Abstract
Background
Treatment of schizophrenia depends heavily on neuroleptic drugs. Hypersalivation is a common side effect when people with schizophrenia are treated with neuroleptic drugs. Hypersalivation can be an embarrassing and stigmatising problem, can affect quality of life and can result in discontinuation of neuroleptic treatment. It can also be difficult to treat.
Objectives
To summarise the best available evidence of the effects of anticholinergic drugs in the treatment of non‐clozapine neuroleptic‐induced hypersalivation in people with schizophrenia. Clozapine‐induced hypersalivation has been addressed in another Cochrane review.
Search methods
We searched the Cochrane Schizophrenia Group Trials Register (15 November 2012) and inspected references of all identified studies for further relevant studies. We were to contact the first author of each included study for information regarding unpublished trials.
Selection criteria
All randomised controlled trials comparing an anticholinergic drug with placebo, no treatment, another anticholinergic drug or any other intervention.
Data collection and analysis
We inspected the results of the search to identify relevant studies. We were to extract data onto standard, simple forms. Disagreements were resolved through discussion. The risk of bias was to be assessed using the Cochrane risk assessment tool. For binary outcomes, we were to calculate a standard estimation of the risk ratio (RR) and its 95% confidence interval (CI). For continuous outcomes, we were to estimate the mean difference between groups.
Main results
The search resulted in four potential studies; after inspection, all were excluded. Three studies were excluded because they involved people with clozapine‐induced hypersalivation ‐ a topic covered in another Cochrane review. The fourth study was excluded because it involved people with schizophrenia, mood disorders or other mental disorders who were suffering from clozapine‐ and non‐clozapine induced hypersalivation and were treated with Chinese medicines with unknown anticholinergic properties. People in the control group received an anticholinergic drug (artane) or an antihistamine (phenergan). It was not possible to separate clozapine‐ from non‐clozapine‐treated people in the intervention group, or to separate artane‐treated people from phenergan‐treated people in the control group.
Authors' conclusions
We have been unable to locate any studies addressing the question raised in this review. Accordingly, this empty review points out an important clinical problem that needs to be investigated via well‐designed and well‐conducted randomised trials. Clinicians and patients are likely to continue with their current dependence on clinical judgement and personal experience. Policy makers have no trial‐based evidence upon which to base guidelines for the treatment of hypersalivation induced by neuroleptics other than clozapine. They are likely to continue to rely on opinion and habit when making recommendations. Funders of studies may wish to make this important subgroup of people a priority in future research.
Keywords: Humans, Antipsychotic Agents, Antipsychotic Agents/adverse effects, Cholinergic Antagonists, Cholinergic Antagonists/therapeutic use, Schizophrenia, Schizophrenia/drug therapy, Sialorrhea, Sialorrhea/chemically induced, Sialorrhea/drug therapy
Plain language summary
Anticholinergic medication for excessive salivation caused by use of antipsychotics other than clozapine
The first line of treatment of schizophrenia is usually antipsychotic drugs. These drugs help in the treatment of the ‘positive symptoms’ of schizophrenia, such as hearing voices, seeing things and having strange beliefs. However, these drugs often have serious side effects, such as weight gain, muscle stiffness, tiredness, apathy and lack of drive. Dribbling or drooling (hypersalivation) is another common side effect, which frequently occurs at night when asleep. This can be an embarrassing and stigmatising problem that can affect quality of life and cause people to stop their medication, which may result in relapse and going back into hospital. Dribbling and drooling can be difficult to treat; however, anticholinergic drugs can decrease production of saliva and dribbling. This review assessed the evidence for the benefit or harm of anticholinergic drugs used in treating hypersalivation caused by antipsychotic or neuroleptic medication. The review excluded the antipsychotic clozapine, as its role in causing hypersalivation has been the subject of another Cochrane review.
The search was carried out 15 November 2012 and resulted in identification of four potential studies, but none could be included. Three of these were excluded because they involved clozapine‐related hypersalivation. The fourth study was excluded because it involved people with mood or other mental disorders and Chinese medicines. Dribbling or hypersalivation is an important problem that needs to be investigated via well‐designed research and randomised trials. Until such time, psychiatrists and patients are likely to continue their treatment of hypersalivation on the basis of daily clinical judgement and personal experience rather than hard evidence. Treatment of hypersalivation caused by antipsychotics or neuroleptics other than clozapine does not seem to have received adequate research attention to help guide practice. The review authors conclude that using anticholinergics to treat dribbling or hypersalivation caused by antipsychotic drugs other than clozapine cannot be justified without further study.
This plain language summary has been written by Benjamin Gray, Service User and Service User Expert: Rethink Mental Illness. Email: ben.gray@rethink.org
Background
Description of the condition
Schizophrenia is a serious, disabling, enduring and relapsing mental illness, the onset of which frequently occurs relatively early in life. It can cause a person to experience problems with the ability to think, feel and perceive things clearly (Carpenter 1994). The worldwide lifetime prevalence of the disorder is about 1% (Almeida‐Filho 1997).
