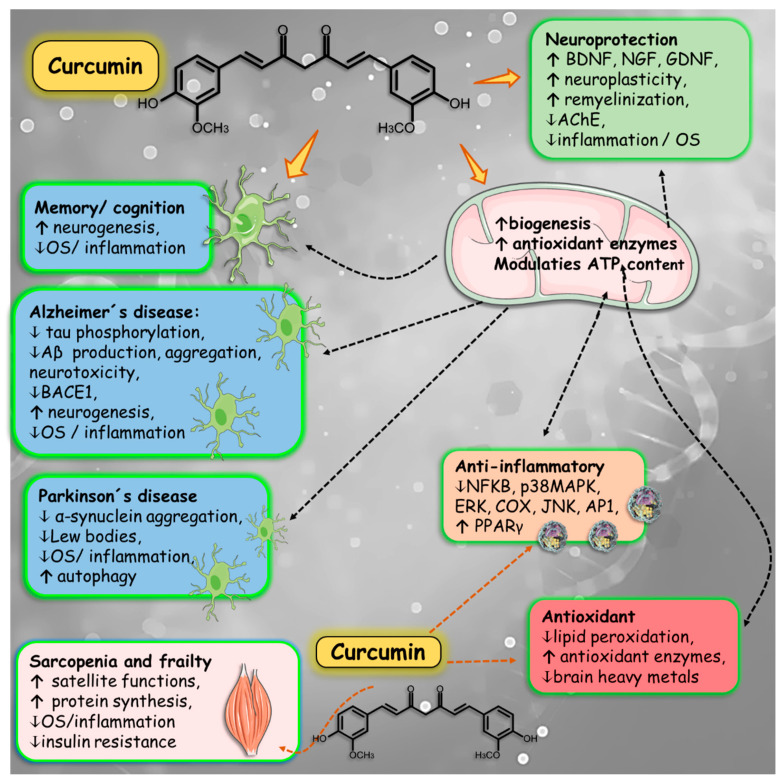
Evidence shows that curcumin is an alternative treatment with the potential for sarcopenia control; it can maintain the number and function of satellite cells, protect the mitochondrial function of muscle cells, and suppress OS and inflammation, thus achieving muscle protection [247]. Figure 7 is a summary of the effects promoted by curcumin in some aging-related conditions discussed above.
Figure 7.
Summary of curcumin effects on some aging-related conditions. Curcumin possesses antioxidant and anti-inflammatory effects that are related to the prevention or treatment of memory loss, neurodegenerative diseases, sarcopenia, and frailty. These effects can play a role in mitochondrial functions that, on the other hand, are also associated with diminishing oxidative stress and inflammation. The results are associated with an increase in the synthesis of neuronal growth factors such as BDNF, NGF, and GDNF, an increase in neuroplasticity, reduction in brain neuroinflammation, and restoration of brain functions. In muscles, there is an increase in protein synthesis and a reduction in its degradation. AChE: acetylcholine esterase; AP-1: activator protein-1; BACE1: β-secretase 1; BDNF: brain-derived neurotrophic factor; COX: cyclooxygenase; ERK: extracellular signal-regulated kinase; GDNF: glial cell-derived neurotrophic factor; JNK: c-Jun N-terminal kinase; NGF: nerve growth factor; NF-κB nuclear factor kappa beta; p38MAPK: p38 mitogen-activated protein kinase; PPAR-γ: peroxisome proliferator-activated receptor gamma.
4.8. Depression
Depression is a chronic, recurrent, and frequent psychiatric disorder that profoundly influences quality of life and reduces the risk of death [257]. According to forecasts from the World Health Organization, it is estimated that it will become the main global burden of disease in the world by 2030 [258,259]. Significant personal suffering and economic loss result from the increased risk of suicide and worldwide morbidity resulting from depression [260,261,262,263,264]. In the elderly, it mainly affects those affected by chronic medical illnesses and cognitive impairment, causing suffering, disability, and family disruption, in addition to aggravating various diseases and increasing mortality. The processes associated with aging and diseases, such as arteriosclerosis and inflammatory, immunological, and endocrine changes, impair the integrity of the frontostriatal pathways, the hippocampus, and the amygdala, increasing susceptibility to depression [265,266].
Currently, there are several types of traditional antidepressant medications commonly used in clinical practice, such as norepinephrine–serotonin reuptake inhibitors, monoamine oxidase inhibitors, norepinephrine–dopamine reuptake inhibitors, tricyclic antidepressants, and selective serotonin reuptake inhibitors. Increasing evidence suggests that curcumin could enhance antidepressant efficacy through several mechanisms of action. With its wide range of pharmacological properties, it is considered a potent antidepressant, as it reduces the inflammatory response [267,268,269,270,271,272,273]; modulates neurotransmitter levels, and inhibits the expression of monoamine oxidase enzymes [17,18,19,20,21]; regulates hypothalamic–pituitary–adrenal (HPA) disorders [274,275,276]; reduces NO [277,278,279,280,281,282]; repairs neurodegeneration and increases neurogenesis and neuronal plasticity, which normally increases BDNF levels [283,284,285,286,287]; regulates mitochondria [288,289,290,291]; and increases antioxidant enzymes [292].
4.9. Clinical Trials Performed with Curcumin and Age-Related Disorders
Some clinical trials have devoted attention to investigating the effects of curcumin on ND. These trials are discussed below and are shown in Table 1. The bias risk for each study can be found in Table 2.
A group of healthy elderly people was administered new bioavailable curcumin to analyze its effects on the management of sarcopenia, and it was found that the use of curcumin increased the strength of hand grip and weight lifting strength, increased distance covered before fatigue, and, at the end of the study, improved walking time for a given distance compared to the initial analysis of the study. The small study sample may be a limiting factor, but the randomized controlled pattern, the double-blind possibility, follow-up for a considerable period, and an age range with little variation are superior factors [293].
A study developed by Ghodsi et al. investigated the possible neuroprotective role of curcumin in patients with PD. The scores Unified Parkinson’s disease Rating Scale (MDS-UPDRS) and Parkinson’s Disease Questionnaire (PDQ-39) were evaluated every 3 months until the end of the intervention. Curcumin was shown to be a well-tolerated natural compound but did not significantly enhance the scores applied, presenting no efficiency in ameliorating PD symptoms. Some negative points in this study are the limited number of participants and the sample heterogeneity, which might have masked curcumin’s effects because of the diverse degrees of severity of the disease included. Consequently, the authors suggested that the results could potentially be more significant if the sample consisted of patients with lower disease severity [294].
The effects of curcumin on physical function in moderately functioning older adults with low-grade inflammation were evaluated. Subjects were divided into curcumin C3 Complex®, receiving twelve 12 weeks of treatment. The results showed large effect sizes in the short physical performance battery measures of knee extension and flexion peak torque in the curcumin C3 Complex® group but small effect sizes of reductions in galectin-3 and IL-6 inflammatory biomarkers. As a positive factor, curcumin was shown to be safe and well tolerated, and the sample presented high adherence levels (>90%) and retention (94%) during the treatment period. However, the sample size was small, consisting only of Caucasian subjects, the intervention lasted a short period of time, and only one dose of curcumin was administered [228].
Cox et al. investigated the effects of curcumin in a solid lipid form on cognition and mood in a healthy older population. The assessment started with a Mini-Mental State Examination, the Beck Depression Inventory-II, and trait scale of the State-Trait Anxiety Inventory; also, a National Adult Reading Test was applied to measure pre-morbid intellect. Subjects then undertook three rounds of assessment batteries consisting of computerized cognitive tasks that were preceded and followed by an evaluation of state mood. Immediately after the first assessment, a single treatment dose was administered, and then the cognitive tasks were repeated at 1 h and 3 h after the administration. The conclusions were significant enhancement in memory and mood, which included fatigue induced by psychological stress and general fatigue and change in state calmness after chronic treatment, a significant effect on alertness and contentedness after acute and chronic treatment, and reduced total cholesterol and LDL levels. Curcumin was shown to be a safe and well-tolerated compound for the elderly population and even the low applied dose (80 mg) promoted significant and positive results. However, it is important to point out that the sample used was small, and the duration of the intervention was short [295].
Thota et al. [296] examined the effects of administering 180 mg/day of curcumin on the insulin resistance of a group of people at high risk of developing DM2, and it was demonstrated that curcumin showed an improvement in insulin resistance index in the lipid profile, fasting insulin, GSK-3β, and islet amyloid polypeptide, the last two being important markers of AD. The randomized controlled design, control of adherence to interventional treatment, and the possibility of double-blinding are strengths of the study; however, the small sample and short follow-up period are limiting factors.
The study by Rainey-Smith et al. [6] analyzed the use of BCM-95 ® CG (Biocurcumax TM) in men and women with good health and no significant cerebral vascular disease or significant cognitive impairments. No significant difference between the group of intervention and placebo was observed in cognitive function, verbal fluency, mood, perceptual–motor speed, the controlled oral word association test, depression anxiety stress scales, and the Wechsler Digit Symbol Scale of the Wechsler Intelligence Scale for Adults Revised. However, the placebo group demonstrated a decline in function at 6 months, which was not observed in the curcumin group. The study has strengths, such as the randomized controlled design and the long interventional period of 12 months, although visits only occur every 3 months.
The analysis by DiSilvestro et al. investigated the use of 80 mg/day of curcumin for 4 weeks in a population of 19 people, involving healthy men and postmenopausal women, and showed that its use was beneficial in antioxidant activity, due to the increase in catalase, plasma myeloperoxidase, and nitric oxide; in addition to inducing a greater capacity to eliminate free radicals, there was also an improvement in the inflammatory and lipid profile, through reductions in triglycerides and soluble intercellular adhesion molecule (sICAM) [297]. The levels of beta-amyloid protein and alanine aminotransferase were significantly reduced. However, the study has limitations due to the small population sample, short intervention period, and unclear methodology.
Baum et al. studied the effects of using 1 g or 4 g of curcumin for 6 months on the lipid profile of a population with cognitive decline or diagnosed with AD for at least 6 weeks [298]. The study did not show any significant results on the lipid profile. The randomized controlled design and the long follow-up period are important points of the study; however, the small sample is a limiting factor.
A study determined the functional effects of CGM (curcumin–galactomannan) on healthy individuals’ brain waves. Subjects were divided into three groups, and assigned to consume 500 mg of CGM, unformulated curcumin (UC), or placebo capsules twice daily for 30 days, and electroencephalogram (EEG) measurement audiovisual reaction time tests were performed, and a working memory test was performed at baseline and after 30 days. The results indicated that the CGM can influence the brain waves of healthy individuals in a manner consistent with the penetration of the blood–brain barrier, and the electroencephalogram results showed a correlation with the improved audiovisual and working memory tests, contributing to demonstrating the contribution of the CGM in the reduction fatigue and improved memory. The study, however, as it is a pilot study, used a small sample of individuals with a wide age range (35–65 years) [299].
A partial replication study was carried out, with the aim of evaluating similar effects at 4 and 12 weeks of supplementation with Longvida©. Outcome measures included cognitive performance, mood, and biomarkers that were assessed at baseline and after 4 and 12 weeks of treatment, which consisted of Longvida© intake for 12 weeks. The results of the study indicated an improvement in aspects of working memory and lower fatigue scores, and in four weeks, lower scores for anger, tension, confusion, and total mood disturbance. A significant increase in glucose in the group that consumed Longvida© was also observed. The large age range among participants was wide (50 to 85 years old) [300].
Another study evaluated the effects of fish oil and curcumin supplementation on cerebrovascular function in older adults. The results did not show modifications regarding Transcranial Doppler ultrasound, blood pressure, heart rate, arterial compliance, fasting glucose, blood lipids, and C-reactive protein. The authors proposed that since the study of the combined effects of fish oil and curcumin in humans is currently limited, the non-significant effects might be related to the dosages applied and unknown interactions between fish oil and curcumin [301].
Curcumin was used in the form of Theracurmin to analyze its effect on brain amyloid and tau accumulation in adults without dementia. During the intervention, several tests were applied, such as vital signs, electrocardiograms, serum electrolytes, thyroid function, and blood counts, as well as Montreal Cognitive Assessment, Beck Depression Inventory, neuropsychological test battery, and memory functioning questionnaires. The main results included improvement in memory and attention, probably associated with decreases in amyloid and tau accumulation in the brain. One limitation of the article was the small sample size. However, regarding the positive points, the study promoted a relatively long treatment duration and applied sensitive cognitive measures to track memory effects [302].
In Pennsylvania, a study evaluated the effects of a highly absorbent curcumin extract dispersed with colloidal nanoparticles (Theracurmin) in treating adults without dementia on memory performance and its potential impact on neurodegeneration by measuring brain deposition of amyloid plaques and tau tangles. Results showed a significant improvement in attention and memory in adult patients without dementia compared to placebo. PET scan examinations suggested that cognitive and behavioral improvement correlates with reduced accumulation of tau plaques and tangles in regions of the brain that regulate mood and behavior [303].
The effectiveness of curcuminoids as a complement to standard antidepressants in patients with major depressive disorder was investigated. Changes in psychological state based on the Hospital Anxiety and Depression Scale (HADS) and the Beck Depression Inventory II (BDI-II) were used to measure the efficacy of combined curcuminoid–piperine supplementation plus standard therapy. Significant reductions in the HADS total score and depression subscales were greater in the curcuminoid group compared to the control group. Reductions in the BDI-II total score and somatic and cognitive subscale scores were also greater in the curcuminoid group compared to the control group. Although the results are promising, the study was not blinded, suggesting a possible interference with the results [304].
Table 1.
Clinical trials performed with curcumin on neurodegenerative conditions.
| Reference | Model/Country | Population | Intervention/Comparison | Outcomes | Side Effects |
|---|---|---|---|---|---|
| Sarcopenia | |||||
| [293] | Randomized, placebo-controlled, double-blind clinical trial. India |
30 healthy elderly individuals, 13♂, 17♀, 69.8 ± 5. | Participants received 500 mg/day of Cureit or placebo for 3 months. | ↑ 1.43% in handgrip strength, a considerable increase of 6.08% in weightlifting strength, and a positive impact on the distance covered before feeling tired (↑ 1.15%, along with speed walking (5.51 m)). | No adverse events were observed. |
| Parkinson’s disease | |||||
| [294] | Pilot, randomized, triple-blind, placebo-controlled, add-on trial. Iran | 60 subjects, 45♂, 15♀, 58.2 ± 11.2 y, with idiopathic PD | Subjects received curcumin nanomicelles in capsules 80 mg/day or placebo/9 months. Then, the scores MDS-UPDRS and PDQ-39 were calculated at 3, 6, and 9 months. | Curcumin group did not have a significant improvement in MDS-UPDRS and PDQ-39 scores compared to placebo group. | Nausea, vomiting, and dyspepsia. |
| Frailty | |||||
| [228] | Pilot, 12-week, randomized trial/United States of America | 17 subjects, 8♀, 9♂, 66–94 y, moderately functioning and sedentary, with low-grade systemic inflammation. |
9 subjects were assigned to Curcumin C3 Complex®, receiving 1000 mg/day or placebo. At 0 and at 12 weeks, patients underwent functional testing and lower-limb strength testing. Also, at the beginning of treatment, 4, 8, and 12 weeks, venous blood was collected for safety blood chemistry analyses and biomarkers of inflammation. | Curcumin C3 Complex® group demonstrated large effect sizes in short physical performance battery (d = 0.75), measures of knee extension (d = 0.69), and flexion peak torque (d = 0.82). Furthermore, effects on galectin-3 and IL-6 levels were smaller in curcumin group compared to placebo. | No adverse events were reported. |
| Dementia | |||||
| [295] | Randomized, double-blind, placebo-controlled parallel-group trial. Australia |
60 healthy subjects, 22♂, 38♀, 60–85 y. | Subjects were divided into curcumin group (80 m solid lipid formulation (Longvida® Curcumin-400 mg) or placebo/1 timeday/4 weeks. Participants performed 3 sets of computerized cognitive tasks preceded and followed by an evaluation of state mood. After the first set, a single treatment dose was used, and then the assessment was repeated at 1 h and 3 h after dose administration. | The results showed that 1 h after administration, the curcumin group presented significantly enhanced performance on sustained attention and working memory tasks, compared with placebo. Also, working memory and mood were significantly better during chronic treatment (4 weeks). Furthermore, curcumin significantly reduced total cholesterol and LDL cholesterol levels. | No adverse events were reported. |
| Alzheimer’s disease | |||||
| [296] | 12-week, 2 × 2 factorial, double-blinded, randomized controlled trial. Australia |
29 participants, 12♂, 17♀ (52.3 ± 1.9 y) at high risk of developing diabetes or with impaired fasting glucose | Participants were divided into 4 groups: the placebo; curcumin (2 × 500 mg of curcumin (Meriva®), providing 180 mg of curcumin plus 2 × 1000 mg of corn oil/day); ω3, 2 × 1000 mg of fish oil + placebo; or double active (1000 mg of curcumin (Meriva®) + 21,000 mg of fish oil. | Curcumin reduced triglyceride levels, fasting insulin, atherogenic index and the HOMA2-IR. There were no significant effects on CRP, TC, HDL-c, LDL-c, fasting glycemia, glycated hemoglobin, and body composition (body weight, muscle mass, body mass index, body fat percentage, circumference waist). | No adverse events were observed. |
| [305] | Randomized, double-blind, placebo-controlled for 12 months. Australia. |
160 healthy individuals; 40–90 y, and no significant cerebral vascular disease; no significant cognitive impairments. | They were randomly assigned to treatment groups with BCM-95 ® CG (Biocurcumax TM) capsule 3 x/day (1500 mg/d) or placebo. | No differences were observed between the placebo and treatment groups in changes in cognitive performance. | Gastrointestinal complaints. |
| [297] | Prospective randomized, 4 weeks. United States of America |
19 healthy participants 17♀, 2 ♂ age 40–60 y | The selected population was assigned to placebo interventions of starch × 80 mg/day of curcumin for 4 weeks | There were no significant effects on TC, LDL-c, HDL-c, superoxide dismutase, and glutathione peroxidase; significant reduction in the levels of TG, intercellular adhesion molecule, and plasma amyloid β protein content. Increased NO, myeloperoxidase, catalase activity, and elimination of free radicals. | No adverse events were observed. |
| [306] | Randomized, double-blind, placebo-controlled for 6 months. China. |
34 individuals 29%♂, 71%♀), aged 73.4 ± 8.8 (progressive decline in memory and cognitive function for at least 6 weeks or diagnosed with AD | They presented 3 groups, one consisting of 10 people (control), the second of 8 people (1 g of curcumin), and the third (4 g of curcumin). | There were no significant effects on the lipid profile (LDL-c, HDL-c, TG, and TC) in both groups receiving curcumin. | Constipation, more, diarrhea, and dizziness. |
| Cognition | |||||
| [299] | Randomized, 30-day, double-blind, placebo-controlled, 3-arm pilot study. India. | 18 healthy participants, 12♂ and 6♀, 35–65 y. | Patients were randomized into 3 groups, CGM (500 mg 2×/day for 30 days of curcuma-galactomannoside complex; UC (500 mg 2×/day for 30 days of curcumin with 95% purity) or placebo | CGM: significant ↑↓ in α and β waves, and in the α/β ratio compared to the unformulated curcumin and placebo groups. Furthermore, CGM showed a significant ↓ in audio reaction time (29.8) compared with placebo and 24.6% with UC. Choice-based visual reaction time was also significantly ↓ (36%) in CGM compared to UC and placebo, which yielded 15.36% and 5.2%, respectively. | No adverse events were reported. |
| [300] | Double-blind, placebo-controlled, 12-week trial/Australia. | 79 participants ♀ and ♂healthy, 50–85 y. | Participants were divided into curcumin group (400 mg Longvida© curcumin capsule with 80 mg of curcumin 1×/day/12 weeks) or placebo. | Curcumin group showed better working memory performance at 12 weeks (Serial Threes, Serial Sevens, and performance on a virtual Morris Water Maze) and lower fatigue scores on the POMS at 4 and 12 weeks, and tension, anger, confusion, and total mood disturbance in just 4 weeks. | No adverse events were reported. |
| [301] | 16-week double-blind, randomized placebo-controlled trial/Australia. | 152 older sedentary overweight/obese adults, 50–80 y. | Subjects were divided into 4 groups: fish oil + curcumin placebo, curcumin + fish oil placebo, fish oil + curcumin or placebo. Then, patients ingested 6 capsules/day consisting of 2 fish oil capsules and 400 mg Longvida® Optimised Curcumin containing 80 mg of curcumin, or placebo, 2×/d. Then, an evaluation of Transcranial Doppler ultrasound, blood, glycemia, heart rate, arterial compliance, blood lipids, and C-RP was performed. | Curcumin did not significantly affect the performed parameters alone or in combination with fish oil. | Digestive problems and reflux. |
| [307] | Randomized, double-blind, placebo-controlled pilot clinical trial/USA. | 12 participants 9♂ and 3♀ with chronic schizophrenia, 5–51 y. |
Patients were randomized into 2 groups: curcumin (180 mg/d) or placebo. A commercially available surface-controlled water-soluble form of 300 mg curcumin (30% formulation: 90 mg pure curcumin) or matching placebo capsules were provided. | Complementary curcumin treatment showed significant improvement in working memory (Z = 2200, p = 0.028) and reduced IL-6 levels (Z = 2402, p = 0.016) compared to placebo. No significant effect of curcumin on PANSS and Calgary Depression scores was found. | No adverse events were reported. |
| [302] | 18-month, randomized, double-blind, two-group parallel design | 40 adults without dementia, 22♀, 18♂ and 50–90 y. | Subjects were divided into placebo group or Theracurmin group (90 mg of curcumin), 2 ×/d/18 months. Depression Inventory and neuropsychological test battery were applied. | Buschke–Fuld Selective Reminding Test presented a consistent long-term retrieval improvement with curcumin (ES = 0.63, p = 0.002). Curcumin also improved visual memory and attention. | Transient abdominal pain, gastritis, nausea, and heat. |
| [303] | Randomized, 18-month, double-blind, placebo-controlled, parallel-group study. EUA | 40 participants, 51–84 y, without dementia. | They were randomized into 2 groups: Theracurmin group: 90 mg of curcumin, 2 times d 18 months or placebo group. | Curcumin significantly improved long-term recovery of SRT, visual memory, and attention compared with placebo. Assessment of neurodegeneration using PET scans significantly reduce in the amygdala with curcumin. | No adverse events were reported. |
| [304] | 6-week open study/Tehran, Iran. | 111 participants, ♀ and ♂ diagnosed with major depressive disorder | They were divided into standard antidepressant therapy + curcuminoids (1000 mg/d—C3 Complex®) or standard antidepressant therapy alone/6 weeks. | Both groups had a reduction in BDI-II total and subscale scores at the end of the study. Significantly greater ↓ in HADS, anxiety, and depression subscales in the curcuminoids versus control group (p < 0.001). | Gastrointestinal symptoms |
AD: Alzheimer’s disease; BDI-II, Beck Depression Inventory II; CRP: C-reactive protein; HADS: Hospital Anxiety and Depression Scale; HDL-c: high-density lipoprotein; HOMA: homeostatic model for insulin resistance; IL: interleukin; LDL-c: low-density lipoprotein; PANSS: Positive and Negative Symptom Scale; PD: Parkinson’s disease; PMS: Profile of Mood States; TC: total cholesterol. ↓: decrease; ↑: increase.
