Abstract
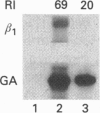
We investigated the effects of phorbol ester (phorbol 12-myristate 13-acetate; PMA) treatment on the adhesive behaviour of three erythroleukaemia cell lines: HEL, LAMA-84 and AP217. In the three cell lines PMA induced an increase in expression of a megakaryocytic marker: alpha IIb beta 3 integrin, but did not promote activation of this receptor. Indeed, an antibody specific for the activated form of alpha IIb beta 3 failed to react with the three cell lines. PMA induction led to different adhesive phenotypes depending on the cell line; in fact LAMA-84 and HEL cells became adherent while AP217 cells remained non-adherent. By studying cell surface receptors we found that the major difference between the adherent and the non-adherent cells was the expression of beta 1 integrins. After PMA induction, beta 1 integrin expression was totally abolished in AP217 cells and the amount of beta 1 mRNA was reduced preventing new synthesis of the subunit. In HEL and LAMA-84 cells, PMA treatment did not alter the overall level of beta 1 integrin but induced a new pattern of alpha-subunit expression: up-regulation of alpha 2 and alpha v subunits and down-regulation of alpha 4 and alpha 5 subunits. Function-perturbing antibodies against beta 1, alpha 4, alpha 5, alpha v and alpha 2 reduced adhesion of HEL cells to fibronectin or collagen, whereas antibodies against beta 3 or alpha v beta 3 did not. Our results favour the involvement of beta 1 integrins in PMA-induced adhesion of erythroleukaemia cells.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aihara H., Asaoka Y., Yoshida K., Nishizuka Y. Sustained activation of protein kinase C is essential to HL-60 cell differentiation to macrophage. Proc Natl Acad Sci U S A. 1991 Dec 15;88(24):11062–11066. doi: 10.1073/pnas.88.24.11062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrieux A., Hudry-Clergeon G., Ryckewaert J. J., Chapel A., Ginsberg M. H., Plow E. F., Marguerie G. Amino acid sequences in fibrinogen mediating its interaction with its platelet receptor, GPIIbIIIa. J Biol Chem. 1989 Jun 5;264(16):9258–9265. [PubMed] [Google Scholar]
- Andrieux A., Rabiet M. J., Chapel A., Concord E., Marguerie G. A highly conserved sequence of the Arg-Gly-Asp-binding domain of the integrin beta 3 subunit is sensitive to stimulation. J Biol Chem. 1991 Aug 5;266(22):14202–14207. [PubMed] [Google Scholar]
- Arroyo A. G., Sánchez-Mateos P., Campanero M. R., Martín-Padura I., Dejana E., Sánchez-Madrid F. Regulation of the VLA integrin-ligand interactions through the beta 1 subunit. J Cell Biol. 1992 May;117(3):659–670. doi: 10.1083/jcb.117.3.659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berry N., Nishizuka Y. Protein kinase C and T cell activation. Eur J Biochem. 1990 Apr 30;189(2):205–214. doi: 10.1111/j.1432-1033.1990.tb15478.x. [DOI] [PubMed] [Google Scholar]
- Bhatia R., Wayner E. A., McGlave P. B., Verfaillie C. M. Interferon-alpha restores normal adhesion of chronic myelogenous leukemia hematopoietic progenitors to bone marrow stroma by correcting impaired beta 1 integrin receptor function. J Clin Invest. 1994 Jul;94(1):384–391. doi: 10.1172/JCI117333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks P. C., Clark R. A., Cheresh D. A. Requirement of vascular integrin alpha v beta 3 for angiogenesis. Science. 1994 Apr 22;264(5158):569–571. doi: 10.1126/science.7512751. [DOI] [PubMed] [Google Scholar]
- Burger S. R., Zutter M. M., Sturgill-Koszycki S., Santoro S. A. Induced cell surface expression of functional alpha 2 beta 1 integrin during megakaryocytic differentiation of K562 leukemic cells. Exp Cell Res. 1992 Sep;202(1):28–35. doi: 10.1016/0014-4827(92)90400-3. [DOI] [PubMed] [Google Scholar]
- Bütler T. M., Ziemiecki A., Friis R. R. Megakaryocytic differentiation of K562 cells is associated with changes in the cytoskeletal organization and the pattern of chromatographically distinct forms of phosphotyrosyl-specific protein phosphatases. Cancer Res. 1990 Oct 1;50(19):6323–6329. [PubMed] [Google Scholar]