Treatment of schizophrenia depends heavily on neuroleptic drugs, which usually are classified into first‐generation 'typical' and second‐generation 'atypical' drugs (Miyamoto 2005). Hypersalivation (or sialorrhoea or drooling) is a significant adverse effect of both typical and atypical neuroleptic drugs. It is most commonly seen in people with schizophrenia treated with clozapine, among whom it affects approximately 31% of patients, usually developing early in the course of treatment, and is more prominent at night (Safferman 1991). Clozapine has been confirmed as a potent cause of hypersalivation in a systematic overview of Cochrane reviews on anticholinergic effects of antipsychotic drugs (Ozbilen 2009). In the short term (up to 12 weeks), only zotepine was more potent than clozapine in inducing hypersalivation. However, the second‐generation 'atypical' drugs tend to cause this problem less frequently than the first‐generation 'typical' drugs, with the exception of perphenazine (Table 1).
1. Prevalence of Increased Salivation, Short‐term Results (by 12 Weeks or Less) (adopted from Ozbilen 2009).
| Compound | n | RCTs | % (95% CI) |
| Placebo | 403 | 10 | 7.7 (5 to 11) |
| Clozapine | 559 | 16 | 32.7 (29 to 37) |
| Zotepine | 53 | 1 | 41.5 (29 to 55) |
| Zuclopenthixol hydrochloride | 66 | 2 | 24.2 (16 to 36) |
| Haloperidol | 1115 | 12 | 18.4 (16 to 21) |
| Thioridazine | 66 | 2 | 15.2 (8 to 26) |
| Chlorpromazine | 499 | 12 | 14.2 (11 to 18) |
| Trifluoperazine | 44 | 6 | 13.6 (6 to 27) |
| Molindone | 42 | 2 | 9.5 (4 to 22) |
| Bromperidol depot | 27 | 1 | 3.7 (1 to 18) |
| Olanzapine | 1857 | 5 | 8.2 (7 to 10) |
| Fluphenazine hydrochloride | 110 | 2 | 8.2 (4 to 15) |
| Amisulpride | 115 | 1 | 7.8 (4 to 14) |
| Sulpiride | 154 | 6 | 7.1 (4 to 12) |
| Risperidone depot | 317 | 2 | 5.7 (4 to 9) |
| Perphenazine | 74 | 3 | 5.4 (2 to 13) |
| Risperidone | 325 | 3 | 3.7 (2 to 6) |
Description of the intervention
Hypersalivation seems paradoxical because antipsychotic drugs tend to cause a dry mouth as a result of anticholinergic adverse effects (Hori 2006). However, several studies suggest that different receptors located on the salivary glands, including adrenergic and muscarinic receptors, can alter salivary flow (Mandel 1975; Ukai 1989; Zorn 1994; Corrigan 1995). Muscarinic and adrenergic receptors have been targeted by pharmacological intervention of neuroleptic‐induced hypersalivation. This review will focus on muscarinic anticholinergic antagonists such as pirenzepine (Fritz 1995), benzhexol (trihexyphenidyl) (Spivak 1997), benztropine (Reinstein 1999), atropine (Antonello 1999), hyoscine hydrobromide and ipratropium bromide (Calderon 2000).
How the intervention might work
Anticholinergic medication for neuroleptic‐induced hypersalivation may work by blocking muscarinic receptors. The combination of an antimuscarinic agent (benztropine) and an adrenergic alpha1 receptor antagonist (terazosin) may be more successful in controlling hypersalivation than either drug alone (Reinstein 1999).
Why it is important to do this review
Hypersalivation is a common effect when people with schizophrenia are treated with neuroleptic drugs (Table 1, Hori 2006). Hypersalivation can be an embarrassing and stigmatising problem, can affect quality of life and can result in discontinuation of neuroleptic treatment. It can also be difficult to treat. Another Cochrane review has focused on the treatment of clozapine‐induced hypersalivation (Syed 2008). This review is important because it aims to evaluate the quality of evidence for the benefit and harm of anticholinergic drugs used in treating hypersalivation induced by neuroleptics other than clozapine.
Objectives
To summarise the best available evidence of the effects of anticholinergic drugs in the treatment of non‐clozapine neuroleptic‐induced hypersalivation in people with schizophrenia. Clozapine‐induced hypersalivation has been addressed in another Cochrane review.
Methods
Criteria for considering studies for this review
Types of studies
All relevant randomised controlled trials. If a trial was described as 'double blind' but implied randomisation, we were to include such trials in a sensitivity analysis (see Sensitivity analysis). If inclusion of such trials did not result in a substantive difference, they would have remained in the analyses. If their inclusion did result in statistically significant differences, we were not to add the data from these lower‐quality studies to the results of the better trials but rather would present such data within a subcategory. We were to exclude quasi‐randomised studies, such as those allocating by alternate days of the week. When people were given additional treatments with an anticholinergic medication, we would have included data only if the adjunct treatment was evenly distributed between groups and if it was only the anticholinergic medication that had been randomly assigned.
Types of participants
Adults, however defined, with schizophrenia or related disorders, including schizophreniform disorder, schizoaffective disorder and delusional disorder, again, by any means of diagnosis with non‐clozapine neuroleptic‐induced hypersalivation, however identified (including recipient, carer and clinician).
We are interested in making sure that information is as relevant to the current care of people with schizophrenia as possible, so we proposed that if possible, we would clearly highlight the current clinical state (acute, early post‐acute, partial remission, remission), as well as the stage (prodromal, first episode, early illness, persistent), and would ascertain whether the studies primarily focused on people with particular problems (e.g. negative symptoms, treatment‐resistant illnesses).