Table 2.
Descriptive table of the biases of the included randomized clinical trials.
| Study | Question Focus |
Allocation Blinding |
Double- Blind |
Losses (>20%) | Prognostic or Demographic Characteristics | Outcomes | Intention to Treat Analysis |
Sample Calculation |
Adequate Follow-Up |
|---|---|---|---|---|---|---|---|---|---|
| [293] | Yes | Yes | Yes | No | Yes | Yes | No | Yes | Yes |
| [294] | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| [228] | Yes | Yes | Yes | No | Yes | Yes | No | No | Yes |
| [295] | Yes | Yes | Yes | No | Yes | Yes | No | No | Yes |
| [296] | Yes | Yes | Yes | No | Yes | Yes | No | Yes | Yes |
| [305] | Yes | No | Yes | Yes | Yes | Yes | No | Yes | Yes |
| [297] | No | No | Yes | No | No | Yes | No | No | Yes |
| [306] | Yes | Yes | Yes | No | Yes | Yes | No | Yes | Yes |
| [299] | Yes | Yes | Yes | No | Yes | Yes | Yes | Yes | Yes |
| [300] | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| [301] | Yes | Yes | Yes | No | Yes | Yes | No | Yes | Yes |
| [307] | Yes | No | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| [302] | Yes | Yes | Yes | No | Yes | Yes | No | No | Yes |
| [303] | Yes | No | Yes | No | Yes | Yes | Yes | No | Yes |
| [304] | Yes | No | No | No | Yes | Yes | Yes | Yes | Yes |
5. Bioavailability and Safety
Curcumin’s bioavailability can be affected by many aspects, such as grinding, drying, and heating processes, and also by the intake of macronutrients, such as dietary lipids, which can interfere with curcumin’s solubility and absorption [308]. Its bioavailability is considered limited due to curcumin’s poor intestinal absorption, high metabolic rate, and fast systemic elimination, contributing to the compound’s low serum levels [309]. However, the development of curcumin and other compound combinations in different formulations enhanced its bioavailability. These formulations include curcumin nanoemulsion, liposomal curcumin, phospholipid curcumin complexes, and even curcumin encapsulation into milk exosomes, which showed higher permeability and bioavailability [20,61,309,310,311,312].
In a study involving male fasting subjects, curcumin was administered in three forms, a completely natural turmeric matrix formulation (CNTMF) and two other commercially available formulations consisting of curcumin with volatile oil and curcumin with phospholipids and cellulose. The analyses showed that the CNTMF form presented the most bioavailability of them [313]. Regarding dosage, in a randomized, double-blind, crossover study, subjects with moderate hyperlipidemia consumed 294 mg of curcuminoids per day in the form of micelles, and this dose was found to be enough to promote accumulation in the blood [314].
Curcumin presents a well-established human safety in the literature [315]. In a randomized and controlled clinical trial involving patients with arthritis, curcumin was administered in doses ranging from 120 to 1500 mg for 4–36 weeks, reduced inflammation and pain levels, and was shown to be a safe treatment method [316]. Also, in a clinical study comparing the administration of curcumin 500 mg (BCM-95®) 3 times/day and diclofenac 50 mg, 2 times/day for 28 days in patients with knee osteoarthritis, patients that received curcumin presented similar improvement in the severity of pain, reduction in flatulence episodes, no requirement of H2 blockers, weight reduction, and anti-ulcer effects, proving to be a very safe treatment method presenting a 13% adverse effect rate versus 38% in the diclofenac group (p < 0.01) [317].
Furthermore, in chronic kidney disease subjects, supplementation with 500 mg of curcumin tablets, two times/day for six months, reduced plasma pro-inflammatory mediators and lipid peroxidation. During the long-term administration treatment, no serious adverse events were observed, confirming the safety profile of this compound [318]. In addition, in patients with non-alcoholic fatty liver disease, phytosomal curcumin supplementation of 1000 mg/day in two doses for eight weeks was considered safe and well tolerated with no report of severe adverse events during the treatment [319].
6. Synthesis and Future Research Endeavors
In the ever-evolving landscape of aging-related disorder research, the exploration of natural compounds as potential therapeutic agents has garnered significant attention. Among these compounds, curcumin has emerged as a promising candidate due to its diverse pharmacological properties and well-documented safety profile. In this section, we delve into the future research directions and endeavors at the intersection of curcumin and aging-related disorders, encompassing a spectrum of scientific inquiries ranging from novel formulations and mechanistic studies to clinical trials and personalized medicine approaches. By elucidating the potential mechanisms of action, optimizing delivery systems, and translating preclinical findings into clinical practice, these endeavors aim to unlock the full therapeutic potential of curcumin in minimizing negative outcomes and improving patient quality of life [13,320,321,322].
6.1. Advancing Curcumin Therapy: Exploring Formulations and Unraveling Mechanisms for Aging-Related Disorders
Firstly, researchers must explore novel formulations aimed at enhancing the bioavailability and efficacy of curcumin. This necessitates investigating various delivery systems such as lipid-based nanoparticles, liposomes, or micelles. By delving into the intricacies of these formulations, researchers can unlock new avenues for optimizing the therapeutic potential of curcumin, potentially leading to breakthroughs in its clinical application. Understanding the dynamics of these delivery systems and their interactions with curcumin is crucial for overcoming the challenges associated with its poor solubility and low bioavailability. Through meticulous experimentation and analysis, researchers can pave the way for developing innovative curcumin formulations with enhanced efficacy and therapeutic outcomes.
Scientists also need to dive deeper into conducting thorough mechanistic investigations to gain a deeper understanding of the complex molecular pathways responsible for curcumin’s properties. This involves exploring how curcumin interacts with crucial proteins, signaling pathways, and cellular processes involved in neurodegeneration, cognition, memory, sarcopenia, fragility, and CVD. For instance, they might study more profoundly its effects on proteins like amyloid beta, tau, or BDNF, signaling pathways such as MAPK or NF-κB, and cellular processes like OS or inflammation within the realm of brain metabolism. By unraveling the precise mechanisms through which curcumin operates, researchers can gain profound insights into its therapeutic potential for such disorders. Employing advanced techniques such as proteomics, transcriptomics, and molecular imaging will be instrumental in dissecting the complex interplay between curcumin and various molecular targets within neuronal and other cells. These mechanistic studies are essential for advancing our understanding of curcumin’s neuroprotective properties and guiding the development of targeted therapeutic interventions aimed at combating diseases [323,324].
6.2. Unveiling Curcumin’s Therapeutic Potential: Insights from Meticulous Clinical Trials and Advanced Neuroimaging Studies in Neurodegenerative Disorders
In this scenario, the design and implementation of meticulously controlled clinical trials to assess the therapeutic efficacy of curcumin in preventing or treating diverse NDs, such as AD and PD, become of particular interest. By systematically evaluating the effects of curcumin on disease progression, cognitive function, motor symptoms, and quality of life outcomes, researchers can ascertain its true clinical potential in NDs. Moreover, incorporating biomarker assessments and neuroimaging techniques can supply crucial insights into the underlying mechanisms of curcumin’s therapeutic action in the human brain. Through collaborative efforts among clinicians, researchers, and pharmaceutical partners, well-designed clinical trials promise to establish curcumin as an effective and safe therapeutic agent for combating NDs.
In the realm of neuroimaging, researchers must delve more deeply into harnessing advanced neuroimaging techniques, such as functional magnetic resonance imaging (fMRI) and positron emission tomography (PET), to explore the impact of curcumin on both the structural and functional aspects of the brain in individuals afflicted with NDs [325,326]. By employing these sophisticated imaging modalities, researchers can visualize and quantify changes in brain activity, connectivity, and metabolism following curcumin administration. fMRI enables the assessment of dynamic alterations in neuronal activity patterns, while PET offers insights into molecular processes by tracking specific biomarkers associated with neurodegeneration. Through neuroimaging studies, researchers can elucidate how curcumin influences neural networks, neurotransmitter systems, and neuroinflammatory responses implicated in the pathogenesis of NDs. Furthermore, integrating neuroimaging data with clinical outcomes can facilitate the identification of biomarkers for predicting treatment response and monitoring disease progression [327,328,329].
6.3. Unlocking Synergistic Therapeutic Strategies: Exploring Curcumin Combinations and Molecular Interactions in Disease Management
Researchers should also explore the potential synergistic effects of curcumin associated with other natural compounds, pharmaceutical agents, or lifestyle interventions for managing aging-related diseases. Investigating these combination therapies offers a multifaceted approach to treatment, leveraging the complementary mechanisms of action of different compounds or interventions. Researchers can uncover novel strategies to enhance therapeutic outcomes while minimizing adverse effects by examining how curcumin interacts with other substances or interventions. Furthermore, exploring combination therapies underscores the importance of integrative approaches to healthcare, where traditional medicine intersects with modern pharmacology and lifestyle modifications. Through rigorous experimentation and clinical trials, researchers can elucidate the optimal combinations, dosages, and treatment regimens that maximize the therapeutic potential of curcumin in synergy with other interventions, ultimately offering hope for more effective management of NDs.
Additionally, future investigations could delve into elucidating the dynamic interplay between curcumin treatment and the intricate molecular networks underlying aging-related diseases employing cutting-edge bioinformatics tools. Using advanced network analysis and pathway modeling, researchers can explore the temporal and spatial effects of curcumin on multi-omics data. Additionally, integrating emerging technologies like single-cell sequencing could provide unprecedented insights into cell-specific responses to curcumin therapy. This comprehensive approach promises to uncover novel biomarkers and therapeutic targets essential for developing personalized interventions against NDs [330].
6.4. Fostering Collaboration for Curcumin Translation: Bridging Academia, Industry, Regulation, and Healthcare for Age-Related Disease Management
Efforts to apply promising preclinical findings into daily clinical practice necessitate collaborative endeavors among academia, industry, regulatory agencies, and healthcare providers [331,332]. By fostering robust partnerships, stakeholders can navigate regulatory frameworks, streamline clinical trials, and ensure curcumin-based interventions’ safe and effective implementation. Additionally, considering the cost-effectiveness of these interventions is crucial for widespread adoption. Balancing efficacy with affordability, this multidisciplinary approach aims to optimize therapeutic outcomes while minimizing economic burdens on patients and healthcare systems. By emphasizing patient-centered care, this strategy aims to enhance the quality of life for individuals afflicted by these debilitating conditions, ensuring equitable and sustainable access to innovative treatments.
6.5. Unraveling the Genetic Basis of Curcumin Response: Genome-Wide Association Studies in Aging-Related Disease Management
Genome-Wide Association Studies (GWASs) present a promising avenue for unraveling the genetic underpinnings of response to curcumin treatment in aging-related diseases such as NDs. By analyzing large cohorts of patients with diverse genetic backgrounds, GWAS can identify genetic variants associated with differential treatment outcomes, including variations in treatment efficacy and susceptibility to adverse effects [333]. Additionally, GWAS can elucidate gene–drug interactions that modulate curcumin metabolism, target engagement, and downstream biological responses.
A comprehensive GWAS in this realm could involve genotyping thousands of individuals with NDs who have undergone curcumin treatment alongside appropriate control groups. Integrating multi-omics data, such as genomic, transcriptomic, and epigenomic profiles, with clinical parameters and treatment responses can provide a holistic understanding of curcumin therapy’s genetic architecture. Furthermore, leveraging advanced bioinformatics and systems biology approaches, such as pathway enrichment analysis and network modeling, can uncover key biological pathways and candidate genes implicated in curcumin-mediated neuroprotection. These findings could inform the development of personalized treatment strategies and facilitate the identification of novel therapeutic targets for intervention.
However, conducting GWASs in this context poses several challenges, including the need for large, well-characterized patient cohorts, rigorous quality control measures to minimize confounding factors, and robust statistical methodologies to account for genetic heterogeneity and population stratification. Additionally, ethical considerations surrounding data privacy, consent, and equitable access to benefits necessitate careful deliberation and adherence to ethical guidelines throughout the study. Despite these challenges, GWASs hold immense potential to speed up the translation of preclinical findings into clinical practice, paving the way for precision medicine approaches tailored to individual genetic profiles and optimizing therapeutic outcomes for patients with NDs undergoing curcumin treatment [334].
7. Conclusions
This systematic review underscores the promising potential of curcumin as a natural compound with diverse therapeutic effects in combating age-related disorders. By targeting key pathways involved in inflammation, OS, and mitochondrial dysfunction, curcumin demonstrates significant benefits in improving cognitive function, reducing neurodegeneration, and enhancing muscle health in the elderly population. The review highlights the importance of further research to elucidate the specific mechanisms of action of curcumin and optimize its dosage and formulation for maximum efficacy. Standardized protocols and well-designed clinical trials are essential to validate the findings and establish curcumin as a safe and effective intervention for promoting healthy aging and preventing age-related conditions. Collaborative efforts among researchers, clinicians, and pharmaceutical partners are crucial in advancing our understanding of curcumin’s therapeutic potential and translating this knowledge into clinical practice. Overall, the evidence presented in this review supports the notion that curcumin holds promise as a valuable adjunct in the management of age-related disorders, offering a natural and potentially effective approach to enhancing the quality of life in aging individuals.
Acknowledgments
The authors attribute the scientific images that were used in this article to Smart Servier (https://smart.servier.com/, accessed on 26 January 2024), under an attribution license of public copyrights (https://creativecommons.org/licenses/by/3.0/, accessed on 26 January 2024) and under disclaimer of warranties. None of Smart Servier’s images were changed in the writing of this article.