- Caligaris-Cappio F., Bergui L., Schena M., Chilosi M., Marchisio P. C. Cell-cell and cell-matrix adhesion structures may influence the growth pattern of chronic lymphoid malignancies. Leukemia. 1989 Mar;3(3):167–169. [PubMed] [Google Scholar]
- Danilov Y. N., Juliano R. L. Phorbol ester modulation of integrin-mediated cell adhesion: a postreceptor event. J Cell Biol. 1989 May;108(5):1925–1933. doi: 10.1083/jcb.108.5.1925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duperray A., Troesch A., Berthier R., Chagnon E., Frachet P., Uzan G., Marguerie G. Biosynthesis and assembly of platelet GPIIb-IIIa in human megakaryocytes: evidence that assembly between pro-GPIIb and GPIIIa is a prerequisite for expression of the complex on the cell surface. Blood. 1989 Oct;74(5):1603–1611. [PubMed] [Google Scholar]
- Gough N. M. Rapid and quantitative preparation of cytoplasmic RNA from small numbers of cells. Anal Biochem. 1988 Aug 15;173(1):93–95. doi: 10.1016/0003-2697(88)90164-9. [DOI] [PubMed] [Google Scholar]
- Grabarek J., Raychowdhury M., Ravid K., Kent K. C., Newman P. J., Ware J. A. Identification and functional characterization of protein kinase C isozymes in platelets and HEL cells. J Biol Chem. 1992 May 15;267(14):10011–10017. [PubMed] [Google Scholar]
- Greenberg S. M., Chandrasekhar C., Golan D. E., Handin R. I. Transforming growth factor beta inhibits endomitosis in the Dami human megakaryocytic cell line. Blood. 1990 Aug 1;76(3):533–537. [PubMed] [Google Scholar]
- Greenwalt D. E., Lipsky R. H., Ockenhouse C. F., Ikeda H., Tandon N. N., Jamieson G. A. Membrane glycoprotein CD36: a review of its roles in adherence, signal transduction, and transfusion medicine. Blood. 1992 Sep 1;80(5):1105–1115. [PubMed] [Google Scholar]
- Harris P., Ralph P. Human leukemic models of myelomonocytic development: a review of the HL-60 and U937 cell lines. J Leukoc Biol. 1985 Apr;37(4):407–422. doi: 10.1002/jlb.37.4.407. [DOI] [PubMed] [Google Scholar]
- Hug H., Sarre T. F. Protein kinase C isoenzymes: divergence in signal transduction? Biochem J. 1993 Apr 15;291(Pt 2):329–343. doi: 10.1042/bj2910329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter T. Cooperation between oncogenes. Cell. 1991 Jan 25;64(2):249–270. doi: 10.1016/0092-8674(91)90637-e. [DOI] [PubMed] [Google Scholar]
- Hynes R. O. Integrins: a family of cell surface receptors. Cell. 1987 Feb 27;48(4):549–554. doi: 10.1016/0092-8674(87)90233-9. [DOI] [PubMed] [Google Scholar]
- Järvinen M., Ylänne J., Vartio T., Virtanen I. Tumor promoter and fibronectin induce actin stress fibers and focal adhesion sites in spreading human erythroleukemia (HEL) cells. Eur J Cell Biol. 1987 Oct;44(2):238–246. [PubMed] [Google Scholar]
- Lindberg F. P., Gresham H. D., Schwarz E., Brown E. J. Molecular cloning of integrin-associated protein: an immunoglobulin family member with multiple membrane-spanning domains implicated in alpha v beta 3-dependent ligand binding. J Cell Biol. 1993 Oct;123(2):485–496. doi: 10.1083/jcb.123.2.485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lok S., Foster D. C. The structure, biology and potential therapeutic applications of recombinant thrombopoietin. Stem Cells. 1994 Nov;12(6):586–598. doi: 10.1002/stem.5530120606. [DOI] [PubMed] [Google Scholar]
- Martin P., Papayannopoulou T. HEL cells: a new human erythroleukemia cell line with spontaneous and induced globin expression. Science. 1982 Jun 11;216(4551):1233–1235. doi: 10.1126/science.6177045. [DOI] [PubMed] [Google Scholar]
- Molla A., Andrieux A., Chapel A., Schweitzer A., Berthier R., Marguerie G. Lack of transcription and expression of the alpha IIb integrin in human early haematopoietic stem cells. Br J Haematol. 1992 Dec;82(4):635–639. doi: 10.1111/j.1365-2141.1992.tb06937.x. [DOI] [PubMed] [Google Scholar]
- Pullman W. E., Bodmer W. F. Cloning and characterization of a gene that regulates cell adhesion. Nature. 1992 Apr 9;356(6369):529–532. doi: 10.1038/356529a0. [DOI] [PubMed] [Google Scholar]