Types of interventions
1. Anticholinergic drugs
Any anticholinergic drug at any dose and by any route of administration. Such drugs include pirenzepine, benzhexol (trihexyphenidyl), benztropine, atropine, hyoscine hydrobromide and ipratropium bromide. We proposed to compare the effects of these with the following.
2. Placebo
This includes placebo or no treatment.
3. Any another anticholinergic drug
4. Any other intervention
Types of outcome measures
All outcomes were to be divided into short‐term (within three months), medium‐term (3 to 12 months) and long‐term (longer than one year).
Primary outcomes
1. Measurement of salivation
1.1 Cured 1.2 Clinically important improvement in hypersalivation
2. Adverse effects-general and specific
2.1 Clinically important general adverse effects
Secondary outcomes
1. Measurement of salivation
1.1 Average endpoint hypersalivation score 1.2 Average change in hypersalivation scores
2. Quality of life (recipient of care or informal carers or professional carers)
2.1 Clinically important change in quality of life 2.2 Average endpoint quality of life score 2.3 Average change in quality of life scores 2.4 Clinically important change in specific aspects of quality of life 2.5 Average endpoint specific aspects of quality of life 2.6 Average change in specific aspects of quality of life
3. Adverse effects-general and specific
3.1 Average endpoint general adverse effect score 3.2 Average change in general adverse effect scores 3.3 Clinically important specific adverse effects 3.4 Average endpoint specific adverse effects 3.5 Average change in specific adverse effects 3.6 Sudden and unexpected death
4. Global state
4.1 Relapse 4.2 Clinically important change in global state (as defined by individual studies) 4.3 Average endpoint global state score 4.4 Average change in global state scores 4.5 Use of other medications
5. Service outcomes
5.1 Hospitalisation 5.2 Time to hospitalisation
6. Mental state (with particular reference to the positive and negative symptoms of schizophrenia)
6.1 Clinically important change in general mental state 6.2 Average endpoint general mental state score 6.3 Average change in general mental state scores 6.4 Clinically important change in specific symptoms (positive symptoms of schizophrenia, negative symptoms of schizophrenia, depression, mania) 6.5 Average endpoint specific symptom score 6.6 Average change in specific symptom scores
7. General functioning
7.1 Clinically important change in general functioning 7.2 Average endpoint general functioning score 7.3 Average change in general functioning scores 7.4 Clinically important change in specific aspects of functioning, such as social or life skills 7.5 Average endpoint specific aspects of functioning, such as social or life skills 7.6 Average change in specific aspects of functioning, such as social or life skills
8. Behaviour
8.1 Clinically important change in general behaviour 8.2 Average endpoint general behaviour score 8.3 Average change in general behaviour scores 8.4 Clinically important change in specific aspects of behaviour 8.5 Average endpoint specific aspects of behaviour 8.6 Average change in specific aspects of behaviour
9. Satisfaction with treatment
9.1 Leaving the studies early 9.2 Recipient of care satisfied with treatment 9.3 Recipient of care average satisfaction score 9.4 Recipient of care average change in satisfaction scores 9.5 Carer satisfaction with treatment 9.6 Carer average satisfaction score 9.7 Carer average change in satisfaction scores
10. Economic outcomes
10.1 Direct costs 10.2 Indirect costs
11. Summary of findings table
We were to use the GRADE approach to interpret findings (Schünemann 2008) and to use the GRADE profiler (GRADE PRO) to import data from RevMan 5.1 (Review Manager) to create 'Summary of findings' tables. These tables provide outcome‐specific information concerning the overall quality of evidence from each included study in the comparison, the magnitude of effect of the interventions examined, and the sum of available data on all outcomes that we will rate as important to patient‐ care and decision making. We were to select the following main outcomes for inclusion in a 'Summary of findings' table.
1. Measurement of salivation (binary) 2. Quality of life 3. Adverse effects 4. Satisfaction with treatment 5. Economic outcome
Search methods for identification of studies
Electronic searches
1. Cochrane Schizophrenia Group Trials Register (November 2012)
We searched the register using the phrase:
[((*hypersaliv* or *drool* or * saliva* or *ptyalism* or *sialism* or *sailorr* in title abstract or index terms of REFERENCE or outcomes of STUDY) or (*hypersaliv* in Helath care conditions of STUDY)) AND (*anticholinergic* OR *atropine* OR *benzatropine* OR *benzhexol* OR *hyoscine hydrobromide* OR *ipratropium bromide* OR *muscarinic* OR *pirenzepine* OR *trihexyphenidyl* in interventions of STUDY)]
This register is compiled by systematic searches of major databases, handsearches and searches of conference proceedings (see group module).
Searching other resources
1. Reference searching
We were to inspect references of all included studies for further relevant studies.
2. Personal contact
We were to contact the first author of each included study for information regarding unpublished trials.
Data collection and analysis
Selection of studies
Review authors AR, MFA, BA and AT independently inspected citations from the searches to identify relevant abstracts. AE and NAH were to independently re‐inspect a random 20% sample to ensure reliability, but this was not needed as the number of studies was low. Full reports of abstracts meeting review criteria or of references/abstracts about which review authors disagreed were to be obtained and inspected by AR, MFA, BA and AT. Again, AE and NAH were to re‐inspect a random 20% of reports to ensure reliable selection. Where it was not possible to resolve disagreement by discussion, we contacted the authors of the study for clarification.