Abbreviations
| AChE | acetylcholinesterase |
| AD | Alzheimer’s disease |
| AMPK | adenosine 5′-monophosphate-activated protein kinase |
| APP | amyloid precursor protein |
| APPsw | APP Swedish Mutant |
| Bax | Bcl-2-associated protein X |
| Bcl-2 | B-cell lymphoma 2 |
| Cdk5 | cyclin-dependent kinase 5 |
| CNTMF | completely natural turmeric matrix formulation |
| COX-2 | cyclooxygenase 2 |
| DM2 | diabetes mellitus type 2 |
| DNA | deoxyribonucleic acid |
| FeONPs-Cur | iron oxide nanoparticles capped with curcumin |
| GSK-3β | glycogen synthase kinase-3β |
| HO-1 | heme oxygenase-1 |
| IL | interleukin |
| JAK/STAT | Janus kinase/signal transducer and activator of transcription |
| JNK | c-Jun N-terminal kinase |
| Keap1 | Kelch-like ECH-associated protein 1 |
| MCT2 | monocarboxylate transporter 2 |
| mTOR | mammalian target of rapamycin |
| NADPH | nicotinamide adenine dinucleotide phosphate |
| NDs | neurodegenerative diseases |
| NF-κβ | nuclear factor-kappabeta |
| NO | nitric oxide |
| Nrf2 | nuclear factor erythroid 2-related factor 2 |
| OS | oxidative stress |
| PD | Parkinson’s disease |
| PI3K | phosphoinositide 3-kinases |
| PI3K/AKT | phosphatidylinositol 3-kinase/protein kinase B |
| PPARγ | peroxisome proliferator-activated receptor gamma |
| RAGE | glycation end products |
| RNS | reactive nitrogen species |
| ROS | oxygen species |
| p- | phosphorylated |
| PPAR-γ | peroxisome proliferator-activated receptor gamma |
| Th17 | T helper 17 |
| TNF | tumor necrosis factor |
| TNF-α | tumor necrosis factor-alpha |
| TLR | Toll-like receptor |
Author Contributions
Conceptualization, S.M.B. and M.J.S.M.; methodology, M.T., N.M.M., E.P.d.L., M.d.S.B. and V.C.S.C.; investigation, Y.C.N., C.B.L., A.C.C., A.C.A., N.M.M. and L.F.L.; writing—original draft preparation, Y.C.N., N.M.M., M.T., L.F.L. and S.M.B.; writing—review and editing, M.T., M.J.S.M. and S.M.B.; visualization, V.C.S.C., J.F.S.H., C.R.P.D. and C.B.L.; supervision, M.J.S.M., S.M.B. and M.T.; project administration, S.M.B. and M.J.S.M. All authors have read and agreed to the published version of the manuscript.
Conflicts of Interest
The authors declare no conflicts of interest.
Funding Statement
This work was supported by the HUN-REN Hungarian Research Network to M. Tanaka.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Cai Y., Song W., Li J., Jing Y., Liang C., Zhang L., Zhang X., Zhang W., Liu B., An Y., et al. The landscape of aging. Sci. China Life Sci. 2022;65:2354–2454. doi: 10.1007/s11427-022-2161-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baig J., Sawant N., Rawat P., Reddy A.P., Reddy P.H., Kshirsagar S. Abnormal interaction of Rlip with mutant APP/Abeta and phosphorylated tau reduces wild-type Rlip levels and disrupt Rlip function in Alzheimer’s disease. Biochim. Biophys. Acta Mol. Basis Dis. 2023;1870:166858. doi: 10.1016/j.bbadis.2023.166858. [DOI] [PubMed] [Google Scholar]
- 3.Chiu C.C., Cheng K.C., Lin Y.H., He C.X., Bow Y.D., Li C.Y., Wu C.Y., Wang H.D., Sheu S.J. Prolonged Exposure to High Glucose Induces Premature Senescence Through Oxidative Stress and Autophagy in Retinal Pigment Epithelial Cells. Arch. Immunol. Ther. Exp. 2023;71:21. doi: 10.1007/s00005-023-00686-9. [DOI] [PubMed] [Google Scholar]
- 4.Sheng Y., Zhu X., Wei L., Zou Y., Qi X., Shi R., Xu W., Wang X., Ding G., Duan Y. Aberrant expression of thyroidal hormone receptor α exasperating mitochondrial dysfunction induced sarcopenia in aged mice. Aging. 2024;16:7141–7152. doi: 10.18632/aging.205748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lagunas-Rangel F.A. SIRT7 in the aging process. Cell. Mol. Life Sci. 2022;79:297. doi: 10.1007/s00018-022-04342-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rosen R.S., Yarmush M.L. Current Trends in Anti-Aging Strategies. Annu. Rev. Biomed. Eng. 2023;25:363–385. doi: 10.1146/annurev-bioeng-120122-123054. [DOI] [PubMed] [Google Scholar]
- 7.Zamboni G., Maramotti R., Salemme S., Tondelli M., Adani G., Vinceti G., Carbone C., Filippini T., Vinceti M., Pagnoni G., et al. Age-specific prevalence of the different clinical presentations of AD and FTD in young-onset dementia. J. Neurol. 2024;271:4326–4335. doi: 10.1007/s00415-024-12364-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Guilbaud E., Sarosiek K.A., Galluzzi L. Inflammation and mitophagy are mitochondrial checkpoints to aging. Nat. Commun. 2024;15:3375. doi: 10.1038/s41467-024-47840-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tanaka M., Tuka B., Vécsei L. Navigating the Neurobiology of Migraine: From Pathways to Potential Therapies. Cells. 2024;13:1098. doi: 10.3390/cells13131098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ropert B., Gallrein C., Schumacher B. DNA repair deficiencies and neurodegeneration. DNA Repair. 2024;138:103679. doi: 10.1016/j.dnarep.2024.103679. [DOI] [PubMed] [Google Scholar]
- 11.McClarty B.M., Rodriguez G., Dong H. Class 1 histone deacetylases differentially modulate memory and synaptic genes in a spatial and temporal manner in aged and APP/PS1 mice. Brain Res. 2024;1837:148951. doi: 10.1016/j.brainres.2024.148951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kong L., Liu Y., Li J., Wang Y., Ji P., Shi Q., Han M., Xu H., Li W., Li W. Ginsenoside Rg1 alleviates chronic inflammation-induced neuronal ferroptosis and cognitive impairments via regulation of AIM2—Nrf2 signaling pathway. J. Ethnopharmacol. 2024;330:118205. doi: 10.1016/j.jep.2024.118205. [DOI] [PubMed] [Google Scholar]
- 13.Battaglia S., Avenanti A., Vécsei L., Tanaka M. Neurodegeneration in Cognitive Impairment and Mood Disorders for Experimental, Clinical and Translational Neuropsychiatry. Biomedicines. 2024;12:574. doi: 10.3390/biomedicines12030574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cho J., Higgason N., Rothman J., Safford M., Pinheiro L.C. “Should I Prioritize My Cancer or My Diabetes?”: Patient-Perceived Barriers to Co-Managing Cancer and Diabetes Mellitus. J. Cancer Educ. Off. J. Am. Assoc. Cancer Educ. 2024;39:437–444. doi: 10.1007/s13187-024-02425-w. [DOI] [PubMed] [Google Scholar]
- 15.Ottosson F., Engström G., Orho-Melander M., Melander O., Nilsson P.M., Johansson M. Plasma Metabolome Predicts Aortic Stiffness and Future Risk of Coronary Artery Disease and Mortality After 23 Years of Follow-Up in the General Population. J. Am. Heart Assoc. 2024;13:e033442. doi: 10.1161/JAHA.123.033442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Huang Z., Zhuang Y., Li W., Ma M., Lei F., Qu Y., Li J., Luo H., Li C., Lu L., et al. Apoptotic vesicles are required to repair DNA damage and suppress premature cellular senescence. J. Extracell. Vesicles. 2024;13:e12428. doi: 10.1002/jev2.12428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Peng L.N., Lin M.H., Tseng S.H., Yen K.H., Lee H.F., Hsiao F.Y., Chen L.K. Protein-enriched soup and weekly exercise improve muscle health: A randomized trial in mid-to-old age with inadequate protein intake. J. Cachexia Sarcopenia Muscle. 2024;4:1348–1357. doi: 10.1002/jcsm.13481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bults M., van Leersum C.M., Olthuis T.J.J., Siebrand E., Malik Z., Liu L., Miguel-Cruz A., Jukema J.S., den Ouden M.E.M. Acceptance of a Digital Assistant (Anne4Care) for Older Adult Immigrants Living With Dementia: Qualitative Descriptive Study. JMIR Aging. 2024;7:e50219. doi: 10.2196/50219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Karimi H., Mahdavi S., Moghaddam S.S., Abbasi-Kangevari M., Soleimani Z., Esfahani Z., Masinaei M., Fateh S.M., Golestani A., Dilmaghani-Marand A., et al. Unveiling the lead exposure attributed burden in Iran from 1990 to 2019 through the lens of the Global Burden of Disease study 2019. Sci. Rep. 2024;14:8688. doi: 10.1038/s41598-024-58823-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Laurindo L.F., de Carvalho G.M., de Oliveira Zanuso B., Figueira M.E., Direito R., de Alvares Goulart R., Buglio D.S., Barbalho S.M. Curcumin-Based Nanomedicines in the Treatment of Inflammatory and Immunomodulated Diseases: An Evidence-Based Comprehensive Review. Pharmaceutics. 2023;15:229. doi: 10.3390/pharmaceutics15010229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pagotto G.L.d.O., Santos L.M.O.d., Osman N., Lamas C.B., Laurindo L.F., Pomini K.T., Guissoni L.M., Lima E.P.d., Goulart R.d.A., Catharin V.M.S. Ginkgo biloba: A Leaf of Hope in the Fight against Alzheimer’s Dementia: Clinical Trial Systematic Review. Antioxidants. 2024;13:651. doi: 10.3390/antiox13060651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Valotto Neto L.J., Reverete de Araujo M., Moretti Junior R.C., Mendes Machado N., Joshi R.K., dos Santos Buglio D., Barbalho Lamas C., Direito R., Fornari Laurindo L., Tanaka M. Investigating the Neuroprotective and Cognitive-Enhancing Effects of Bacopa monnieri: A Systematic Review Focused on Inflammation, Oxidative Stress, Mitochondrial Dysfunction, and Apoptosis. Antioxidants. 2024;13:393. doi: 10.3390/antiox13040393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kasprzak-Drozd K., Oniszczuk T., Gancarz M., Kondracka A., Rusinek R., Oniszczuk A. Curcumin and Weight Loss: Does It Work? Int. J. Mol. Sci. 2022;23:639. doi: 10.3390/ijms23020639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Razavi B.M., Ghasemzadeh Rahbardar M., Hosseinzadeh H. A review of therapeutic potentials of turmeric (Curcuma longa) and its active constituent, curcumin, on inflammatory disorders, pain, and their related patents. Phytother. Res. 2021;35:6489–6513. doi: 10.1002/ptr.7224. [DOI] [PubMed] [Google Scholar]
- 25.Scazzocchio B., Minghetti L., D’Archivio M. Interaction between Gut Microbiota and Curcumin: A New Key of Understanding for the Health Effects of Curcumin. Nutrients. 2020;12:2499. doi: 10.3390/nu12092499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Moskwa J., Bronikowska M., Socha K., Markiewicz-Żukowska R. Vegetable as a Source of Bioactive Compounds with Photoprotective Properties: Implication in the Aging Process. Nutrients. 2023;15:3594. doi: 10.3390/nu15163594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ren Y., Qu S. Constituent isoflavones of Puerariae radix as a potential neuroprotector in cognitive impairment: Evidence from preclinical studies. Ageing Res. Rev. 2023;90:102040. doi: 10.1016/j.arr.2023.102040. [DOI] [PubMed] [Google Scholar]
- 28.Chainoglou E., Hadjipavlou-Litina D. Curcumin in Health and Diseases: Alzheimer’s Disease and Curcumin Analogues, Derivatives, and Hybrids. Int. J. Mol. Sci. 2020;21:1975. doi: 10.3390/ijms21061975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Budhathoki R., Timilsina A.P., Regmi B.P., Sharma K.R., Aryal N., Parajuli N. Metabolome Mining of Curcuma longa L. Using HPLC-MS/MS and Molecular Networking. Metabolites. 2023;13:898. doi: 10.3390/metabo13080898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cacciola N.A., Cuciniello R., Petillo G.D., Piccioni M., Filosa S., Crispi S. An Overview of the Enhanced Effects of Curcumin and Chemotherapeutic Agents in Combined Cancer Treatments. Int. J. Mol. Sci. 2023;24:12587. doi: 10.3390/ijms241612587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhi H.W., Jia Y.Z., Bo H.Q., Li H.T., Zhang S.S., Wang Y.H., Yang J., Hu M.Z., Wu H.Y., Cui W.Q., et al. Curcumin alleviates orofacial allodynia and improves cognitive impairment via regulating hippocampal synaptic plasticity in a mouse model of trigeminal neuralgia. Aging. 2023;15:4984. doi: 10.18632/aging.204984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Marton L.T., Barbalho S.M., Sloan K.P., Sloan L.A., Goulart R.A., Araújo A.C., Bechara M.D. Curcumin, autoimmune and inflammatory diseases: Going beyond conventional therapy—A systematic review. Crit. Rev. Food Sci. Nutr. 2022;62:2140–2157. doi: 10.1080/10408398.2020.1850417. [DOI] [PubMed] [Google Scholar]
- 33.Akaberi M., Sahebkar A., Emami S.A. Turmeric and Curcumin: From Traditional to Modern Medicine. Adv. Exp. Med. Biol. 2021;1291:15–39. doi: 10.1007/978-3-030-56153-6_2. [DOI] [PubMed] [Google Scholar]
- 34.Wong S.C., Kamarudin M.N.A., Naidu R. Anticancer Mechanism of Curcumin on Human Glioblastoma. Nutrients. 2021;13:950. doi: 10.3390/nu13030950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cunha Neto F., Marton L.T., de Marqui S.V., Lima T.A., Barbalho S.M. Curcuminoids from Curcuma longa: New adjuvants for the treatment of crohn’s disease and ulcerative colitis? Crit. Rev. Food Sci. Nutr. 2019;59:2136–2143. doi: 10.1080/10408398.2018.1456403. [DOI] [PubMed] [Google Scholar]
- 36.Mazieiro R., Frizon R.R., Barbalho S.M., Goulart R.A. Is Curcumin a Possibility to Treat Inflammatory Bowel Diseases? J. Med. Food. 2018;21:1077–1085. doi: 10.1089/jmf.2017.0146. [DOI] [PubMed] [Google Scholar]
- 37.Den Hartogh D.J., Gabriel A., Tsiani E. Antidiabetic Properties of Curcumin I: Evidence from In Vitro Studies. Nutrients. 2020;12:118. doi: 10.3390/nu12010118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Górka M., Białoń N., Bieczek D., Górka D. Neuroprotective effect of curcumin and its potential use in the treatment of neurodegenerative diseases. Postep. Biochem. 2023;69:18–25. doi: 10.18388/pb.2021_472. [DOI] [PubMed] [Google Scholar]
- 39.Jafari-Nozad A.M., Jafari A., Yousefi S., Bakhshi H., Farkhondeh T., Samarghandian S. Anti-gout and urate-lowering potentials of curcumin: A review from bench to beside. Curr. Med. Chem. 2023;31:3715–3732. doi: 10.2174/0929867331666230721154653. [DOI] [PubMed] [Google Scholar]
- 40.Boonla O., Kukongviriyapan U., Pakdeechote P., Kukongviriyapan V., Pannangpetch P., Prachaney P., Greenwald S.E. Curcumin improves endothelial dysfunction and vascular remodeling in 2K-1C hypertensive rats by raising nitric oxide availability and reducing oxidative stress. Nitric Oxide. 2014;42:44–53. doi: 10.1016/j.niox.2014.09.001. [DOI] [PubMed] [Google Scholar]
- 41.Izadi M., Sadri N., Abdi A., Zadeh M.M.R., Jalaei D., Ghazimoradi M.M., Shouri S., Tahmasebi S. Longevity and anti-aging effects of curcumin supplementation. GeroScience. 2024;46:2933–2950. doi: 10.1007/s11357-024-01092-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Xu J., Du P., Liu X., Xu X., Ge Y., Zhang C. Curcumin supplementation increases longevity and antioxidant capacity in Caenorhabditis elegans. Front. Pharmacol. 2023;14:1195490. doi: 10.3389/fphar.2023.1195490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kumar A., Prakash A., Dogra S. Protective effect of curcumin (Curcuma longa) against D-galactose-induced senescence in mice. J. Asian Nat. Prod. Res. 2011;13:42–55. doi: 10.1080/10286020.2010.544253. [DOI] [PubMed] [Google Scholar]
- 44.Lee J., Kim Y.S., Kim E., Kim Y., Kim Y. Curcumin and hesperetin attenuate D-galactose-induced brain senescence in vitro and in vivo. Nutr. Res. Pract. 2020;14:438–452. doi: 10.4162/nrp.2020.14.5.438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Li J.H., Wei T.T., Guo L., Cao J.H., Feng Y.K., Guo S.N., Liu G.H., Ding Y., Chai Y.R. Curcumin protects thymus against D-galactose-induced senescence in mice. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2021;394:411–420. doi: 10.1007/s00210-020-01945-8. [DOI] [PubMed] [Google Scholar]
- 46.Moher D., Liberati A., Tetzlaff J., Altman D.G., Group P. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA Statement. Open Med. 2009;3:e123–e130. [PMC free article] [PubMed] [Google Scholar]
- 47.Page M.J., McKenzie J.E., Bossuyt P.M., Boutron I., Hoffmann T.C., Mulrow C.D., Shamseer L., Tetzlaff J.M., Akl E.A., Brennan S.E., et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. doi: 10.1136/bmj.n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Higgins J.P., Thomas J., Chandler J., Cumpston M., Li T., Page M.J., Welch V.A. Cochrane Handbook for Systematic Reviews of Interventions. John Wiley & Sons; Hoboken, NJ, USA: 2019. [Google Scholar]
- 49.Peng Y., Ao M., Dong B., Jiang Y., Yu L., Chen Z., Hu C., Xu R. Anti-inflammatory effects of curcumin in the inflammatory diseases: Status, limitations and countermeasures. Drug Des. Dev. Ther. 2021;15:4503–4525. doi: 10.2147/DDDT.S327378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.de Lima E.P., Moretti R.C., Jr., Torres Pomini K., Laurindo L.F., Sloan K.P., Sloan L.A., Castro M.V.M., Baldi E., Jr., Ferraz B.F.R., de Souza Bastos Mazuqueli Pereira E., et al. Glycolipid Metabolic Disorders, Metainflammation, Oxidative Stress, and Cardiovascular Diseases: Unraveling Pathways. Biology. 2024;13:519. doi: 10.3390/biology13070519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bishayee A., Kavalakatt J., Sunkara C., Johnson O., Zinzuwadia S.S., Collignon T.E., Banerjee S., Barbalho S.M. Litchi (Litchi chinensis Sonn.): A comprehensive and critical review on cancer prevention and intervention. Food Chem. 2024;457:140142. doi: 10.1016/j.foodchem.2024.140142. [DOI] [PubMed] [Google Scholar]