- Rosemblatt M., Vuillet-Gaugler M. H., Leroy C., Coulombel L. Coexpression of two fibronectin receptors, VLA-4 and VLA-5, by immature human erythroblastic precursor cells. J Clin Invest. 1991 Jan;87(1):6–11. doi: 10.1172/JCI115002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santoro S. A. Identification of a 160,000 dalton platelet membrane protein that mediates the initial divalent cation-dependent adhesion of platelets to collagen. Cell. 1986 Sep 12;46(6):913–920. doi: 10.1016/0092-8674(86)90073-5. [DOI] [PubMed] [Google Scholar]
- Seigneurin D., Champelovier P., Mouchiroud G., Berthier R., Leroux D., Prenant M., McGregor J., Starck J., Morle F., Micouin C. Human chronic myeloid leukemic cell line with positive Philadelphia chromosome exhibits megakaryocytic and erythroid characteristics. Exp Hematol. 1987 Sep;15(8):822–832. [PubMed] [Google Scholar]
- Shattil S. J., Ginsberg M. H., Brugge J. S. Adhesive signaling in platelets. Curr Opin Cell Biol. 1994 Oct;6(5):695–704. doi: 10.1016/0955-0674(94)90096-5. [DOI] [PubMed] [Google Scholar]
- Shattil S. J., Haimovich B., Cunningham M., Lipfert L., Parsons J. T., Ginsberg M. H., Brugge J. S. Tyrosine phosphorylation of pp125FAK in platelets requires coordinated signaling through integrin and agonist receptors. J Biol Chem. 1994 May 20;269(20):14738–14745. [PubMed] [Google Scholar]
- Siegel J. N., Klausner R. D., Rapp U. R., Samelson L. E. T cell antigen receptor engagement stimulates c-raf phosphorylation and induces c-raf-associated kinase activity via a protein kinase C-dependent pathway. J Biol Chem. 1990 Oct 25;265(30):18472–18480. [PubMed] [Google Scholar]
- Smyth S. S., Joneckis C. C., Parise L. V. Regulation of vascular integrins. Blood. 1993 Jun 1;81(11):2827–2843. [PubMed] [Google Scholar]
- Soligo D., Schiró R., Luksch R., Manara G., Quirici N., Parravicini C., Lambertenghi Deliliers G. Expression of integrins in human bone marrow. Br J Haematol. 1990 Nov;76(3):323–332. doi: 10.1111/j.1365-2141.1990.tb06363.x. [DOI] [PubMed] [Google Scholar]
- Symington B. E., Symington F. W., Rohrschneider L. R. Phorbol ester induces increased expression, altered glycosylation, and reduced adhesion of K562 erythroleukemia cell fibronectin receptors. J Biol Chem. 1989 Aug 5;264(22):13258–13266. [PubMed] [Google Scholar]
- Sánchez-Aparicio P., Dominguez-Jiménez C., Garcia-Pardo A. Activation of the alpha 4 beta 1 integrin through the beta 1 subunit induces recognition of the RGDS sequence in fibronectin. J Cell Biol. 1994 Jul;126(1):271–279. doi: 10.1083/jcb.126.1.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tonks N. K., Charbonneau H., Diltz C. D., Fischer E. H., Walsh K. A. Demonstration that the leukocyte common antigen CD45 is a protein tyrosine phosphatase. Biochemistry. 1988 Nov 29;27(24):8695–8701. doi: 10.1021/bi00424a001. [DOI] [PubMed] [Google Scholar]
- Troesch A., Duperray A., Polack B., Marguerie G. Comparative study of the glycosylation of platelet glycoprotein GPIIb/IIIa and the vitronectin receptor. Differential processing of their beta-subunit. Biochem J. 1990 May 15;268(1):129–133. doi: 10.1042/bj2680129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinacker A., Chen A., Agrez M., Cone R. I., Nishimura S., Wayner E., Pytela R., Sheppard D. Role of the integrin alpha v beta 6 in cell attachment to fibronectin. Heterologous expression of intact and secreted forms of the receptor. J Biol Chem. 1994 Mar 4;269(9):6940–6948. [PubMed] [Google Scholar]
- Werb Z., Tremble P. M., Behrendtsen O., Crowley E., Damsky C. H. Signal transduction through the fibronectin receptor induces collagenase and stromelysin gene expression. J Cell Biol. 1989 Aug;109(2):877–889. doi: 10.1083/jcb.109.2.877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ylänne J., Cheresh D. A., Virtanen I. Localization of beta 1, beta 3, alpha 5, alpha v, and alpha IIb subunits of the integrin family in spreading human erythroleukemia cells. Blood. 1990 Aug 1;76(3):570–577. [PubMed] [Google Scholar]