Data extraction and management
1. Extraction
Review authors AR, MFA, BA and AT were to extract data from all included studies. In addition, to ensure reliability, AE and NAH were to independently extract data from a random sample of these studies, constituting 50% of the total. Again, any disagreement would have been discussed and decisions documented, and, if necessary, we were to contact the authors of studies for clarification. With remaining problems, AE and NAH would have helped to clarify issues, and we would have documented these final decisions. We were to extract data presented only in graphs and figures whenever possible, but we were to include the data only if two review authors independently achieved the same result. We would have attempted to contact authors through an open‐ended request to obtain missing information or for clarification whenever necessary. If studies were multi‐centre, where possible, we were to extract data relevant to each component centre separately.
2. Management
2.1 Forms
We extracted data onto standard, simple forms.
2.2 Scale‐derived data
We were to include continuous data from rating scales only if: a. the psychometric properties of the measuring instrument had been described in a peer‐reviewed journal (Marshall 2000); and b. the measuring instrument had not been written or modified by one of the trialists for that particular trial.
Ideally the measuring instrument should be a self‐report or should be completed by an independent rater or relative (not the therapist). We realise that this often is not reported clearly; in 'Description of studies', we will note whether or not this is the case.
2.3 Endpoint versus change data
Both endpoint and change data provide advantages. Change data can remove a component of between‐person variability from the analysis. On the other hand, calculation of change needs two assessments (baseline and endpoint), which can be difficult to perform in unstable and difficult to measure conditions such as schizophrenia. We have decided to use primarily endpoint data and to use change data only if the former are not available. We were to combine endpoint and change data in the analysis, as we were to use mean differences (MDs) rather than standardised mean differences (SMDs) throughout (Higgins 2011).
2.4 Skewed data
Continuous data on clinical and social outcomes often are not normally distributed. To avoid the pitfall of applying parametric tests to non‐parametric data, we aimed to apply the following standards to all data before inclusion: (a) standard deviations (SDs) and means are reported in the paper or are obtainable from the authors; (b) when a scale starts from the finite number zero, the SD, when multiplied by two, is less than the mean (as otherwise, the mean is unlikely to be an appropriate measure of the centre of the distribution (Altman 1996)); and (c) if a scale started from a positive value (such as the Positive and Negative Syndrome Scale (PANSS), which can have values from 30 to 210), we will modify the calculation described above to take the scale starting point into account. In these cases, skew is present if 2 SD > (S - S min), where S is the mean score and S min is the minimum score. Endpoint scores on scales often have finite start and end points, and these rules can be applied. We were to enter skewed endpoint data from studies of fewer than 200 participants as other data within the data and analyses section rather than into a statistical analysis. Skewed endpoint data pose less of a problem when means are used if the sample size is large and if we synthesise such data.
When continuous data are presented on a scale that includes a possibility of negative values (such as change data), it is difficult to tell whether or not data are skewed. We were to enter skewed change data into analyses.
2.5 Common measure
To facilitate comparison between trials, we intended to convert variables that can be reported in different metrics, such as days in hospital (mean days per year, per week or per month), to a common metric (e.g. mean days per month).
2.6 Conversion of continuous to binary
Where possible, efforts were to be made to convert outcome measures to dichotomous data. This can be done by identifying cut‐off points on rating scales and dividing participants accordingly into 'clinically improved' and 'not clinically improved'. It is generally assumed that if a 50% reduction is seen in a scale‐derived score such as the Brief Psychiatric Rating Scale (BPRS; Overall 1962) or the PANSS (Kay 1986), this could be considered a clinically significant response (Leucht 2005, Leucht 2005a). If data based on these thresholds were not available, we were to use the primary cut‐off presented by the original authors.
2.7 Direction of graphs
Where possible, we were to enter data in such a way that the area to the left of the line of no effect indicates a favourable outcome for anticholinergic medication. Where keeping to this makes it impossible to avoid outcome titles with clumsy double‐negatives (e.g. 'Not improved'), we were to report data in such a way that the area to the left of the line indicates an unfavourable outcome. We were to note this in the relevant graphs.
Assessment of risk of bias in included studies
Again AR, MFA, BA and AT worked independently to assess risk of bias by using criteria described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011) to assess trial quality. This set of criteria is based on evidence of associations between overestimate of effect and high risk of bias of the article, such as sequence generation, allocation concealment, blinding, incomplete outcome data and selective reporting.
If the raters disagreed, we were to make the final rating by consensus, with the involvement of AE and NAH. Where inadequate details of randomisation and other characteristics of trials were provided, we contacted the authors of the studies to obtain further information. We were to report non‐concurrence in quality assessment, but if disputes arose regarding to which category a trial was to be allocated, we were to resolve them by discussion.
We were to note the level of risk of bias in both the text of the review and 'Summary of findings' table.
Measures of treatment effect
1. Binary data
For binary outcomes, we were to calculate a standard estimation of the risk ratio (RR) and its 95% confidence interval (CI). It has been shown that RR is more intuitive (Boissel 1999) than odds ratios (ORs) and that ORs tend to be interpreted as RR by clinicians (Deeks 2000).
2. Continuous data
For continuous outcomes, we were to estimate the MD between groups. We preferred not to calculate effect size measures (SMD). However, if scales of very considerable similarity were used, we would have presumed a small difference in measurement, and we would have calculated effect size and transformed the effect back to the units of one or more of the specific instruments.