- 52.Direito R., Barbalho S.M., Sepodes B., Figueira M.E. Plant-Derived Bioactive Compounds: Exploring Neuroprotective, Metabolic, and Hepatoprotective Effects for Health Promotion and Disease Prevention. Pharmaceutics. 2024;16:577. doi: 10.3390/pharmaceutics16050577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Barbalho S.M., Bueno Ottoboni A.M.M., Fiorini A.M.R., Guiguer E.L., Nicolau C.C.T., Goulart R.d.A., Flato U.A.P. Grape juice or wine: Which is the best option? Crit. Rev. Food Sci. Nutr. 2020;60:3876–3889. doi: 10.1080/10408398.2019.1710692. [DOI] [PubMed] [Google Scholar]
- 54.Bosso H., Barbalho S.M., de Alvares Goulart R., Otoboni A.M.M.B. Nutrition. Green coffee: Economic relevance and a systematic review of the effects on human health. Crit. Rev. Food Sci. Nutr. 2023;63:394–410. doi: 10.1080/10408398.2021.1948817. [DOI] [PubMed] [Google Scholar]
- 55.Laurindo L.F., Direito R., Bueno Otoboni A.M., Goulart R.A., Quesada K., Barbalho S.M. Grape processing waste: Effects on inflammatory bowel disease and colorectal cancer. Food Rev. Int. 2024;40:336–369. doi: 10.1080/87559129.2023.2168281. [DOI] [Google Scholar]
- 56.Derochette S., Franck T., Mouithys-Mickalad A., Ceusters J., Deby-Dupont G., Lejeune J.-P., Neven P., Serteyn D. Curcumin and resveratrol act by different ways on NADPH oxidase activity and reactive oxygen species produced by equine neutrophils. Chem. Biol. Interact. 2013;206:186–193. doi: 10.1016/j.cbi.2013.09.011. [DOI] [PubMed] [Google Scholar]
- 57.Cordero-Herrera I., Martín M.A., Goya L., Ramos S. Cocoa flavonoids protect hepatic cells against high-glucose-induced oxidative stress: Relevance of MAPKs. Mol. Nutr. Food Res. 2015;59:597–609. doi: 10.1002/mnfr.201400492. [DOI] [PubMed] [Google Scholar]
- 58.He Y., Yue Y., Zheng X., Zhang K., Chen S., Du Z. Curcumin, inflammation, and chronic diseases: How are they linked? Molecules. 2015;20:9183–9213. doi: 10.3390/molecules20059183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Pirunkaset E., Boonyarat C., Maneenet J., Khamphukdee C., Daodee S., Monthakantirat O., Awale S., Kijjoa A., Chulikhit Y. Effect of Diacetylcurcumin Manganese Complex on Rotenone-Induced Oxidative Stress, Mitochondria Dysfunction, and Inflammation in the SH-SY5Y Parkinson’s Disease Cell Model. Molecules. 2024;29:957. doi: 10.3390/molecules29050957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Nair B., Adithya J.K., Chandrababu G., Lakshmi P.K., Koshy J.J., Manoj S.V., Ambiliraj D.B., Vinod B.S., Sethi G., Nath L.R. Modulation of carcinogenesis with selected GRAS nutraceuticals via Keap1-Nrf2 signaling pathway. Phytother. Res. PTR. 2023;37:4398–4413. doi: 10.1002/ptr.7940. [DOI] [PubMed] [Google Scholar]
- 61.Matias J.N., Achete G., Campanari G., Guiguer É.L., Araújo A.C., Buglio D.S., Barbalho S.M. A systematic review of the antidepressant effects of curcumin: Beyond monoamines theory. Aust. N. Z. J. Psychiatry. 2021;55:451–462. doi: 10.1177/0004867421998795. [DOI] [PubMed] [Google Scholar]
- 62.Goulart R.A., Barbalho S.M., Lima V.M., Souza G.A., Matias J.N., Araújo A.C., Rubira C.J., Buchaim R.L., Buchaim D.V., Carvalho A.C.A., et al. Effects of the Use of Curcumin on Ulcerative Colitis and Crohn’s Disease: A Systematic Review. J. Med. Food. 2021;24:675–685. doi: 10.1089/jmf.2020.0129. [DOI] [PubMed] [Google Scholar]
- 63.Hu P., Li K., Peng X.-X., Kan Y., Yao T.-J., Wang Z.-Y., Li Z., Liu H.-Y., Cai D. Curcumin derived from medicinal homologous foods: Its main signals in immunoregulation of oxidative stress, inflammation, and apoptosis. Front. Immunol. 2023;14:1233652. doi: 10.3389/fimmu.2023.1233652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Cai Z., Wang W., Zhang Y., Zeng Y. Curcumin alleviates imiquimod-induced psoriasis-like inflammation and regulates gut microbiota of mice. Immun. Inflamm. Dis. 2023;11:e967. doi: 10.1002/iid3.967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Barbalho S.M., de Sousa Gonzaga H.F., de Souza G.A., de Alvares Goulart R., de Sousa Gonzaga M.L., de Alvarez Rezende B. Dermatological effects of Curcuma species: A systematic review. Clin. Exp. Dermatol. 2021;46:825–833. doi: 10.1111/ced.14584. [DOI] [PubMed] [Google Scholar]
- 66.Islam T., Koboziev I., Albracht-Schulte K., Mistretta B., Scoggin S., Yosofvand M., Moussa H., Zabet-Moghaddam M., Ramalingam L., Gunaratne P.H. Curcumin reduces adipose tissue inflammation and alters gut microbiota in diet-induced obese male mice. Mol. Nutr. Food Res. 2021;65:2100274. doi: 10.1002/mnfr.202100274. [DOI] [PubMed] [Google Scholar]
- 67.Gao J., Liu Y., Chen J., Tong C., Wang Q., Piao Y. Curcumin treatment attenuates cisplatin-induced gastric mucosal inflammation and apoptosis through the NF-κ B and MAPKs signaling pathway. Hum. Human. Exp. Toxicol. 2022;41:09603271221128738. doi: 10.1177/09603271221128738. [DOI] [PubMed] [Google Scholar]
- 68.Rathore A.S., Singh S.S., Birla H., Zahra W., Keshri P.K., Dilnashin H., Singh R., Singh S., Singh S.P. Curcumin Modulates p62-Keap1-Nrf2-Mediated Autophagy in Rotenone-Induced Parkinson’s Disease Mouse Models. ACS Chem. Neurosci. 2023;14:1412–1423. doi: 10.1021/acschemneuro.2c00706. [DOI] [PubMed] [Google Scholar]
- 69.Chauhan W., Zennadi R. Keap1-Nrf2 Heterodimer: A Therapeutic Target to Ameliorate Sickle Cell Disease. Antioxidants. 2023;12:740. doi: 10.3390/antiox12030740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Abdullah G., Akpan A., Phelan M.M., Wright H.L. New insights into healthy ageing, inflammageing and frailty using metabolomics. Front. Aging. 2024;5:1426436. doi: 10.3389/fragi.2024.1426436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Cianciulli A., Calvello R., Ruggiero M., Panaro M.A. Inflammaging and Brain: Curcumin and Its Beneficial Potential as Regulator of Microglia Activation. Molecules. 2022;27:341. doi: 10.3390/molecules27020341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kujundžić R.N., Stepanić V., Milković L., Gašparović A., Tomljanović M., Trošelj K.G. Curcumin and its Potential for Systemic Targeting of Inflamm-Aging and Metabolic Reprogramming in Cancer. Int. J. Mol. Sci. 2019;20:1180. doi: 10.3390/ijms20051180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Devita M., Debiasi G., Anglani M., Ceolin C., Mazzonetto I., Begliomini C., Cauzzo S., Raffaelli C., Lazzarin A., Ravelli A., et al. The Role of Cognitive Reserve in Protecting Cerebellar Volumes of Older Adults with mild Cognitive Impairment. Cerebellum. 2024:1–9. doi: 10.1007/s12311-024-01695-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Csiszar A., Ungvari A., Patai R., Gulej R., Yabluchanskiy A., Benyo Z., Kovacs I., Sotonyi P., Kirkpartrick A.C., Prodan C.I., et al. Atherosclerotic burden and cerebral small vessel disease: Exploring the link through microvascular aging and cerebral microhemorrhages. GeroScience. 2024:1–30. doi: 10.1007/s11357-024-01139-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Silva I.F.D., Bragante W.R., Junior R.C.M., Laurindo L.F., Guiguer E.L., Araújo A.C., Fiorini A.M.R., Nicolau C.C.T., Oshiiwa M., Lima E.P., et al. Effects of Smallanthus sonchifolius Flour on Metabolic Parameters: A Systematic Review. Pharmaceuticals. 2024;17:658. doi: 10.3390/ph17050658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Laurindo L.F., Rodrigues V.D., Minniti G., de Carvalho A.C.A., Zutin T.L.M., DeLiberto L.K., Bishayee A., Barbalho S.M. Pomegranate (Punica granatum L.) phytochemicals target the components of metabolic syndrome. J. Nutr. Biochem. 2024;131:109670. doi: 10.1016/j.jnutbio.2024.109670. [DOI] [PubMed] [Google Scholar]
- 77.Wen R., Huang X., Long J., Guo Y., Wei Y., Lin P., Xie S., Zhao Z., Zhang L., Fan A.Y., et al. Advances in traditional Chinese herbal medicine and their pharmacodynamic mechanisms in cancer immunoregulation: A narrative review. Transl. Cancer Res. 2024;13:1166–1187. doi: 10.21037/tcr-23-1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Minniti G., Laurindo L.F., Machado N.M., Duarte L.G., Guiguer E.L., Araujo A.C., Dias J.A., Lamas C.B., Nunes Y.C., Bechara M.D., et al. Mangifera indica L., By-Products, and Mangiferin on Cardio-Metabolic and Other Health Conditions: A Systematic Review. Life. 2023;13:2270. doi: 10.3390/life13122270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Nunes Y.C., Santos G.O., Machado N.M., Otoboni A., Laurindo L.F., Bishayee A., Fimognari C., Bishayee A., Barbalho S.M. Peanut (Arachis hypogaea L.) seeds and by-products in metabolic syndrome and cardiovascular disorders: A systematic review of clinical studies. Phytomedicine. 2024;123:155170. doi: 10.1016/j.phymed.2023.155170. [DOI] [PubMed] [Google Scholar]
- 80.Nishikito D.F., Borges A.C.A., Laurindo L.F., Otoboni A., Direito R., Goulart R.A., Nicolau C.C.T., Fiorini A.M.R., Sinatora R.V., Barbalho S.M. Anti-Inflammatory, Antioxidant, and Other Health Effects of Dragon Fruit and Potential Delivery Systems for Its Bioactive Compounds. Pharmaceutics. 2023;15:159. doi: 10.3390/pharmaceutics15010159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Guo X., Xin Q., Wei P., Hua Y., Zhang Y., Su Z., She G., Yuan R. Antioxidant and anti-aging activities of Longan crude and purified polysaccharide (LP-A) in nematode Caenorhabditis elegans. Int. J. Biol. Macromol. 2024;267:131634. doi: 10.1016/j.ijbiomac.2024.131634. [DOI] [PubMed] [Google Scholar]
- 82.Xu M., Wang W., Cheng J., Qu H., Xu M., Wang L. Effects of mitochondrial dysfunction on cellular function: Role in atherosclerosis. Biomed. Pharmacother. 2024;174:116587. doi: 10.1016/j.biopha.2024.116587. [DOI] [PubMed] [Google Scholar]
- 83.Kong J., Fan R., Zhang Y., Jia Z., Zhang J., Pan H., Wang Q. Oxidative stress in the brain-lung crosstalk: Cellular and molecular perspectives. Front. Aging Neurosci. 2024;16:1389454. doi: 10.3389/fnagi.2024.1389454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Tanaka M., Vécsei L. Monitoring the redox status in multiple sclerosis. Biomedicines. 2020;8:406. doi: 10.3390/biomedicines8100406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Stolp H.B., Solito E. Developmental priming of early cerebrovascular ageing: Implications across a lifetime. Int. J. Geriatr. Psychiatry. 2024;39:e6090. doi: 10.1002/gps.6090. [DOI] [PubMed] [Google Scholar]
- 86.Hirunsai M., Srikuea R. Differential effects of cholecalciferol and calcitriol on muscle proteolysis and oxidative stress in angiotensin II-induced C2C12 myotube atrophy. Physiol. Rep. 2024;12:e16011. doi: 10.14814/phy2.16011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Li S., Liu Y., Lu S., Xu J., Liu X., Yang D., Yang Y., Hou L., Li N. A crazy trio in Parkinson’s disease: Metabolism alteration, α-synuclein aggregation, and oxidative stress. Mol. Cell. Biochem. :2024. doi: 10.1007/s11010-024-04985-3. [DOI] [PubMed] [Google Scholar]
- 88.Duan D., Li H., Chai S., Zhang L., Fan T., Hu Z., Feng Y. The relationship between cardiac oxidative stress, inflammatory cytokine response, cardiac pump function, and prognosis post-myocardial infarction. Sci. Rep. 2024;14:8985. doi: 10.1038/s41598-024-59344-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Muhammad I., Khan A., Mustafa A., Elshikh M.S., Shen W. Elucidating the modulatory effect of melatonin on enzyme activity and oxidative stress in wheat: A global meta-analysis. Physiol. Plant. 2024;176:e14294. doi: 10.1111/ppl.14294. [DOI] [PubMed] [Google Scholar]
- 90.Ezim O.E., Nyeche J., Nebeolisa C.E., Belonwu C.D., Abarikwu S.O. Ascorbic acid attenuates gasoline-induced testicular toxicity, sperm quality deterioration, and testosterone imbalance in rats. Toxicol. Ind. Health. 2024;40:323–336. doi: 10.1177/07482337241245154. [DOI] [PubMed] [Google Scholar]
- 91.Fišar Z., Hroudová J. CoQ(10) and Mitochondrial Dysfunction in Alzheimer’s Disease. Antioxidants. 2024;13:191. doi: 10.3390/antiox13020191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Speers A.B., Wright K.M., Brandes M.S., Kedjejian N., Matthews D.G., Caruso M., Harris C.J., Koike S., Nguyen T., Quinn J.F., et al. Mode of administration influences plasma levels of active Centella asiatica compounds in 5xFAD mice while markers of neuroinflammation remain unaltered. Front. Neurosci. 2024;18:1277626. doi: 10.3389/fnins.2024.1277626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Ferrara F., Yan X., Pecorelli A., Guiotto A., Colella S., Pasqui A., Ivarrson J., Lynch S., Anderias S., Choundhary H., et al. Combined exposure to UV and PM affect skin oxinflammatory responses and it is prevented by antioxidant mix topical application: Evidences from clinical study. J. Cosmet. Dermatol. 2024;8:2644–2656. doi: 10.1111/jocd.16321. [DOI] [PubMed] [Google Scholar]
- 94.Novoselova E.G., Lunin S.M., Khrenov M.O., Glushkova O.V., Novoselova T.V., Parfenyuk S.B. Pancreas Β-Cells in Type 1 and Type 2 Diabetes: Cell Death, Oxidative Stress and Immune Regulation. Recently Appearing Changes in Diabetes Consequences. Cell. Physiol. Biochem. Int. J. Exp. Cell. Physiol. Biochem. Pharmacol. 2024;58:144–155. doi: 10.33594/000000690. [DOI] [PubMed] [Google Scholar]
- 95.Uysal F., Sukur G., Bozdemir N., Cinar O. Antioxidant supplementation may effect DNA methylation patterns, apoptosis, and ROS levels in developing mouse embryos. Histochem. Cell Biol. 2024:1–10. doi: 10.1007/s00418-024-02286-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Zia A., Farkhondeh T., Pourbagher-Shahri A.M., Samarghandian S. The role of curcumin in aging and senescence: Molecular mechanisms. Biomed. Pharmacother. 2021;134:111119. doi: 10.1016/j.biopha.2020.111119. [DOI] [PubMed] [Google Scholar]
- 97.Ren L., Zhan P., Wang Q., Wang C., Liu Y., Yu Z., Zhang S. Curcumin upregulates the Nrf2 system by repressing inflammatory signaling-mediated Keap1 expression in insulin-resistant conditions. Biochem. Biophys. Res. Commun. 2019;514:691–698. doi: 10.1016/j.bbrc.2019.05.010. [DOI] [PubMed] [Google Scholar]
- 98.Scuto M.C., Mancuso C., Tomasello B., Ontario M.L., Cavallaro A., Frasca F., Maiolino L., Salinaro A.T., Calabrese E.J., Calabrese V. Curcumin, hormesis and the nervous system. Nutrients. 2019;11:2417. doi: 10.3390/nu11102417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Méndez-García L.A., Martinez-Castillo M., Villegas-Sepúlveda N., Orozco L., Córdova E.J. Curcumin induces p53-independent inactivation of Nrf2 during oxidative stress–induced apoptosis. Hum. Human. Exp. Toxicol. 2019;38:951–961. doi: 10.1177/0960327119845035. [DOI] [PubMed] [Google Scholar]
- 100.Azzini E., Peña-Corona S.I., Hernández-Parra H., Chandran D., Saleena L.A.K., Sawikr Y., Peluso I., Dhumal S., Kumar M., Leyva-Gómez G., et al. Neuroprotective and anti-inflammatory effects of curcumin in Alzheimer’s disease: Targeting neuroinflammation strategies. Phytother. Res. 2024;38:3169–3189. doi: 10.1002/ptr.8200. [DOI] [PubMed] [Google Scholar]
- 101.Hou D., Liao H., Hao S., Liu R., Huang H., Duan C. Curcumin simultaneously improves mitochondrial dynamics and myocardial cell bioenergy after sepsis via the SIRT1-DRP1/PGC-1α pathway. Heliyon. 2024;10:e28501. doi: 10.1016/j.heliyon.2024.e28501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Osawa T., Kato Y. Protective role of antioxidative food factors in oxidative stress caused by hyperglycemia. Ann. N. Y. Acad. Sci. 2005;1043:440–451. doi: 10.1196/annals.1333.050. [DOI] [PubMed] [Google Scholar]
- 103.Ashrafizadeh M., Zarrabi A., Hushmandi K., Zarrin V., Moghadam E.R., Hashemi F., Makvandi P., Samarghandian S., Khan H., Hashemi F., et al. Toward Regulatory Effects of Curcumin on Transforming Growth Factor-Beta Across Different Diseases: A Review. Front. Pharmacol. 2020;11:585413. doi: 10.3389/fphar.2020.585413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Amalraj A., Pius A., Gopi S., Gopi S. Biological activities of curcuminoids, other biomolecules from turmeric and their derivatives—A review. J. Tradit. Complement. Med. 2017;7:205–233. doi: 10.1016/j.jtcme.2016.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Kocaadam B., Şanlier N. Curcumin, an active component of turmeric (Curcuma longa), and its effects on health. Crit. Rev. Food Sci. Nutr. 2017;57:2889–2895. doi: 10.1080/10408398.2015.1077195. [DOI] [PubMed] [Google Scholar]
- 106.Nazarian M., Aramjoo H., Roshanravan B., Samarghandian S., Farkhondeh T. Protective Effects of Curcumin and Nanomicelle Curcumin on Chlorpyrifos-induced Oxidative Damage and Inflammation in the Uterus, Ovary and Brain of Rats. Curr. Pharm. Biotechnol. :2024. doi: 10.2174/0113892010297408240319073735. [DOI] [PubMed] [Google Scholar]
- 107.Farkhondeh T., Zardast M., Rajabi S., Abdollahi-Karizno M., Roshanravan B., Havangi J., Aschner M., Samarghandian S. Neuroprotective Effects of Curcumin against Chronic ChlorpyrifosInduced Oxidative Damage in Rat Brain Tissue. Curr. Aging Sci. 2024:17. doi: 10.2174/0118746098244014240119112706. [DOI] [PubMed] [Google Scholar]
- 108.Zhang M.W., Sun X., Xu Y.W., Meng W., Tang Q., Gao H., Liu L., Chen S.H. Curcumin relieves oxaliplatin-induced neuropathic pain via reducing inflammation and activating antioxidant response. Cell Biol. Int. 2024;6:872–882. doi: 10.1002/cbin.12153. [DOI] [PubMed] [Google Scholar]
- 109.Sathyabhama M., Priya Dharshini L.C., Karthikeyan A., Kalaiselvi S., Min T. The credible role of curcumin in oxidative stress-mediated mitochondrial dysfunction in mammals. Biomolecules. 2022;12:1405. doi: 10.3390/biom12101405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Liu Y., Zeng J.M., Zhao H., Ao C.Y., Ao L.H., Ban J.Q., Li J. Mechanism of KAT2A regulation of H3K36ac in manganese-induced oxidative damage to mitochondria in the nervous system and intervention by curcumin. Ecotoxicol. Environ. Saf. 2024;273:116155. doi: 10.1016/j.ecoenv.2024.116155. [DOI] [PubMed] [Google Scholar]
- 111.Wang D., Yang Y., Zou X., Zheng Z., Zhang J. Curcumin ameliorates CKD-induced mitochondrial dysfunction and oxidative stress through inhibiting GSK-3β activity. J. Nutr. Biochem. 2020;83:108404. doi: 10.1016/j.jnutbio.2020.108404. [DOI] [PubMed] [Google Scholar]
- 112.Saghari Y., Movahedi M., Tebianian M., Entezari M. The Neuroprotective Effects of Curcumin Nanoparticles on The Cerebral Ischemia-Reperfusion Injury in The Rats-The Roles of The Protein Kinase RNA-Like ER Kinase/Extracellular Signal-Regulated Kinase and Transcription Factor EB proteins. Cell J. 2024;26:62–69. doi: 10.22074/cellj.2023.1995696.1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Wang X., Zhang Y., Yang Y., Zhang W., Luo L., Han F., Guan H., Tao K., Hu D. Curcumin pretreatment protects against hypoxia/reoxgenation injury via improvement of mitochondrial function, destabilization of HIF-1α and activation of Epac1-Akt pathway in rat bone marrow mesenchymal stem cells. Biomed. Pharmacother. 2019;109:1268–1275. doi: 10.1016/j.biopha.2018.11.005. [DOI] [PubMed] [Google Scholar]
- 114.Liu J., Hu S., Zhu B., Shao S., Yuan L. Grape seed procyanidin suppresses inflammation in cigarette smoke-exposed pulmonary arterial hypertension rats by the PPAR-γ/COX-2 pathway. Nutr. Metab. Cardiovasc. Dis. 2020;30:347–354. doi: 10.1016/j.numecd.2019.09.022. [DOI] [PubMed] [Google Scholar]
- 115.Kim Y., Lim J., Oh J. Taming neuroinflammation in Alzheimer’s disease: The protective role of phytochemicals through the gut-brain axis. Biomed. Pharmacother.=Biomed. Pharmacother. 2024;178:117277. doi: 10.1016/j.biopha.2024.117277. [DOI] [PubMed] [Google Scholar]
- 116.Chiu Y.J., Yang J.S., Tsai F.J., Chiu H.Y., Juan Y.N., Lo Y.H., Chiang J.H. Curcumin suppresses cell proliferation and triggers apoptosis in vemurafenib-resistant melanoma cells by downregulating the EGFR signaling pathway. Environ. Toxicol. 2022;37:868–879. doi: 10.1002/tox.23450. [DOI] [PubMed] [Google Scholar]
- 117.Petiti J., Rosso V., Lo Iacono M., Panuzzo C., Calabrese C., Signorino E., Pironi L., Cartellà A., Bracco E., Pergolizzi B., et al. Curcumin induces apoptosis in JAK2-mutated cells by the inhibition of JAK2/STAT and mTORC1 pathways. J. Cell. Mol. Med. 2019;23:4349–4357. doi: 10.1111/jcmm.14326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Ren B.C., Zhang Y.F., Liu S.S., Cheng X.J., Yang X., Cui X.G., Zhao X.R., Zhao H., Hao M.F., Li M.D., et al. Curcumin alleviates oxidative stress and inhibits apoptosis in diabetic cardiomyopathy via Sirt1-Foxo1 and PI3K-Akt signalling pathways. J. Cell. Mol. Med. 2020;24:12355–12367. doi: 10.1111/jcmm.15725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Zhu H., Wang X., Wang X., Liu B., Yuan Y., Zuo X. Curcumin attenuates inflammation and cell apoptosis through regulating NF-κB and JAK2/STAT3 signaling pathway against acute kidney injury. Cell Cycle. 2020;19:1941–1951. doi: 10.1080/15384101.2020.1784599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Souza P.V.S.d., Pinto W.B.V.d.R., Oliveira A.S.B. C9orf72-related disorders: Expanding the clinical and genetic spectrum of neurodegenerative diseases. Arq. Neuro-Psiquiatr. 2015;73:246–256. doi: 10.1590/0004-282X20140229. [DOI] [PubMed] [Google Scholar]
- 121.Bulle Oliveira A.S., Batista Pereira R.D. Amyotrophic Lateral Sclerosis (ALS): Three Letters That Change The People’s Life. Arq. Neuro-Psiquiatr. 2009;67:750–782. doi: 10.1590/S0004-282X2009000400040. [DOI] [PubMed] [Google Scholar]
- 122.Song J., Li J., Pei X., Chen J., Wang L. Identification of cuproptosis-realated key genes and pathways in Parkinson’s disease via bioinformatics analysis. PLoS ONE. 2024;19:e0299898. doi: 10.1371/journal.pone.0299898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Zhou Y., Sanchez V.B., Xu P., Roule T., Flores-Mendez M., Ciesielski B., Yoo D., Teshome H., Jimenez T., Liu S., et al. Altered lipid homeostasis is associated with cerebellar neurodegeneration in SNX14 deficiency. JCI Insight. 2024;9:e168594. doi: 10.1172/jci.insight.168594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Kovalová M., Gottfriedová N., Mrázková E., Janout V., Janoutová J. Cognitive impairment, neurodegenerative disorders, and olfactory impairment: A literature review. Otolaryngol. Pol. 2024;78:1–17. doi: 10.5604/01.3001.0053.6158. [DOI] [PubMed] [Google Scholar]
- 125.Bássoli R., Audi D., Ramalho B., Audi M., Quesada K., Barbalho S. The Effects of Curcumin on Neurodegenerative Diseases: A Systematic Review. J. Herb. Herbal. Med. 2023;42:100771. doi: 10.1016/j.hermed.2023.100771. [DOI] [Google Scholar]
- 126.Gunnarsson L.-G., Bodin L. Occupational exposures and neurodegenerative diseases—A systematic literature review and meta-analyses. Int. J. Environ. Res. Public. Health. 2019;16:337. doi: 10.3390/ijerph16030337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Rekatsina M., Paladini A., Piroli A., Zis P., Pergolizzi J.V., Varrassi G. Pathophysiology and therapeutic perspectives of oxidative stress and neurodegenerative diseases: A narrative review. Adv. Ther. 2020;37:113–139. doi: 10.1007/s12325-019-01148-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Katsnelson A., De Strooper B., Zoghbi H.Y. Neurodegeneration: From cellular concepts to clinical applications. Sci. Transl. Med. 2016;8:ps318–ps364. doi: 10.1126/scitranslmed.aal2074. [DOI] [PubMed] [Google Scholar]
- 129.Yadav S., Mansoori A., Sisodiya J. Current pathologic determinants of complex neurodegenerative diseases: A review. Int. J. Pharm. Technol. 2013;5:2607–2621. [Google Scholar]
- 130.Jette N., Maxwell C.J., Fiest K.M., Hogan D.B. Systematic reviews and meta-analyses of the incidence and prevalence of dementia and its commoner neurodegenerative causes. Can. J. Neurol. Sci. 2016;43:S1–S2. doi: 10.1017/cjn.2016.38. [DOI] [PubMed] [Google Scholar]
- 131.González H., Pacheco R. T-cell-mediated regulation of neuroinflammation involved in neurodegenerative diseases. J. Neuroinflammation. 2014;11:1–11. doi: 10.1186/s12974-014-0201-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Tanaka M., Chen C. Towards a mechanistic understanding of depression, anxiety, and their comorbidity: Perspectives from cognitive neuroscience. Front. Behav. Neurosci. 2023;17:1268156. doi: 10.3389/fnbeh.2023.1268156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Bagheri H., Ghasemi F., Barreto G.E., Rafiee R., Sathyapalan T., Sahebkar A. Effects of curcumin on mitochondria in neurodegenerative diseases. Biofactors. 2020;46:5–20. doi: 10.1002/biof.1566. [DOI] [PubMed] [Google Scholar]
- 134.Kobayashi H., Ohnishi T., Nakagawa R., Yoshizawa K. The comparative efficacy and safety of cholinesterase inhibitors in patients with mild-to-moderate Alzheimer’s disease: A Bayesian network meta-analysis. Int. J. Geriatr. Psychiatry. 2016;31:892–904. doi: 10.1002/gps.4405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Mehla J., Gupta P., Pahuja M., Diwan D., Diksha D. Indian medicinal herbs and formulations for Alzheimer’s disease, from traditional knowledge to scientific assessment. Brain Sci. 2020;10:964. doi: 10.3390/brainsci10120964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Popa-Wagner A., Dumitrascu D.I., Capitanescu B., Petcu E.B., Surugiu R., Fang W.-H., Dumbrava D.-A. Dietary habits, lifestyle factors and neurodegenerative diseases. Neural Regen. Res. 2020;15:394–400. doi: 10.4103/1673-5374.266045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Fasihi M., Samimi-Badabi M., Robat-Jazi B., Bitarafan S., Moghadasi A.N., Mansouri F., Yekaninejad M.S., Izad M., Saboor-Yaraghi A.A. Immunoregulatory Effects of the Active Form of Vitamin D (Calcitriol), Individually and in Combination with Curcumin, on Peripheral Blood Mononuclear Cells (PBMCs) of Multiple Sclerosis (MS) Patients. Anti-Inflamm. Anti-Allergy Agents Med. Chem. 2024;23 doi: 10.2174/0118715230293847240314073359. [DOI] [PubMed] [Google Scholar]
- 138.Chae J., Choi Y., Hong J., Kim N., Kim J., Lee H.Y., Choi J. Anticancer and Antibacterial Properties of Curcumin-Loaded Mannosylated Solid Lipid Nanoparticles for the Treatment of Lung Diseases. ACS Appl. Bio. Mater. 2024;7:2175–2185. doi: 10.1021/acsabm.3c01145. [DOI] [PubMed] [Google Scholar]
- 139.Guo J., Tang X., Deng P., Hui H., Chen B., An J., Zhang G., Shi K., Wang J., He Y., et al. Interleukin-4 from curcumin-activated OECs emerges as a central modulator for increasing M2 polarization of microglia/macrophage in OEC anti-inflammatory activity for functional repair of spinal cord injury. Cell Commun. Signal. CCS. 2024;22:162. doi: 10.1186/s12964-024-01539-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Harada C.N., Natelson Love M.C., Triebel K.L. Normal cognitive aging. Clin. Geriatr. Med. 2013;29:737–752. doi: 10.1016/j.cger.2013.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Bliss E.S., Wong R.H., Howe P.R., Mills D.E. Benefits of exercise training on cerebrovascular and cognitive function in ageing. J. Cereb. Blood Flow. Metab. 2021;41:447–470. doi: 10.1177/0271678X20957807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Ahmad M. Protective effects of curcumin against lithium-pilocarpine induced status epilepticus, cognitive dysfunction and oxidative stress in young rats. Saudi J. Biol. Sci. 2013;20:155–162. doi: 10.1016/j.sjbs.2013.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Sarker M.R., Franks S.F. Efficacy of curcumin for age-associated cognitive decline: A narrative review of preclinical and clinical studies. Geroscience. 2018;40:73–95. doi: 10.1007/s11357-018-0017-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Cho J.A., Park S.H., Cho J., Kim J.O., Yoon J.H., Park E. Exercise and Curcumin in Combination Improves Cognitive Function and Attenuates ER Stress in Diabetic Rats. Nutrients. 2020;12:1309. doi: 10.3390/nu12051309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Kodali M., Hattiangady B., Shetty G.A., Bates A., Shuai B., Shetty A.K. Curcumin treatment leads to better cognitive and mood function in a model of Gulf War Illness with enhanced neurogenesis, and alleviation of inflammation and mitochondrial dysfunction in the hippocampus. Brain Behav. Immun. 2018;69:499–514. doi: 10.1016/j.bbi.2018.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Grimm A., Eckert A. Brain aging and neurodegeneration: From a mitochondrial point of view. J. Neurochem. 2017;143:418–431. doi: 10.1111/jnc.14037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Ionescu-Tucker A., Cotman C.W. Emerging roles of oxidative stress in brain aging and Alzheimer’s disease. Neurobiol. Aging. 2021;107:86–95. doi: 10.1016/j.neurobiolaging.2021.07.014. [DOI] [PubMed] [Google Scholar]
- 148.Yi L.T., Dong S.Q., Wang S.S., Chen M., Li C.F., Geng D., Zhu J.X., Liu Q., Cheng J. Curcumin attenuates cognitive impairment by enhancing autophagy in chemotherapy. Neurobiol. Dis. 2020;136:104715. doi: 10.1016/j.nbd.2019.104715. [DOI] [PubMed] [Google Scholar]
- 149.Rueda N., Vidal V., García-Cerro S., Puente A., Campa V., Lantigua S., Narcís O., Bartesaghi R., Martínez-Cué C. Prenatal, but not Postnatal, Curcumin Administration Rescues Neuromorphological and Cognitive Alterations in Ts65Dn Down Syndrome Mice. J. Nutr. 2020;150:2478–2489. doi: 10.1093/jn/nxaa207. [DOI] [PubMed] [Google Scholar]
- 150.Noorafshan A., Abdollahifar M.A., Karbalay-Doust S., Asadi-Golshan R., Rashidian-Rashidabadi A. Protective effects of curcumin and sertraline on the behavioral changes in chronic variable stress-induced rats. Exp. Neurobiol. 2013;22:96–106. doi: 10.5607/en.2013.22.2.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Sorrenti V., Contarini G., Sut S., Dall’Acqua S., Confortin F., Pagetta A., Giusti P., Zusso M. Curcumin Prevents Acute Neuroinflammation and Long-Term Memory Impairment Induced by Systemic Lipopolysaccharide in Mice. Front. Pharmacol. 2018;9:183. doi: 10.3389/fphar.2018.00183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Vorhees C.V., Williams M.T. Tests for learning and memory in rodent regulatory studies. Curr. Res. Toxicol. 2024;6:100151. doi: 10.1016/j.crtox.2024.100151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Lang B., Kahnau P., Hohlbaum K., Mieske P., Andresen N.P., Boon M.N., Thöne-Reineke C., Lewejohann L., Diederich K. Challenges and advanced concepts for the assessment of learning and memory function in mice. Front. Behav. Neurosci. 2023;17:1230082. doi: 10.3389/fnbeh.2023.1230082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Denninger J.K., Smith B.M., Kirby E.D. Novel Object Recognition and Object Location Behavioral Testing in Mice on a Budget. J. Vis. Exp. 2018:141. doi: 10.3791/58593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Battaglia S., Avenanti A., Vécsei L., Tanaka M. Neural correlates and molecular mechanisms of memory and learning. Int. J. Mol. Sci. 2024;25:2724. doi: 10.3390/ijms25052724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Changlek S., Rana M.N., Phyu M.P., Karim N., Majima H.J., Tangpong J. Curcumin Suppresses Lead-Induced Inflammation and Memory Loss in Mouse Model and In Silico Molecular Docking. Foods. 2022;11:856. doi: 10.3390/foods11060856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Lu W.T., Sun S.Q., Li Y., Xu S.Y., Gan S.W., Xu J., Qiu G.P., Zhuo F., Huang S.Q., Jiang X.L., et al. Curcumin Ameliorates Memory Deficits by Enhancing Lactate Content and MCT2 Expression in APP/PS1 Transgenic Mouse Model of Alzheimer’s Disease. Anat. Rec. 2019;302:332–338. doi: 10.1002/ar.23969. [DOI] [PubMed] [Google Scholar]
- 158.Sarlak Z., Oryan S., Moghaddasi M. Interaction between the antioxidant activity of curcumin and cholinergic system on memory retention in adult male Wistar rats. Iran. J. Basic. Med. Sci. 2015;18:398–403. [PMC free article] [PubMed] [Google Scholar]
- 159.Ikram M., Saeed K., Khan A., Muhammad T., Khan M.S., Jo M.G., Rehman S.U., Kim M.O. Natural Dietary Supplementation of Curcumin Protects Mice Brains against Ethanol-Induced Oxidative Stress-Mediated Neurodegeneration and Memory Impairment via Nrf2/TLR4/RAGE Signaling. Nutrients. 2019;11:1082. doi: 10.3390/nu11051082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Zhang L., Fang Y., Xu Y., Lian Y., Xie N., Wu T., Zhang H., Sun L., Zhang R., Wang Z. Curcumin Improves Amyloid β-Peptide (1–42) Induced Spatial Memory Deficits through BDNF-ERK Signaling Pathway. PLoS ONE. 2015;10:e0131525. doi: 10.1371/journal.pone.0131525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Gouras G.K., Olsson T.T., Hansson O. β-Amyloid peptides and amyloid plaques in Alzheimer’s disease. Neurotherapeutics. 2015;12:3–11. doi: 10.1007/s13311-014-0313-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Serrano-Pozo A., Frosch M.P., Masliah E., Hyman B.T. Neuropathological alterations in Alzheimer disease. Cold Spring Harb. Perspect. Med. 2011;1:a006189. doi: 10.1101/cshperspect.a006189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Metaxas A., Kempf S.J. Neurofibrillary tangles in Alzheimer’s disease: Elucidation of the molecular mechanism by immunohistochemistry and tau protein phospho-proteomics. Neural Regen. Res. 2016;11:1579–1581. doi: 10.4103/1673-5374.193234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Minter M.R., Taylor J.M., Crack P.J. The contribution of neuroinflammation to amyloid toxicity in Alzheimer’s disease. J. Neurochem. 2016;136:457–474. doi: 10.1111/jnc.13411. [DOI] [PubMed] [Google Scholar]