Unit of analysis issues
1. Cluster trials
Studies increasingly employ 'cluster randomisation' (such as randomisation by clinician or practice), but analysis and pooling of clustered data can pose problems. First, authors often fail to account for intraclass correlation in clustered studies, leading to a 'unit of analysis' error (Divine 1992) whereby P values are spuriously low, CIs unduly narrow and statistical significance overestimated. This causes type I errors (Bland 1997; Gulliford 1999).
If clustering was not accounted for in primary studies, we would have presented data in a table, with an (*) symbol to indicate the presence of a probable unit of analysis error. In subsequent versions of this review, we will seek to contact the first authors of such studies to obtain intraclass correlation coefficients (ICCs) for their clustered data and to adjust for these by using accepted methods (Gulliford 1999).
If clustering had been incorporated into the analysis of primary studies, we would have presented these data as if from a non‐cluster randomised study with adjustment for the clustering effect.
We have sought statistical advice and have been advised that the binary data as presented in a report should be divided by a 'design effect'. This is calculated by using the mean number of participants per cluster (m) and the ICC [Design effect = 1 + (m - 1) * ICC] (Donner 2002). If the ICC was not reported, we were to assume that it was 0.1 (Ukoumunne 1999).
If cluster studies had been appropriately analysed by taking into account ICCs and relevant data documented in the report, synthesis with other studies would have been possible through the generic inverse variance technique.
2. Cross‐over trials
A major concern of cross‐over trials is the carry‐over effect. It occurs if an effect (e.g. pharmacological, physiological, psychological) of the treatment in the first phase is carried over to the second phase. As a consequence, on entry to the second phase, participants can differ systematically from their initial state despite a wash‐out phase. For the same reason, cross‐over trials are not appropriate if the condition of interest is unstable (Elbourne 2002). As both effects are very likely in severe mental illness, we were to use only data from the first phase of cross‐over studies.
3. Studies with multiple treatment groups
Where a study involved more than two treatment arms, if relevant, we were to present the additional treatment arms in comparisons. If data were binary, we were to simply add and combine them within the two‐by‐two table. If data were continuous, we were to combine data in accordance with the formula in Section 7.7.3.8 (Combining groups) of the Cochrane Handbook for Systematic Reviews of Interventions. Where the additional treatment arms were not relevant, we were not to reproduce the data.
Dealing with missing data
1. Overall loss of credibility
At some degree of loss of follow‐up, data must lose credibility (Xia 2009). We chose that for any particular outcome, should more than 50% of data be unaccounted for, we would not have reproduced these data or used them within analyses, except for the outcome of leaving the study early. If, however, more than 50% of those in one arm of a study were lost, but the total loss was less than 50%, we would have marked such data with an (*) to indicate that such a result may well be prone to bias.
2. Binary
In the case where attrition for a binary outcome was between 0% and 50% and where these data were not clearly described, we were to present the data on a 'once‐randomised‐always‐analyse' basis (an intention‐to‐treat analysis). All of those leaving the study early would have been assumed to have the same rates of negative outcome as those who completed the study. We were to undertake a sensitivity analysis to test how prone the primary outcomes were to change when 'completer' data only were compared with the intention‐to‐treat analysis using the above assumptions.
3. Continuous
3.1 Attrition
In cases where attrition for a continuous outcome was between 0% and 50% and completer‐only data were reported, we were to reproduce these data.
3.2 Standard deviations
If SDs were not reported, we were to try first to obtain the missing values from the authors. If these data were not available and measures of variance for continuous data were missing, but an exact standard error (SE) and CIs were available for group means, and either P or t values were available for differences in mean, we were to calculate them according to the rules described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011): When only the SE is reported, SDs are calculated by the formula SD = SE * square root (n). Chapters 7.7.3 and 16.1.3 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011) present detailed formulas for estimating SDs from P values, t or F values, CIs, ranges or other statistics. If these formula did not apply, we were to calculate the SDs according to a validated imputation method based on the SDs of the other included studies (Furukawa 2006). Although some of these imputation strategies can introduce error, the alternative would be to exclude a given study’s outcome and thus to lose information. We nevertheless were to examine the validity of the imputations in a sensitivity analysis while excluding imputed values.
3.3 Last observation carried forward
We anticipated that in some studies, the method of last observation carried forward (LOCF) would have been employed within the study report. As with all methods of imputation used to deal with missing data, LOCF introduces uncertainty about the reliability of the results (Leucht 2007). Therefore, where LOCF data had been used in the trial, if less than 50% of the data had been assumed, we would have reproduced these data and indicated that they are the product of LOCF assumptions.
Assessment of heterogeneity
1. Clinical heterogeneity
We were to consider all included studies initially, without seeing comparison data, to judge clinical heterogeneity. We were simply to inspect all studies for clearly outlying people or situations that we had not predicted would arise. When such situations or participant groups were noted, they were to be fully discussed.
2. Methodological heterogeneity
We were to consider all included studies initially, without seeing comparison data, to judge methodological heterogeneity. We were simply to inspect all studies for clearly outlying methods that we had not predicted would arise. When such methodological outliers were noted, they were to be fully discussed.
3. Statistical heterogeneity
3.1 Visual inspection
We were to visually inspect graphs to investigate the possibility of statistical heterogeneity.