- 165.Trejo-Lopez J.A., Yachnis A.T., Prokop S. Neuropathology of Alzheimer’s Disease. Neurotherapeutics. 2022;19:173–185. doi: 10.1007/s13311-021-01146-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Domingues C., da Cruz E., Silva O.A.B., Henriques A.G. Impact of Cytokines and Chemokines on Alzheimer’s Disease Neuropathological Hallmarks. Curr. Alzheimer Res. 2017;14:870–882. doi: 10.2174/1567205014666170317113606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Al-Ghraiybah N.F., Wang J., Alkhalifa A.E., Roberts A.B., Raj R., Yang E., Kaddoumi A. Glial Cell-Mediated Neuroinflammation in Alzheimer’s Disease. Int. J. Mol. Sci. 2022;23:10572. doi: 10.3390/ijms231810572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Twarowski B., Herbet M. Inflammatory Processes in Alzheimer’s Disease-Pathomechanism, Diagnosis and Treatment: A Review. Int. J. Mol. Sci. 2023;24:6518. doi: 10.3390/ijms24076518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.González-Reyes R.E., Nava-Mesa M.O., Vargas-Sánchez K., Ariza-Salamanca D., Mora-Muñoz L. Involvement of Astrocytes in Alzheimer’s Disease from a Neuroinflammatory and Oxidative Stress Perspective. Front. Mol. Neurosci. 2017;10:427. doi: 10.3389/fnmol.2017.00427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Ono K., Hasegawa K., Naiki H., Yamada M. Curcumin has potent anti-amyloidogenic effects for Alzheimer’s beta-amyloid fibrils in vitro. J. Neurosci. Res. 2004;75:742–750. doi: 10.1002/jnr.20025. [DOI] [PubMed] [Google Scholar]
- 171.Lim G.P., Chu T., Yang F., Beech W., Frautschy S.A., Cole G.M. The curry spice curcumin reduces oxidative damage and amyloid pathology in an Alzheimer transgenic mouse. J. Neurosci. 2001;21:8370–8377. doi: 10.1523/JNEUROSCI.21-21-08370.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Hoppe J.B., Coradini K., Frozza R.L., Oliveira C.M., Meneghetti A.B., Bernardi A., Pires E.S., Beck R.C., Salbego C.G. Free and nanoencapsulated curcumin suppress β-amyloid-induced cognitive impairments in rats: Involvement of BDNF and Akt/GSK-3β signaling pathway. Neurobiol. Learn. Mem. 2013;106:134–144. doi: 10.1016/j.nlm.2013.08.001. [DOI] [PubMed] [Google Scholar]
- 173.Garcia-Alloza M., Borrelli L.A., Rozkalne A., Hyman B.T., Bacskai B.J. Curcumin labels amyloid pathology in vivo, disrupts existing plaques, and partially restores distorted neurites in an Alzheimer mouse model. J. Neurochem. 2007;102:1095–1104. doi: 10.1111/j.1471-4159.2007.04613.x. [DOI] [PubMed] [Google Scholar]
- 174.Goozee K.G., Shah T.M., Sohrabi H.R., Rainey-Smith S.R., Brown B., Verdile G., Martins R.N. Examining the potential clinical value of curcumin in the prevention and diagnosis of Alzheimer’s disease. Br. J. Nutr. 2016;115:449–465. doi: 10.1017/S0007114515004687. [DOI] [PubMed] [Google Scholar]
- 175.Tiwari S.K., Agarwal S., Seth B., Yadav A., Nair S., Bhatnagar P., Karmakar M., Kumari M., Chauhan L.K., Patel D.K., et al. Curcumin-loaded nanoparticles potently induce adult neurogenesis and reverse cognitive deficits in Alzheimer’s disease model via canonical Wnt/β-catenin pathway. ACS Nano. 2014;8:76–103. doi: 10.1021/nn405077y. [DOI] [PubMed] [Google Scholar]
- 176.Martos D., Lőrinczi B., Szatmári I., Vécsei L., Tanaka M. The Impact of C-3 Side Chain Modifications on Kynurenic Acid: A Behavioral Analysis of Its Analogs in the Motor Domain. Int. J. Mol. Sci. 2024;25:3394. doi: 10.3390/ijms25063394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Tanaka M., Bohár Z., Vécsei L. Are kynurenines accomplices or principal villains in dementia? Maintenance of kynurenine metabolism. Molecules. 2020;25:564. doi: 10.3390/molecules25030564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Yang F., Lim G.P., Begum A.N., Ubeda O.J., Simmons M.R., Ambegaokar S.S., Chen P.P., Kayed R., Glabe C.G., Frautschy S.A., et al. Curcumin inhibits formation of amyloid beta oligomers and fibrils, binds plaques, and reduces amyloid in vivo. J. Biol. Chem. 2005;280:5892–5901. doi: 10.1074/jbc.M404751200. [DOI] [PubMed] [Google Scholar]
- 179.Pan R., Qiu S., Lu D.X., Dong J. Curcumin improves learning and memory ability and its neuroprotective mechanism in mice. Chin. Med. J. 2008;121:832–839. doi: 10.1097/00029330-200805010-00015. [DOI] [PubMed] [Google Scholar]
- 180.Xie Y., Zhao Q.Y., Li H.Y., Zhou X., Liu Y., Zhang H. Curcumin ameliorates cognitive deficits heavy ion irradiation-induced learning and memory deficits through enhancing of Nrf2 antioxidant signaling pathways. Pharmacol. Biochem. Behav. 2014;126:181–186. doi: 10.1016/j.pbb.2014.08.005. [DOI] [PubMed] [Google Scholar]
- 181.Hacioglu C., Kar F., Kar E., Kara Y., Kanbak G. Effects of Curcumin and Boric Acid Against Neurodegenerative Damage Induced by Amyloid Beta (1–42) Biol. Trace Elem. Res. 2021;199:3793–3800. doi: 10.1007/s12011-020-02511-2. [DOI] [PubMed] [Google Scholar]
- 182.Ahlijanian M.K., Barrezueta N.X., Williams R.D., Jakowski A., Kowsz K.P., McCarthy S., Coskran T., Carlo A., Seymour P.A., Burkhardt J.E., et al. Hyperphosphorylated tau and neurofilament and cytoskeletal disruptions in mice overexpressing human p25, an activator of cdk5. Proc. Natl. Acad. Sci. USA. 2000;97:2910–2915. doi: 10.1073/pnas.040577797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Chow H.M., Guo D., Zhou J.C., Zhang G.Y., Li H.F., Herrup K., Zhang J. CDK5 activator protein p25 preferentially binds and activates GSK3β. Proc. Natl. Acad. Sci. USA. 2014;111:E4887–E4895. doi: 10.1073/pnas.1402627111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Alamro A.A., Alsulami E.A., Almutlaq M., Alghamedi A., Alokail M., Haq S.H. Therapeutic Potential of Vitamin D and Curcumin in an. J. Cent. Nerv. Syst. Dis. 2020;12:1179573520924311. doi: 10.1177/1179573520924311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Das T.K., Jana P., Chakrabarti S.K., Abdul Hamid M.R.W. Curcumin Downregulates GSK3 and Cdk5 in Scopolamine-Induced Alzheimer’s Disease Rats Abrogating Aβ. J. Alzheimers Dis. Rep. 2019;3:257–267. doi: 10.3233/ADR-190135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Li Y., Li J., Li S., Wang X., Liu B., Fu Q., Ma S. Curcumin attenuates glutamate neurotoxicity in the hippocampus by suppression of ER stress-associated TXNIP/NLRP3 inflammasome activation in a manner dependent on AMPK. Toxicol. Appl. Pharmacol. 2015;286:53–63. doi: 10.1016/j.taap.2015.03.010. [DOI] [PubMed] [Google Scholar]
- 187.Zhou J., Wu N., Lin L. Curcumin Suppresses Apoptosis and Inflammation in Hypoxia/Reperfusion-Exposed Neurons via Wnt Signaling Pathway. Med. Sci. Monit. 2020;26:e920445. doi: 10.12659/MSM.920445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Park J.S., Lee W.K., Kim H.S., Seo J.A., Kim D.H., Han H.C., Min B.H. Clusterin overexpression protects against western diet-induced obesity and NAFLD. Sci. Rep. 2020;10:17484. doi: 10.1038/s41598-020-73927-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Shao S., Ye X., Su W., Wang Y. Curcumin alleviates Alzheimer’s disease by inhibiting inflammatory response, oxidative stress and activating the AMPK pathway. J. Chem. Neuroanat. 2023;134:102363. doi: 10.1016/j.jchemneu.2023.102363. [DOI] [PubMed] [Google Scholar]
- 190.Yavarpour-Bali H., Ghasemi-Kasman M., Pirzadeh M. Curcumin-loaded nanoparticles: A novel therapeutic strategy in treatment of central nervous system disorders. Int. J. Nanomed. 2019;14:4449–4460. doi: 10.2147/IJN.S208332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Panzarini E., Mariano S., Tacconi S., Carata E., Tata A.M., Dini L. Novel Therapeutic Delivery of Nanocurcumin in Central Nervous System Related Disorders. Nanomaterials. 2020;11:2. doi: 10.3390/nano11010002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Ruan Y., Xiong Y., Fang W., Yu Q., Mai Y., Cao Z., Wang K., Lei M., Xu J., Liu Y., et al. Highly sensitive Curcumin-conjugated nanotheranostic platform for detecting amyloid-beta plaques by magnetic resonance imaging and reversing cognitive deficits of Alzheimer’s disease via NLRP3-inhibition. J. Nanobiotechnol. 2022;20:322. doi: 10.1186/s12951-022-01524-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Cheng K.K., Yeung C.F., Ho S.W., Chow S.F., Chow A.H., Baum L. Highly stabilized curcumin nanoparticles tested in an in vitro blood-brain barrier model and in Alzheimer’s disease Tg2576 mice. AAPS J. 2013;15:324–336. doi: 10.1208/s12248-012-9444-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Lee D.-Y., Chun Y.-S., Kim J.-K., Lee J.-O., Ku S.-K., Shim S.-M. Curcumin attenuates sarcopenia in chronic forced exercise executed aged mice by regulating muscle degradation and protein synthesis with antioxidant and anti-inflammatory effects. J. Agric. Food Chem. 2021;69:6214–6228. doi: 10.1021/acs.jafc.1c00699. [DOI] [PubMed] [Google Scholar]
- 195.Miller D.B., O’Callaghan J.P. Biomarkers of Parkinson’s disease: Present and future. Metabolism. 2015;64:S40–S46. doi: 10.1016/j.metabol.2014.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Cabreira V., Massano J. Doença de Parkinson: Revisão clínica e atualização [Parkinson’s disease: Clinical review and update] Acta Med. Port. 2019;32:661–670. doi: 10.20344/amp.11978. [DOI] [PubMed] [Google Scholar]
- 197.Tanaka M., Vécsei L. Revolutionizing our understanding of Parkinson’s disease: Dr. Heinz Reichmann’s pioneering research and future research direction. J. Neural. Transm. 2024 doi: 10.1007/s00702-024-02812-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Buglio D.S., Marton L.T., Laurindo L.F., Guiguer E.L., Araújo A.C., Buchaim R.L., Goulart R.A., Rubira C.J., Barbalho S.M. The Role of Resveratrol in Mild Cognitive Impairment and Alzheimer’s Disease: A Systematic Review. J. Med. Food. 2022;25:797–806. doi: 10.1089/jmf.2021.0084. [DOI] [PubMed] [Google Scholar]
- 199.de Oliveira Zanuso B., de Oliveira Dos Santos A.R., Miola V.F.B., Guissoni Campos L.M., Spilla C.S.G., Barbalho S.M. Panax ginseng and aging related disorders: A systematic review. Exp. Gerontol. 2022;161:111731. doi: 10.1016/j.exger.2022.111731. [DOI] [PubMed] [Google Scholar]
- 200.Solleiro-Villavicencio H., Rivas-Arancibia S. Effect of chronic oxidative stress on neuroinflammatory response mediated by CD4+ T cells in neurodegenerative diseases. Front. Cell. Neurosci. 2018;12:114. doi: 10.3389/fncel.2018.00114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Puspita L., Chung S.Y., Shim J.-w. Oxidative stress and cellular pathologies in Parkinson’s disease. Mol. Brain. 2017;10:53. doi: 10.1186/s13041-017-0340-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Martinez B., Peplow P.V. Neuroprotection by immunomodulatory agents in animal models of Parkinson’s disease. Neural Regen. Res. 2018;13:1493–1506. doi: 10.4103/1673-5374.237108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Achete de Souza G., de Marqui S.V., Matias J.N., Guiguer E.L., Barbalho S.M. Effects of Ginkgo biloba on Diseases Related to Oxidative Stress. Planta Medica. 2020;86:376–386. doi: 10.1055/a-1109-3405. [DOI] [PubMed] [Google Scholar]
- 204.Barbalho S.M., Direito R., Laurindo L.F., Marton L.T., Guiguer E.L., Goulart R.A., Tofano R.J., Carvalho A.C.A., Flato U.A.P., Capelluppi Tofano V.A., et al. Ginkgo biloba in the Aging Process: A Narrative Review. Antioxidants. 2022;11:525. doi: 10.3390/antiox11030525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Dhouib I.B., Annabi A., Doghri R., Rejeb I., Dallagi Y., Bdiri Y., Lasram M.M., Elgaaied A., Marrakchi R., Fazaa S. Neuroprotective effects of curcumin against acetamiprid-induced neurotoxicity and oxidative stress in the developing male rat cerebellum: Biochemical, histological, and behavioral changes. Environ. Sci. Pollut. Res. 2017;24:27515–27524. doi: 10.1007/s11356-017-0331-5. [DOI] [PubMed] [Google Scholar]
- 206.Mamun A.A., Shao C., Geng P., Wang S., Xiao J. Polyphenols Targeting NF-κB Pathway in Neurological Disorders: What We Know So Far? Int. J. Biol. Sci. 2024;20:1332–1355. doi: 10.7150/ijbs.90982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Reglodi D., Renaud J., Tamas A., Tizabi Y., Socías S.B., Del-Bel E., Raisman-Vozari R. Novel tactics for neuroprotection in Parkinson’s disease: Role of antibiotics, polyphenols and neuropeptides. Prog. Progress. Neurobiol. 2017;155:120–148. doi: 10.1016/j.pneurobio.2015.10.004. [DOI] [PubMed] [Google Scholar]
- 208.Bhat A., Mahalakshmi A.M., Ray B., Tuladhar S., Hediyal T.A., Manthiannem E., Padamati J., Chandra R., Chidambaram S.B., Sakharkar M.K. Benefits of curcumin in brain disorders. BioFactors. 2019;45:666–689. doi: 10.1002/biof.1533. [DOI] [PubMed] [Google Scholar]
- 209.Sharma N., Nehru B.J.I. Curcumin affords neuroprotection and inhibits α-synuclein aggregation in lipopolysaccharide-induced Parkinson’s disease model. Inflammopharmacology. 2018;26:349–360. doi: 10.1007/s10787-017-0402-8. [DOI] [PubMed] [Google Scholar]
- 210.Khadrawy Y.A., Hosny E.N., Eldein Mohamed H.S. Assessment of the neuroprotective effect of green synthesized iron oxide nanoparticles capped with curcumin against a rat model of Parkinson’s disease. Iran. J. Basic. Med. Sci. 2024;27:81–89. doi: 10.22038/ijbms.2023.73124.15892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Bandeen-Roche K., Seplaki C.L., Huang J., Buta B., Kalyani R.R., Varadhan R., Xue Q.-L., Walston J.D., Kasper J.D. Frailty in older adults: A nationally representative profile in the United States. J. Gerontol. Ser. A Biomed. Sci. Med. Sci. 2015;70:1427–1434. doi: 10.1093/gerona/glv133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Proietti M., Cesari M. Frailty and Cardiovascular Diseases. Springer; Cham, Switzerland: 2020. Frailty: What is it? pp. 1–7. Advances in Experimental Medicine and Biology. [Google Scholar]
- 213.Cesari M., Calvani R., Marzetti E. Frailty in older persons. Clin. Geriatr. Med. 2017;33:293–303. doi: 10.1016/j.cger.2017.02.002. [DOI] [PubMed] [Google Scholar]
- 214.Asavamongkolkul A., Adulkasem N., Chotiyarnwong P., Vanitcharoenkul E., Chandhanayingyong C., Laohaprasitiporn P., Soparat K., Unnanuntana A. Prevalence of osteoporosis, sarcopenia, and high falls risk in healthy community-dwelling Thai older adults: A nationwide cross-sectional study. JBMR Plus. 2024;8:ziad020. doi: 10.1093/jbmrpl/ziad020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Hoogendijk E.O., Afilalo J., Ensrud K.E., Kowal P., Onder G., Fried L.P. Frailty: Implications for clinical practice and public health. Lancet. 2019;394:1365–1375. doi: 10.1016/S0140-6736(19)31786-6. [DOI] [PubMed] [Google Scholar]
- 216.Bandeen-Roche K., Xue Q.-L., Ferrucci L., Walston J., Guralnik J.M., Chaves P., Zeger S.L., Fried L.P. Phenotype of frailty: Characterization in the women’s health and aging studies. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2006;61:262–266. doi: 10.1093/gerona/61.3.262. [DOI] [PubMed] [Google Scholar]
- 217.Xue Q.-L., Walston J.D., Fried L.P., Beamer B.A. Prediction of risk of falling, physical disability, and frailty by rate of decline in grip strength: The women’s health and aging study. Arch. Intern. Med. 2011;171:1119–1121. doi: 10.1001/archinternmed.2011.252. [DOI] [PubMed] [Google Scholar]
- 218.Ensrud K.E., Ewing S.K., Taylor B.C., Fink H.A., Stone K.L., Cauley J.A., Tracy J.K., Hochberg M.C., Rodondi N., Cawthon P.M. Frailty and risk of falls, fracture, and mortality in older women: The study of osteoporotic fractures. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2007;62:744–751. doi: 10.1093/gerona/62.7.744. [DOI] [PubMed] [Google Scholar]
- 219.Makary M.A., Segev D.L., Pronovost P.J., Syin D., Bandeen-Roche K., Patel P., Takenaga R., Devgan L., Holzmueller C.G., Tian J. Frailty as a predictor of surgical outcomes in older patients. J. Am. Coll. Surg. 2010;210:901–908. doi: 10.1016/j.jamcollsurg.2010.01.028. [DOI] [PubMed] [Google Scholar]
- 220.McAdams-DeMarco M.A., Suresh S., Law A., Salter M.L., Gimenez L.F., Jaar B.G., Walston J.D., Segev D.L. Frailty and falls among adult patients undergoing chronic hemodialysis: A prospective cohort study. BMC Nephrol. 2013;14:224. doi: 10.1186/1471-2369-14-224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Cawthon P.M., Marshall L.M., Michael Y., Dam T.T., Ensrud K.E., Barrett-Connor E., Orwoll E.S., Group O.F.i.M.R. Frailty in older men: Prevalence, progression, and relationship with mortality. J. Am. Geriatr. Soc. 2007;55:1216–1223. doi: 10.1111/j.1532-5415.2007.01259.x. [DOI] [PubMed] [Google Scholar]