3.2 Employing the I2 statistic
We were to investigate heterogeneity between studies by considering the I2 method alongside the Chi2 P value. The I2 statistic provides an estimate of the percentage of inconsistency thought to be due to chance (Higgins 2003). The importance of the observed value of I2 depends on the magnitude and direction of effects and the strength of the evidence for heterogeneity (e.g. P value from Chi2 test, or CI for I2). We will interpret an I2 estimate greater than or equal to around 50% accompanied by a statistically significant Chi2 statistic as evidence of substantial levels of heterogeneity (Section 9.5.2-Higgins 2011). When substantial levels of heterogeneity were found in the primary outcome, we were to explore reasons for heterogeneity (Subgroup analysis and investigation of heterogeneity).
Assessment of reporting biases
1. Protocol versus full study
Reporting biases arise when the dissemination of research findings is influenced by the nature and direction of results. These are described in Section 10.1 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We were to try to locate protocols of included randomised trials. If the protocol was available, we would have compared the outcomes in the protocol with those in the published report. If the protocol was not available, we would have compared outcomes listed in the methods section of the trial report with actual reported results.
2. Funnel plot
Publication biases arise when the dissemination of research findings is influenced by the nature and direction of results (Egger 1997). These again are described in Section 10 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We are aware that funnel plots may be useful in investigating publication biases but are of limited power for detecting small study effects. We were not to use funnel plots for outcomes where 10 or fewer studies were included, or where all studies were of similar size. In other cases, where funnel plots were possible, we were to seek statistical advice in their interpretation.
Data synthesis
We understood that there was no closed argument regarding preference for use of fixed‐effect or random‐effects models. The random‐effects method incorporates an assumption that different studies are estimating different, yet related, intervention effects. This often seems to be true to us, and the random‐effects model takes into account differences between studies even if no statistically significant heterogeneity is noted. However, the random‐effects model provides a disadvantage. It puts added weight onto small studies, which often are the most biased ones. Depending on the direction of effect, these studies can inflate or deflate the effect size. We intended to use a fixed‐effect model for all analyses.
Subgroup analysis and investigation of heterogeneity
1. Subgroup analyses-only primary outcomes
1.1 Intervention
We anticipated subgroup analyses to investigate the use of anticholinergic drugs in combination with other drugs for hypersalivation.
1.2 Clinical state, stage or problem
We proposed to undertake this review and provide an overview of the effects of anticholinergic medication among people with schizophrenia in general. In addition, we were to try to report data on subgroups of people in the same clinical state or stage and with similar problems.
2. Investigation of heterogeneity
If inconsistency was high, we were to report it. First, we would have investigated whether data had been entered correctly. Second, if data were correct, we would have visually inspected the graph and removed outlying studies to see whether heterogeneity could be restored. For this review, we had decided that should this occur with data contributing to the summary finding of no more than around 10% of the total weighting, we would have presented the data. If not, we would not have pooled data but would have discussed the relevant issues in the text. We knew of no supporting research for this 10% cut‐off but were investigating the use of prediction intervals as an alternative to this unsatisfactory state.
When unanticipated clinical or methodological heterogeneity was obvious, we simply would have stated hypotheses regarding this for future reviews or future versions of this review. We did not anticipate undertaking analyses related to these.
Sensitivity analysis
1. Implication of randomisation
We aimed to include trials in a sensitivity analysis if they were described in some way as to imply randomisation. For primary outcomes, we were to include these studies; if no substantive difference was noted when the implied randomised studies would have been added to those with better descriptions of randomisation, we were to use all data from these studies.
2. Assumptions for lost binary data
Where assumptions have to be made regarding people lost to follow‐up (see Dealing with missing data), we were to compare the findings of primary outcomes when we use our assumption with completer data only. If a substantial difference was noted, we were to report results and discuss them but continue to employ our assumption.
Where assumptions have to be made regarding missing SD data (see Dealing with missing data), we were to compare the findings of primary outcomes when we use our assumption with completer data only. We were to undertake a sensitivity analysis to test how prone results were to change when 'completer' data only are compared with imputed data using the above assumption. If a substantial difference was noted, we were to report results and discuss them but continue to employ our assumption.
3. Risk of bias
We were to analyse the effects of excluding trials that had been judged to be at high risk of bias across one or more of the domains of randomisation (implied as randomised with no further details available), allocation concealment, blinding and outcome reporting for the meta‐analysis of the primary outcome. If the exclusion of trials at high risk of bias did not substantially alter the direction of effect or the precision of effect estimates, we would have included data from these trials in the analysis.
4. Imputed values
We were to undertake a sensitivity analysis to assess the effects of including data from trials where we used imputed values for ICC in calculating the design effect in cluster‐randomised trials.
If substantial differences were noted in the direction or precision of effect estimates in any of the sensitivity analyses listed above, we would have not pooled data from the excluded trials with those of other trials contributing to the outcome but would have presented them separately.
5. Fixed‐effect and random‐effects models
We were to synthesise all data using a fixed‐effect model; however, we were also to synthesise data for the primary outcome using a random‐effects model to evaluate whether the greater weight assigned to larger trials with greater event rates altered the significance of the results compared with more evenly distributed weight in the random‐effects model.
Results
Description of studies
Results of the search
The search yielded only four references, all of which were randomised controlled studies. See also Figure 1.
1.
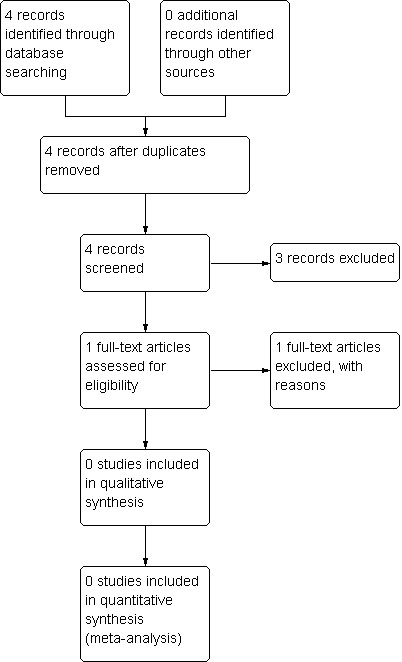
Study flow diagram.
Included studies
No included studies are provided in this review.
Excluded studies
Three studies (Reinstein 1999; Bai 2001; ISRCTN47146067 2010) were excluded because they involved people with clozapine‐induced hypersalivation ‐ a topic covered in another Cochrane review (Syed 2008). The fourth study (Hui 2002) was excluded because it investigated people with schizophrenia, mood disorders or other mental disorders who were suffering from clozapine‐ and non‐clozapine‐induced hypersalivation and were treated with Chinese medicines with unknown anticholinergic properties. People in the control group received an anticholinergic drug (artane) or an antihistamine (phenergan). It was not possible to separate clozapine‐ from non‐clozapine‐treated people in the intervention group or to separate artane‐treated people from phenergan‐treated people in the control group. We have sought this information but have not yet received an answer.
Risk of bias in included studies
No included studies are provided in this review.
Allocation
No included studies are provided in this review.
Blinding
No included studies are provided in this review.
Incomplete outcome data
No included studies are provided in this review.
Selective reporting
No included studies are provided in this review.
Other potential sources of bias
No included studies are provided in this review.
Effects of interventions
No included studies are provided in this review.
Discussion
Treatment of schizophrenia depends heavily on neuroleptic drugs. Hypersalivation is a common side effect when people with schizophrenia are treated with neuroleptic drugs. Hypersalivation can be an embarrassing and stigmatising problem, can affect quality of life and can result in discontinuation of neuroleptic treatment. It can also be difficult to treat. The aim of this review was to evaluate the quality of evidence for the benefit and harm of anticholinergic drugs used in treating hypersalivation induced by neuroleptics other than clozapine.
We have been unable to locate any studies addressing the question raised in this review. Accordingly, this may be considered an "empty review", pointing at an important clinical problem that needs to be investigated (Yaffe 2012). Well‐designed and well‐conducted RCTs in the treatment of hypersalivation induced by neuroleptics other than clozapine are needed. Such trials are feasible as demonstrated by the excluded studies described in this review.
Summary of main results
The main result is that no randomised controlled trials have investigated the effects of anticholinergic drugs for non‐clozapine neuroleptic‐induced hypersalivation.
Overall completeness and applicability of evidence
No included studies are provided in this review. The evidence is not complete, and no randomised controlled trials could guide treatment decisions in this area.
Quality of the evidence
No included studies are provided in this review.
Potential biases in the review process
No included studies are provided in this review. We might have missed some studies, but this is unlikely because we searched the Schizophrenia Group Trial Register, which is compiled by systematic searches of major databases, handsearches and searches of conference proceedings
Agreements and disagreements with other studies or reviews
We were unable to locate any studies, and we are not aware of another systematic review that has addressed the effects of anticholinergic medication for non‐clozapine neuroleptic‐induced hypersalivation.
Authors' conclusions
Implications for practice.
1. For clinicians
Treatment of hypersalivation induced by neuroleptics other than clozapine does not seem to have received adequate research attention, and no RCT‐based evidence is available to help guide practice. Clinicians are likely to continue with their current practices of using clinical judgement.
2. For people with schizophrenia
Patients suffering from hypersalivation induced by neuroleptics other than clozapine and their relatives are justified to be disappointed in the medical/research fraternity.
3. For policy makers
Policy makers have no RCT‐based evidence upon which to base guidelines for the treatment of hypersalivation induced by neuroleptics other than clozapine. They are likely to continue to rely on opinion and habit when making recommendations.
Implications for research.
The treatment of hypersalivation induced by neuroleptics other than clozapine cannot be justified without well‐designed, well‐conducted and well‐reported randomised studies. At present, no convincing evidence supports any intervention for hypersalivation induced by neuroleptics other than clozapine. Clinically meaningful randomised studies are needed to help guide clinicians in the management of people suffering from hypersalivation induced by neuroleptics other than clozapine. Available publications prove that such studies are possible.
Funders of studies may wish to make this important subgroup of people a priority in future research.
Acknowledgements
The Cochrane Schizophrenia Group Editorial Base in Nottingham produces and maintains standard text for use in the Methods sections of their reviews. We have used this text as the basis of what appears here and have adapted it as required.
We would like to acknowledge the contribution of Maher Sharaf, who helped develop the protocol and assisted in writing the review.