- 222.Collard R.M., Boter H., Schoevers R.A., Oude Voshaar R.C. Prevalence of frailty in community-dwelling older persons: A systematic review. J. Am. Geriatr. Soc. 2012;60:1487–1492. doi: 10.1111/j.1532-5415.2012.04054.x. [DOI] [PubMed] [Google Scholar]
- 223.Santos-Eggimann B., Cuénoud P., Spagnoli J., Junod J. Prevalence of frailty in middle-aged and older community-dwelling Europeans living in 10 countries. J. Gerontol. Ser. A Biomed. Sci. Med. Sci. 2009;64:675–681. doi: 10.1093/gerona/glp012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Ikegami S., Uehara M., Tokida R., Nishimura H., Sakai N., Horiuchi H., Kato H., Takahashi J. Male-female disparity in clinical features and significance of mild vertebral fractures in community-dwelling residents aged 50 and over. Sci. Rep. 2024;14:5602. doi: 10.1038/s41598-024-56379-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Nicol L.M., Rowlands D.S., Fazakerly R., Kellett J. Curcumin supplementation likely attenuates delayed onset muscle soreness (DOMS) Eur. J. Appl. Physiol. 2015;115:1769–1777. doi: 10.1007/s00421-015-3152-6. [DOI] [PubMed] [Google Scholar]
- 226.Drobnic F., Riera J., Appendino G., Togni S., Franceschi F., Valle X., Pons A., Tur J. Reduction of delayed onset muscle soreness by a novel curcumin delivery system (Meriva®): A randomised, placebo-controlled trial. J. Int. Soc. Sports Nutr. 2014;11:31. doi: 10.1186/1550-2783-11-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Perna S., Alalwan T.A., Al-Thawadi S., Negro M., Parimbelli M., Cerullo G., Gasparri C., Guerriero F., Infantino V., Diana M. Evidence-based role of nutrients and antioxidants for chronic pain management in musculoskeletal frailty and sarcopenia in aging. Geriatrics. 2020;5:16. doi: 10.3390/geriatrics5010016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Mankowski R.T., Sibille K.T., Leeuwenburgh C., Lin Y., Hsu F.C., Qiu P., Sandesara B., Anton S.D. Effects of Curcumin C3 Complex® on Physical Function in Moderately Functioning Older Adults with Low-Grade Inflammation—A Pilot Trial. J. Frailty Aging. 2023;12:143–149. doi: 10.14283/jfa.2023.26. [DOI] [PubMed] [Google Scholar]
- 229.Saud Gany S.L., Chin K.-Y., Tan J.K., Aminuddin A., Makpol S. Curcumin as a therapeutic agent for sarcopenia. Nutrients. 2023;15:2526. doi: 10.3390/nu15112526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Cruz-Jentoft A.J., Baeyens J.P., Bauer J.M., Boirie Y., Cederholm T., Landi F., Martin F.C., Michel J.-P., Rolland Y., Schneider S.M. Sarcopenia: European consensus on definition and diagnosis: Report of the European Working Group on Sarcopenia in Older People. Age Ageing. 2010;39:412–423. doi: 10.1093/ageing/afq034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Morley J.E., Abbatecola A.M., Argiles J.M., Baracos V., Bauer J., Bhasin S., Cederholm T., Coats A.J.S., Cummings S.R., Evans W.J. Sarcopenia with limited mobility: An international consensus. J. Am. Med. Dir. Assoc. 2011;12:403–409. doi: 10.1016/j.jamda.2011.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Studenski S.A., Peters K.W., Alley D.E., Cawthon P.M., McLean R.R., Harris T.B., Ferrucci L., Guralnik J.M., Fragala M.S., Kenny A.M. The FNIH sarcopenia project: Rationale, study description, conference recommendations, and final estimates. J. Gerontol. Ser. A Biomed. Sci. Med. Sci. 2014;69:547–558. doi: 10.1093/gerona/glu010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Mellen R.H., Girotto O.S., Marques E.B., Laurindo L.F., Grippa P.C., Mendes C.G., Garcia L.N.H., Bechara M.D., Barbalho S.M., Sinatora R.V., et al. Insights into Pathogenesis, Nutritional and Drug Approach in Sarcopenia: A Systematic Review. Biomedicines. 2023;11:136. doi: 10.3390/biomedicines11010136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Minniti G., Pescinini-Salzedas L.M., Minniti G., Laurindo L.F., Barbalho S.M., Vargas Sinatora R., Sloan L.A., Haber R.S.A., Araújo A.C., Quesada K., et al. Organokines, Sarcopenia, and Metabolic Repercussions: The Vicious Cycle and the Interplay with Exercise. Int. J. Mol. Sci. 2022;23:13452. doi: 10.3390/ijms232113452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Barbalho S.M., Flato U.A.P., Tofano R.J., Goulart R.A., Guiguer E.L., Detregiachi C.R.P., Buchaim D.V., Araújo A.C., Buchaim R.L., Reina F.T.R., et al. Physical Exercise and Myokines: Relationships with Sarcopenia and Cardiovascular Complications. Int. J. Mol. Sci. 2020;21:3607. doi: 10.3390/ijms21103607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Park J., Park S. Association of Handgrip Strength and Cardiovascular Disease Risk Among Middle-Aged Postmenopausal Women: An Analysis of the Korea National Health and Nutrition Examination Survey 2014-2019. Vasc. Health Risk Manag. 2024;20:183–194. doi: 10.2147/VHRM.S442277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Barbalho S.M., Prado Neto E.V., De Alvares Goulart R., Bechara M.D., Baisi Chagas E.F., Audi M., Guissoni Campos L.M., Landgraf Guiger E., Buchaim R.L., Buchaim D.V., et al. Myokines: A descriptive review. J. Sports Med. Phys. Fit. 2020;60:1583–1590. doi: 10.23736/S0022-4707.20.10884-3. [DOI] [PubMed] [Google Scholar]
- 238.Ferri E., Marzetti E., Calvani R., Picca A., Cesari M., Arosio B. Role of age-related mitochondrial dysfunction in sarcopenia. Int. J. Mol. Sci. 2020;21:5236. doi: 10.3390/ijms21155236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Dong H., Ni W., Bai Y., Yuan X., Zhang Y., Zhang H., Sun Y., Xu J. Cross-sectional and longitudinal associations of apolipoprotein A1 and B with glycosylated hemoglobin in Chinese adults. Sci. Rep. 2022;12:2751. doi: 10.1038/s41598-022-06829-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Ticinesi A., Nouvenne A., Cerundolo N., Parise A., Meschi T. Accounting gut microbiota as the mediator of beneficial effects of dietary (poly) phenols on skeletal muscle in aging. Nutrients. 2023;15:2367. doi: 10.3390/nu15102367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Goates S., Du K., Arensberg M., Gaillard T., Guralnik J., Pereira S.L. Economic impact of hospitalizations in US adults with sarcopenia. J. Frailty Aging. 2019;8:93–99. doi: 10.14283/jfa.2019.10. [DOI] [PubMed] [Google Scholar]
- 242.Dalle S., Rossmeislova L., Koppo K. The role of inflammation in age-related sarcopenia. Front. Physiol. 2017;8:311540. doi: 10.3389/fphys.2017.01045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Bian A.-L., Hu H.-Y., Rong Y.-D., Wang J., Wang J.-X., Zhou X.-Z. A study on relationship between elderly sarcopenia and inflammatory factors IL-6 and TNF-α. Eur. J. Med. Res. 2017;22:25. doi: 10.1186/s40001-017-0266-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Schaap L.A., Pluijm S.M., Deeg D.J., Visser M. Inflammatory markers and loss of muscle mass (sarcopenia) and strength. Am. J. Med. 2006;119:526.e9–526.e17. doi: 10.1016/j.amjmed.2005.10.049. [DOI] [PubMed] [Google Scholar]
- 245.Baylis D., Bartlett D.B., Syddall H.E., Ntani G., Gale C.R., Cooper C., Lord J.M., Sayer A.A. Immune-endocrine biomarkers as predictors of frailty and mortality: A 10-year longitudinal study in community-dwelling older people. Age. 2013;35:963–971. doi: 10.1007/s11357-012-9396-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Aggarwal B.B., Kumar A., Bharti A.C. Anticancer potential of curcumin: Preclinical and clinical studies. Anticancer. Res. 2003;23:363–398. [PubMed] [Google Scholar]
- 247.Gorza L., Germinario E., Tibaudo L., Vitadello M., Tusa C., Guerra I., Bondì M., Salmaso S., Caliceti P., Vitiello L. Chronic systemic curcumin administration antagonizes murine sarcopenia and presarcopenia. Int. J. Mol. Sci. 2021;22:11789. doi: 10.3390/ijms222111789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Shen Y., Zhang C., Dai C., Zhang Y., Wang K., Gao Z., Chen X., Yang X., Sun H., Yao X., et al. Nutritional Strategies for Muscle Atrophy: Current Evidence and Underlying Mechanisms. Mol. Nutr. Food Res. 2024;68:e2300347. doi: 10.1002/mnfr.202300347. [DOI] [PubMed] [Google Scholar]
- 249.Das S., Preethi B., Kushwaha S., Shrivastava R. Therapeutic strategies to modulate gut microbial health: Approaches for sarcopenia management. Histol. Histopathol. 2024:18730. doi: 10.14670/hh-18-730. [DOI] [PubMed] [Google Scholar]
- 250.Kawamori T., Lubet R., Steele V.E., Kelloff G.J., Kaskey R.B., Rao C.V., Reddy B.S. Chemopreventive effect of curcumin, a naturally occurring anti-inflammatory agent, during the promotion/progression stages of colon cancer. Cancer Res. 1999;59:597–601. [PubMed] [Google Scholar]
- 251.Asai A., Miyazawa T. Occurrence of orally administered curcuminoid as glucuronide and glucuronide/sulfate conjugates in rat plasma. Life Sci. 2000;67:2785–2793. doi: 10.1016/S0024-3205(00)00868-7. [DOI] [PubMed] [Google Scholar]
- 252.Öner-İyidoğan Y., Tanrıkulu-Küçük S., Seyithanoğlu M., Koçak H., Doğru-Abbasoğlu S., Aydin A.F., Beyhan-Özdaş Ş., Yapişlar H., Koçak-Toker N. Effect of curcumin on hepatic heme oxygenase 1 expression in high fat diet fed rats: Is there a triangular relationship? Can. J. Physiol. Pharmacol. 2014;92:805–812. doi: 10.1139/cjpp-2014-0174. [DOI] [PubMed] [Google Scholar]
- 253.Receno C.N., Liang C., Korol D.L., Atalay M., Heffernan K.S., Brutsaert T.D., DeRuisseau K.C. Effects of Prolonged Dietary Curcumin Exposure on Skeletal Muscle Biochemical and Functional Responses of Aged Male Rats. Int. J. Mol. Sci. 2019;5:1178. doi: 10.3390/ijms20051178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Liang Y.J., Yang I.H., Lin Y.W., Lin J.N., Wu C.C., Chiang C.Y., Lai K.H., Lin F.H. Curcumin-Loaded Hydrophobic Surface-Modified Hydroxyapatite as an Antioxidant for Sarcopenia Prevention. Antioxid. 2021;10:616. doi: 10.3390/antiox10040616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Wang M.Y., Yang J.M., Wu Y., Li H., Zhong Y.B., Luo Y., Xie R.L. Curcumin-activated Wnt5a pathway mediates Ca(2+) channel opening to affect myoblast differentiation and skeletal muscle regeneration. J. Cachexia Sarcopenia Muscle. 2024 doi: 10.1002/jcsm.13535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Sani A., Hasegawa K., Yamaguchi Y., Panichayupakaranant P., Pengjam Y. Inhibitory effects of curcuminoids on dexamethasone-induced muscle atrophy in differentiation of C2C12 cells. Phytomed. Plus. 2021;1:100012. doi: 10.1016/j.phyplu.2020.100012. [DOI] [Google Scholar]
- 257.Sivertsen H., Bjørkløf G.H., Engedal K., Selbæk G., Helvik A.S. Depression and Quality of Life in Older Persons: A Review. Dement. Geriatr. Cogn. Disord. 2015;40:311–339. doi: 10.1159/000437299. [DOI] [PubMed] [Google Scholar]
- 258.Michaud C.M., Murray C.J.L., Bloom B.R. Burden of Disease—Implications for Future Research. JAMA. 2001;285:535–539. doi: 10.1001/jama.285.5.535. [DOI] [PubMed] [Google Scholar]
- 259.Malhi G.S., Mann J.J. Depression. Lancet. 2018;392:2299–2312. doi: 10.1016/S0140-6736(18)31948-2. [DOI] [PubMed] [Google Scholar]
- 260.He Z.F., Tan W.Y., Ma H., Shuai Y., Shan Z., Zhai J., Qiu Y., Zeng H., Chen X.L., Wang S.B., et al. Prevalence and factors associated with depression and anxiety among older adults: A large-scale cross-sectional study in China. J. Affect. Disord. 2024;346:135–143. doi: 10.1016/j.jad.2023.11.022. [DOI] [PubMed] [Google Scholar]
- 261.Ferrari A.J., Somerville A.J., Baxter A.J., Norman R., Patten S.B., Vos T., Whiteford H.A. Global variation in the prevalence and incidence of major depressive disorder: A systematic review of the epidemiological literature. Psychol. Med. 2013;43:471–481. doi: 10.1017/S0033291712001511. [DOI] [PubMed] [Google Scholar]
- 262.Hussain Z., Wegmann E., Yang H., Montag C. Social Networks Use Disorder and Associations With Depression and Anxiety Symptoms: A Systematic Review of Recent Research in China. Front. Psychol. 2020;11:211. doi: 10.3389/fpsyg.2020.00211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Monroe S.M., Harkness K.L. Major Depression and Its Recurrences: Life Course Matters. Annu. Rev. Clin. Psychol. 2022;18:329–357. doi: 10.1146/annurev-clinpsy-072220-021440. [DOI] [PubMed] [Google Scholar]
- 264.Bai Y., Cai Y., Chang D., Li D., Huo X., Zhu T. Immunotherapy for depression: Recent insights and future targets. Pharmacol. Ther. 2024;257:108624. doi: 10.1016/j.pharmthera.2024.108624. [DOI] [PubMed] [Google Scholar]
- 265.Alexopoulos G.S. Depression in the elderly. Lancet. 2005;365:1961–1970. doi: 10.1016/S0140-6736(05)66665-2. [DOI] [PubMed] [Google Scholar]
- 266.Giovannini S., Onder G., van der Roest H.G., Topinkova E., Gindin J., Cipriani M.C., Denkinger M.D., Bernabei R., Liperoti R. Use of antidepressant medications among older adults in European long-term care facilities: A cross-sectional analysis from the SHELTER study. BMC Geriatr. 2020;20:310. doi: 10.1186/s12877-020-01730-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Ng A., Tam W.W., Zhang M.W., Ho C.S., Husain S.F., McIntyre R.S., Ho R.C. IL-1β, IL-6, TNF- α and CRP in Elderly Patients with Depression or Alzheimer’s disease: Systematic Review and Meta-Analysis. Sci. Rep. 2018;8:12050. doi: 10.1038/s41598-018-30487-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Liu Y., Ho R.C., Mak A. Interleukin (IL)-6, tumour necrosis factor alpha (TNF-α) and soluble interleukin-2 receptors (sIL-2R) are elevated in patients with major depressive disorder: A meta-analysis and meta-regression. J. Affect. Disord. 2012;139:230–239. doi: 10.1016/j.jad.2011.08.003. [DOI] [PubMed] [Google Scholar]
- 269.Haapakoski R., Mathieu J., Ebmeier K.P., Alenius H., Kivimäki M. Cumulative meta-analysis of interleukins 6 and 1β, tumour necrosis factor α and C-reactive protein in patients with major depressive disorder. Brain Behav. Immun. 2015;49:206–215. doi: 10.1016/j.bbi.2015.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Hannestad J., DellaGioia N., Bloch M. The effect of antidepressant medication treatment on serum levels of inflammatory cytokines: A meta-analysis. Neuropsychopharmacology. 2011;36:2452–2459. doi: 10.1038/npp.2011.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Kiraly D.D., Horn S.R., Van Dam N.T., Costi S., Schwartz J., Kim-Schulze S., Patel M., Hodes G.E., Russo S.J., Merad M., et al. Altered peripheral immune profiles in treatment-resistant depression: Response to ketamine and prediction of treatment outcome. Transl. Psychiatry. 2017;7:e1065. doi: 10.1038/tp.2017.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Zhang W.Y., Guo Y.J., Han W.X., Yang M.Q., Wen L.P., Wang K.Y., Jiang P. Curcumin relieves depressive-like behaviors via inhibition of the NLRP3 inflammasome and kynurenine pathway in rats suffering from chronic unpredictable mild stress. Int. Immunopharmacol. 2019;67:138–144. doi: 10.1016/j.intimp.2018.12.012. [DOI] [PubMed] [Google Scholar]
- 273.Yu J.J., Pei L.B., Zhang Y., Wen Z.Y., Yang J.L. Chronic Supplementation of Curcumin Enhances the Efficacy of Antidepressants in Major Depressive Disorder: A Randomized, Double-Blind, Placebo-Controlled Pilot Study. J. Clin. Psychopharmacol. 2015;35:406–410. doi: 10.1097/JCP.0000000000000352. [DOI] [PubMed] [Google Scholar]
- 274.Liu D., Wang Z., Gao Z., Xie K., Zhang Q., Jiang H., Pang Q. Effects of curcumin on learning and memory deficits, BDNF, and ERK protein expression in rats exposed to chronic unpredictable stress. Behav. Brain Res. 2014;271:116–121. doi: 10.1016/j.bbr.2014.05.068. [DOI] [PubMed] [Google Scholar]
- 275.Mizoguchi K., Sun N., Jin X.L., Kase Y., Takeda S., Maruyama W., Tabira T. Saikokaryukotsuboreito, a herbal medicine, prevents chronic stress-induced dysfunction of glucocorticoid negative feedback system in rat brain. Pharmacol. Biochem. Behav. 2007;86:55–61. doi: 10.1016/j.pbb.2006.12.007. [DOI] [PubMed] [Google Scholar]
- 276.Huang Z., Zhong X.M., Li Z.Y., Feng C.R., Pan A.J., Mao Q.Q. Curcumin reverses corticosterone-induced depressive-like behavior and decrease in brain BDNF levels in rats. Neurosci. Lett. 2011;493:145–148. doi: 10.1016/j.neulet.2011.02.030. [DOI] [PubMed] [Google Scholar]
- 277.Michel T.M., Pülschen D., Thome J. The role of oxidative stress in depressive disorders. Curr. Pharm. Des. 2012;18:5890–5899. doi: 10.2174/138161212803523554. [DOI] [PubMed] [Google Scholar]
- 278.Naik S.R., Thakare V.N., Patil S.R. Protective effect of curcumin on experimentally induced inflammation, hepatotoxicity and cardiotoxicity in rats: Evidence of its antioxidant property. Exp. Toxicol. Pathol. 2011;63:419–431. doi: 10.1016/j.etp.2010.03.001. [DOI] [PubMed] [Google Scholar]
- 279.Wei Q.Y., Chen W.F., Zhou B., Yang L., Liu Z.L. Inhibition of lipid peroxidation and protein oxidation in rat liver mitochondria by curcumin and its analogues. Biochim. Biophys. Acta. 2006;1760:70–77. doi: 10.1016/j.bbagen.2005.09.008. [DOI] [PubMed] [Google Scholar]
- 280.Al-Rubaei Z.M., Mohammad T.U., Ali L.K. Effects of local curcumin on oxidative stress and total antioxidant capacity in vivo study. Pak. J. Biol. Sci. 2014;17:1237–1241. doi: 10.3923/pjbs.2014.1237.1241. [DOI] [PubMed] [Google Scholar]
- 281.Gilhotra N., Dhingra D. GABAergic and nitriergic modulation by curcumin for its antianxiety-like activity in mice. Brain Res. 2010;1352:167–175. doi: 10.1016/j.brainres.2010.07.007. [DOI] [PubMed] [Google Scholar]
- 282.Głombik K., Stachowicz A., Trojan E., Ślusarczyk J., Suski M., Chamera K., Kotarska K., Olszanecki R., Basta-Kaim A. Mitochondrial proteomics investigation of frontal cortex in an animal model of depression: Focus on chronic antidepressant drugs treatment. Pharmacol. Rep. 2018;70:322–330. doi: 10.1016/j.pharep.2017.11.016. [DOI] [PubMed] [Google Scholar]
- 283.Wang R., Xu Y., Wu H.L., Li Y.B., Li Y.H., Guo J.B., Li X.J. The antidepressant effects of curcumin in the forced swimming test involve 5-HT1 and 5-HT2 receptors. Eur. J. Pharmacol. 2008;578:43–50. doi: 10.1016/j.ejphar.2007.08.045. [DOI] [PubMed] [Google Scholar]
- 284.Kulkarni S.K., Bhutani M.K., Bishnoi M. Antidepressant activity of curcumin: Involvement of serotonin and dopamine system. Psychopharmacology. 2008;201:435–442. doi: 10.1007/s00213-008-1300-y. [DOI] [PubMed] [Google Scholar]
- 285.Bhutani M.K., Bishnoi M., Kulkarni S.K. Anti-depressant like effect of curcumin and its combination with piperine in unpredictable chronic stress-induced behavioral, biochemical and neurochemical changes. Pharmacol. Biochem. Behav. 2009;92:39–43. doi: 10.1016/j.pbb.2008.10.007. [DOI] [PubMed] [Google Scholar]
- 286.Xu Y., Ku B.S., Yao H.Y., Lin Y.H., Ma X., Zhang Y.H., Li X.J. Antidepressant effects of curcumin in the forced swim test and olfactory bulbectomy models of depression in rats. Pharmacol. Biochem. Behav. 2005;82:200–206. doi: 10.1016/j.pbb.2005.08.009. [DOI] [PubMed] [Google Scholar]
- 287.Lin T.Y., Lu C.W., Wang C.C., Wang Y.C., Wang S.J. Curcumin inhibits glutamate release in nerve terminals from rat prefrontal cortex: Possible relevance to its antidepressant mechanism. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2011;35:1785–1793. doi: 10.1016/j.pnpbp.2011.06.012. [DOI] [PubMed] [Google Scholar]
- 288.Gardner A., Boles R.G. Comment on treatment of psychiatric illness in patients with mitochondrial disease. Psychosomatics. 2011;52:497–498. doi: 10.1016/j.psym.2011.01.025. [DOI] [PubMed] [Google Scholar]
- 289.Sequeira A., Rollins B., Magnan C., van Oven M., Baldi P., Myers R.M., Barchas J.D., Schatzberg A.F., Watson S.J., Akil H., et al. Mitochondrial mutations in subjects with psychiatric disorders. PLoS ONE. 2015;10:e0127280. doi: 10.1371/journal.pone.0127280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Wang X., Muhammad I., Sun X., Han M., Hamid S., Zhang X. Protective role of curcumin in ameliorating AFB(1)-induced apoptosis via mitochondrial pathway in liver cells. Mol. Biol. Rep. 2018;45:881–891. doi: 10.1007/s11033-018-4234-4. [DOI] [PubMed] [Google Scholar]
- 291.Jiang H., Tian X., Guo Y., Duan W., Bu H., Li C. Activation of nuclear factor erythroid 2-related factor 2 cytoprotective signaling by curcumin protect primary spinal cord astrocytes against oxidative toxicity. Biol. Pharm. Bull. 2011;34:1194–1197. doi: 10.1248/bpb.34.1194. [DOI] [PubMed] [Google Scholar]
- 292.Zhang Y., Li L., Zhang J. Curcumin in antidepressant treatments: An overview of potential mechanisms, pre-clinical/clinical trials and ongoing challenges. Basic. Clin. Pharmacol. Toxicol. 2020;127:243–253. doi: 10.1111/bcpt.13455. [DOI] [PubMed] [Google Scholar]
- 293.Varma K., Amalraj A., Divya C., Gopi S. The Efficacy of the Novel Bioavailable Curcumin (Cureit) in the Management of Sarcopenia in Healthy Elderly Subjects: A Randomized, Placebo-Controlled, Double-Blind Clinical Study. J. Med. Food. 2021;24:40–49. doi: 10.1089/jmf.2020.4778. [DOI] [PubMed] [Google Scholar]
- 294.Ghodsi H., Rahimi H.R., Aghili S.M., Saberi A., Shoeibi A. Evaluation of curcumin as add-on therapy in patients with Parkinson’s disease: A pilot randomized, triple-blind, placebo-controlled trial. Clin. Neurol. Neurosurg. 2022;218:107300. doi: 10.1016/j.clineuro.2022.107300. [DOI] [PubMed] [Google Scholar]
- 295.Cox K.H., Pipingas A., Scholey A.B. Investigation of the effects of solid lipid curcumin on cognition and mood in a healthy older population. J. Psychopharmacol. 2015;29:642–651. doi: 10.1177/0269881114552744. [DOI] [PubMed] [Google Scholar]
- 296.Thota R.N., Acharya S.H., Garg M.L. Curcumin and/or omega-3 polyunsaturated fatty acids supplementation reduces insulin resistance and blood lipids in individuals with high risk of type 2 diabetes: A randomised controlled trial. Lipids Health Dis. 2019;18:31. doi: 10.1186/s12944-019-0967-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297.DiSilvestro R.A., Joseph E., Zhao S., Bomser J. Diverse effects of a low dose supplement of lipidated curcumin in healthy middle aged people. Nutr. J. 2012;11:79. doi: 10.1186/1475-2891-11-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.Baum L., Cheung S.K., Mok V.C., Lam L.C., Leung V.P., Hui E., Ng C.C., Chow M., Ho P.C., Lam S., et al. Curcumin effects on blood lipid profile in a 6-month human study. Pharmacol. Res. 2007;56:509–514. doi: 10.1016/j.phrs.2007.09.013. [DOI] [PubMed] [Google Scholar]
- 299.Khanna A., Das S.S., Kannan R., Swick A.G., Matthewman C., Maliakel B., Ittiyavirah S.P., Krishnakumar I.M. The effects of oral administration of curcumin-galactomannan complex on brain waves are consistent with brain penetration: A randomized, double-blinded, placebo-controlled pilot study. Nutr. Neurosci. 2022;25:1240–1249. doi: 10.1080/1028415X.2020.1853410. [DOI] [PubMed] [Google Scholar]
- 300.Cox K.H.M., White D.J., Pipingas A., Poorun K., Scholey A. Further Evidence of Benefits to Mood and Working Memory from Lipidated Curcumin in Healthy Older People: A 12-Week, Double-Blind, Placebo-Controlled, Partial Replication Study. Nutrients. 2020;12:1678. doi: 10.3390/nu12061678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.Kuszewski J.C., Wong R.H.X., Wood L.G., Howe P.R.C. Effects of fish oil and curcumin supplementation on cerebrovascular function in older adults: A randomized controlled trial. Nutr. Metab. Cardiovasc. Dis. 2020;30:625–633. doi: 10.1016/j.numecd.2019.12.010. [DOI] [PubMed] [Google Scholar]
- 302.Small G.W., Siddarth P., Li Z., Miller K.J., Ercoli L., Emerson N.D., Martinez J., Wong K.P., Liu J., Merrill D.A., et al. Memory and Brain Amyloid and Tau Effects of a Bioavailable Form of Curcumin in Non-Demented Adults: A Double-Blind, Placebo-Controlled 18-Month Trial. Am. J. Geriatr. Psychiatry Off. J. Am. Assoc. Geriatr. Psychiatry. 2018;26:266–277. doi: 10.1016/j.jagp.2017.10.010. [DOI] [PubMed] [Google Scholar]
- 303.Ross S.M. Curcuma longa (Theracumin®): A Bioavailable Form of Curcumin and Its Cognitive Benefits. Holist. Nurs. Pr. Pract. 2018;32:217–220. doi: 10.1097/HNP.0000000000000281. [DOI] [PubMed] [Google Scholar]
- 304.Panahi Y., Badeli R., Karami G.R., Sahebkar A. Investigation of the efficacy of adjunctive therapy with bioavailability-boosted curcuminoids in major depressive disorder. Phytother. Res. 2015;29:17–21. doi: 10.1002/ptr.5211. [DOI] [PubMed] [Google Scholar]
- 305.Rainey-Smith S.R., Brown B.M., Sohrabi H.R., Shah T., Goozee K.G., Gupta V.B., Martins R.N. Curcumin and cognition: A randomised, placebo-controlled, double-blind study of community-dwelling older adults. Br. J. Nutr. 2016;115:2106–2113. doi: 10.1017/S0007114516001203. [DOI] [PubMed] [Google Scholar]
- 306.Yaikwawong M., Jansarikit L., Jirawatnotai S., Chuengsamarn S. The Effect of Curcumin on Reducing Atherogenic Risks in Obese Patients with Type 2 Diabetes: A Randomized Controlled Trial. Nutrients. 2024;16:2441. doi: 10.3390/nu16152441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307.Kucukgoncu S., Guloksuz S., Tek C. Effects of Curcumin on Cognitive Functioning and Inflammatory State in Schizophrenia: A Double-Blind, Placebo-Controlled Pilot Trial. J. Clin. Psychopharmacol. 2019;39:182–184. doi: 10.1097/JCP.0000000000001012. [DOI] [PubMed] [Google Scholar]
- 308.Dei Cas M., Ghidoni R. Dietary Curcumin: Correlation between Bioavailability and Health Potential. Nutrients. 2019;11:2147. doi: 10.3390/nu11092147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309.Kunnumakkara A.B., Harsha C., Banik K., Vikkurthi R., Sailo B.L., Bordoloi D., Gupta S.C., Aggarwal B.B. Is curcumin bioavailability a problem in humans: Lessons from clinical trials. Expert. Opin. Drug Metab. Toxicol. 2019;15:705–733. doi: 10.1080/17425255.2019.1650914. [DOI] [PubMed] [Google Scholar]
- 310.Vashisht M., Rani P., Onteru S.K., Singh D. Curcumin Encapsulated in Milk Exosomes Resists Human Digestion and Possesses Enhanced Intestinal Permeability in Vitro. Appl. Biochem. Biotechnol. 2017;183:993–1007. doi: 10.1007/s12010-017-2478-4. [DOI] [PubMed] [Google Scholar]
- 311.Marton L.T., Pescinini E.S.L.M., Camargo M.E.C., Barbalho S.M., Haber J., Sinatora R.V., Detregiachi C.R.P., Girio R.J.S., Buchaim D.V., Cincotto Dos Santos Bueno P. The Effects of Curcumin on Diabetes Mellitus: A Systematic Review. Front. Endocrinol. 2021;12:669448. doi: 10.3389/fendo.2021.669448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 312.Akuri M.C., Barbalho S.M., Val R.M., Guiguer E.L. Reflections about Osteoarthritis and Curcuma longa. Pharmacogn. Rev. 2017;11:8–12. doi: 10.4103/phrev.phrev_54_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 313.Gopi S., Jacob J., Varma K., Jude S., Amalraj A., Arundhathy C.A., George R., Sreeraj T.R., Divya C., Kunnumakkara A.B., et al. Comparative Oral Absorption of Curcumin in a Natural Turmeric Matrix with Two Other Curcumin Formulations: An Open-label Parallel-arm Study. Phytother. Res. 2017;31:1883–1891. doi: 10.1002/ptr.5931. [DOI] [PubMed] [Google Scholar]
- 314.Kocher A., Bohnert L., Schiborr C., Frank J. Highly bioavailable micellar curcuminoids accumulate in blood, are safe and do not reduce blood lipids and inflammation markers in moderately hyperlipidemic individuals. Mol. Nutr. Food Res. 2016;60:1555–1563. doi: 10.1002/mnfr.201501034. [DOI] [PubMed] [Google Scholar]
- 315.Sharifi-Rad J., Rayess Y.E., Rizk A.A., Sadaka C., Zgheib R., Zam W., Sestito S., Rapposelli S., Neffe-Skocińska K., Zielińska D., et al. Turmeric and Its Major Compound Curcumin on Health: Bioactive Effects and Safety Profiles for Food, Pharmaceutical, Biotechnological and Medicinal Applications. Front. Pharmacol. 2020;11:01021. doi: 10.3389/fphar.2020.01021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 316.Zeng L., Yang T., Yang K., Yu G., Li J., Xiang W., Chen H. Efficacy and Safety of Curcumin and Curcuma longa Extract in the Treatment of Arthritis: A Systematic Review and Meta-Analysis of Randomized Controlled Trial. Front. Immunol. 2022;13:891822. doi: 10.3389/fimmu.2022.891822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 317.Shep D., Khanwelkar C., Gade P., Karad S. Safety and efficacy of curcumin versus diclofenac in knee osteoarthritis: A randomized open-label parallel-arm study. Trials. 2019;20:214. doi: 10.1186/s13063-019-3327-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 318.Pivari F., Mingione A., Piazzini G., Ceccarani C., Ottaviano E., Brasacchio C., Dei Cas M., Vischi M., Cozzolino M.G., Fogagnolo P., et al. Curcumin Supplementation (Meriva®) Modulates Inflammation, Lipid Peroxidation and Gut Microbiota Composition in Chronic Kidney Disease. Nutrients. 2022;14:231. doi: 10.3390/nu14010231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 319.Panahi Y., Kianpour P., Mohtashami R., Jafari R., Simental-Mendía L.E., Sahebkar A. Efficacy and Safety of Phytosomal Curcumin in Non-Alcoholic Fatty Liver Disease: A Randomized Controlled Trial. Drug Res. 2017;67:244–251. doi: 10.1055/s-0043-100019. [DOI] [PubMed] [Google Scholar]
- 320.Tanaka M., Vécsei L. A Decade of Dedication: Pioneering Perspectives on Neurological Diseases and Mental Illnesses. Biomedicines. 2024;12:1083. doi: 10.3390/biomedicines12051083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 321.Tanaka M., Battaglia S., Giménez-Llort L., Chen C., Hepsomali P., Avenanti A., Vécsei L. Innovation at the intersection: Emerging translational research in neurology and psychiatry. Cells. 2024;13:790. doi: 10.3390/cells13100790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 322.Tanaka M., Vécsei L. From Lab to Life: Exploring Cutting-Edge Models for Neurological and Psychiatric Disorders. Biomedicines. 2024;12:613. doi: 10.3390/biomedicines12030613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 323.Tajti J., Szok D., Csáti A., Szabó Á., Tanaka M., Vécsei L. Exploring novel therapeutic targets in the common pathogenic factors in migraine and neuropathic pain. Int. J. Mol. Sci. 2023;24:4114. doi: 10.3390/ijms24044114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 324.Tanaka M., Török N., Tóth F., Szabó Á., Vécsei L. Co-players in chronic pain: Neuroinflammation and the tryptophan-kynurenine metabolic pathway. Biomedicines. 2021;9:897. doi: 10.3390/biomedicines9080897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 325.Battaglia S., Schmidt A., Hassel S., Tanaka M. Case reports in neuroimaging and stimulation. Front. Psychiatry. 2023;14:1264669. doi: 10.3389/fpsyt.2023.1264669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 326.Balogh L., Tanaka M., Török N., Vécsei L., Taguchi S. Crosstalk between existential phenomenological psychotherapy and neurological sciences in mood and anxiety disorders. Biomedicines. 2021;9:340. doi: 10.3390/biomedicines9040340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 327.Jászberényi M., Thurzó B., Bagosi Z., Vécsei L., Tanaka M. The Orexin/Hypocretin System, the Peptidergic Regulator of Vigilance, Orchestrates Adaptation to Stress. Biomedicines. 2024;12:448. doi: 10.3390/biomedicines12020448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 328.Tanaka M., Diano M., Battaglia S. Insights into structural and functional organization of the brain: Evidence from neuroimaging and non-invasive brain stimulation techniques. Front. Psychiatry. 2023;14:1225755. doi: 10.3389/fpsyt.2023.1225755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 329.Liloia D., Zamfira D.A., Tanaka M., Manuello J., Crocetta A., Keller R., Cozzolino M., Duca S., Cauda F., Costa T. Disentangling the role of gray matter volume and concentration in autism spectrum disorder: A meta-analytic investigation of 25 years of voxel-based morphometry research. Neurosci. Biobehav. Rev. 2024;164:105791. doi: 10.1016/j.neubiorev.2024.105791. [DOI] [PubMed] [Google Scholar]
- 330.Tanaka M., Szabó Á., Körtési T., Szok D., Tajti J., Vécsei L. From CGRP to PACAP, VIP, and beyond: Unraveling the next chapters in migraine treatment. Cells. 2023;12:2649. doi: 10.3390/cells12222649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 331.Tanaka M., Szabó Á., Vécsei L. Preclinical modeling in depression and anxiety: Current challenges and future research directions. Adv. Clin. Exp. Med. 2023;32:505–509. doi: 10.17219/acem/165944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 332.Polyák H., Galla Z., Nánási N., Cseh E.K., Rajda C., Veres G., Spekker E., Szabó Á., Klivényi P., Tanaka M. The tryptophan-kynurenine metabolic system is suppressed in cuprizone-induced model of demyelination simulating progressive multiple sclerosis. Biomedicines. 2023;11:945. doi: 10.3390/biomedicines11030945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 333.Török N., Maszlag-Török R., Molnár K., Szolnoki Z., Somogyvári F., Boda K., Tanaka M., Klivényi P., Vécsei L. Single Nucleotide Polymorphisms of Indoleamine 2, 3-Dioxygenase1 Influenced the Age Onset of Parkinson’s Disease. Front. Biosci. 2022;27:265. doi: 10.31083/j.fbl2709265. [DOI] [PubMed] [Google Scholar]
- 334.Tanaka M., Szabó Á., Vécsei L., Giménez-Llort L. Emerging Translational Research in Neurological and Psychiatric Diseases: From In Vitro to In Vivo Models. Int. J. Mol. Sci. 2023;24:15739. doi: 10.3390/ijms242115739. [DOI] [PMC free article] [PubMed] [Google Scholar]