Characteristics of studies
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Bai 2001 | Allocation: randomised. Participants: people with clozapine‐induced hypersalivation. |
| Hui 2002 | Allocation: randomised. Participants: people with schizophrenia, mood disorders or other mental disorders and patients with clozapine‐induced and non-clozapine‐induced hypersalivation. Interventions.
|
| ISRCTN47146067 2010 | Allocation: randomised. Participants: people with clozapine‐induced hypersalivation. |
| Reinstein 1999 | Allocation: randomised. Participants: people with clozapine‐induced hypersalivation. |
Differences between protocol and review
None
Contributions of authors
Adib Essali ‐ developed protocol and wrote review.
Nahla Alhaj Hasan ‐ developed protocol and wrote review.
Anas Rihawi ‐ developed protocol and wrote review.
Bishr Alhafez ‐ developed protocol and wrote review.
Amjad Tarboush ‐ developed protocol and wrote review.
Mohammad Fakhri Al‐tujjar ‐ help with selection of trials and data extraction.
Sources of support
Internal sources
-
Association for Evidence‐Based Medicine, Syrian Arab Republic.
Training
External sources
No sources of support supplied
Declarations of interest
None.
New
References
References to studies excluded from this review
Bai 2001 {published data only}
- Bai YM, Lin CC, Chen JY, Liu WC. Therapeutic effect of pirenzepine for clozapine‐induced hypersalivation: a randomized, double‐blind, placebo‐controlled, cross‐over study. Journal of Clinical Psychopharmacology 2001;21(6):608‐11. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Hui 2002 {published data only (unpublished sought but not used)}
- Flaws B. The Chinese medical treatment of hypersalivation as an adverse reaction to anti‐pyschotic medication. Blue Poppy Press 2003. [http://bluepoppy.com/cfwebstore/index.cfm/feature/898/the‐chinese‐medical‐treatment‐of‐hypersalivation‐as‐an‐adverse‐reaction‐to‐anti‐pyschotic‐medication.cfm]
- Hui L, Xingxing P. Treatment of neuroleptic salivation by Chinese Luijunazi Wan: a clinical observation of 56 cases. China Academic Journal 2002;8:19‐29. [Google Scholar]
ISRCTN47146067 2010 {published data only}
- ISRCTN47146067. Reducing excess salivation in clozapine treatment: hyoscine for the treatment of clozapine induced nocturnal sialorrhoea, 2010. http://www.controlled‐trials.com.
Reinstein 1999 {published data only}
- Reinstein MJ, Sirotovskaya LA, Chasanov MA, Jones LE, Mohan S. Comparative efficacy and tolerability of benzatropine and terazosin in the treatment of hypersalivation secondary to clozapine. Clinical Drug Investigation 1999;17(2):97‐102. [Google Scholar]
Additional references
Almeida‐Filho 1997
- Almeida‐Filho N, Mari JJ, Coutinho ESF, França J, Fernandes J, Andreoli SB, et al. Brazilian multicentric study of psychiatry morbidity: methodological features and prevalence estimates [Estudo multicêntrico de morbidade psiquiátrica em áreas urbanas brasileiras (Brasilia, São Paulo, Porto Alegre)]. British Journal of Psychiatry 1997;171:524‐9. [DOI] [PubMed] [Google Scholar]
Altman 1996
- Altman DG, Bland JM. Detecting skewness from summary information. BMJ 1996;313(7066):1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
Antonello 1999
- Antonello C. Clozapine and sialorrhea: a new intervention for this bothersome and potentially dangerous side effect. Journal of Psychiatry and Neuroscience 1999;24:250. [PMC free article] [PubMed] [Google Scholar]
Bland 1997
- Bland JM. Statistics notes. Trials randomised in clusters. BMJ 1997;315:600. [DOI] [PMC free article] [PubMed] [Google Scholar]
Boissel 1999
- Boissel JP, Cucherat M, Li W, Chatellier G, Gueyffier F, Buyse M, et al. The problem of therapeutic efficacy indices. 3. Comparison of the indices and their use [Apercu sur la problematique des indices d'efficacite therapeutique, 3: comparaison des indices et utilisation. Groupe d'etude des Indices d'efficacite]. Therapie 1999;54(4):405‐11. [PUBMED: 10667106] [PubMed] [Google Scholar]
Calderon 2000
- Calderon J, Robin E, Sobota WL. Potential use of ipatropium bromide for the treatment of clozapine‐induced hypersalivation: a preliminary report. International Clinical Psychopharmacology 2000;15:49‐52. [DOI] [PubMed] [Google Scholar]
Carpenter 1994
- Carpenter WT Jr, Buchanam RW. Schizophrenia. New England Journal of Medicine 1994;330:681‐90. [DOI] [PubMed] [Google Scholar]
Corrigan 1995
- Corrigan F, MacDonald S, Reynolds G. Clozapine‐induced hypersalivation and the alpha2 adrenoceptor. British Journal of Psychiatry 1995;167:412. [DOI] [PubMed] [Google Scholar]
Deeks 2000
- Deeks J. Issues in the selection for meta‐analyses of binary data. Proceedings of the 8th International Cochrane Colloquium; 2000 Oct 25‐28; Cape Town. Cape Town: The Cochrane Collaboration, 2000.
Divine 1992
- Divine GW, Brown JT, Frazier LM. The unit of analysis error in studies about physicians' patient care behavior. Journal of General Internal Medicine 1992;7(6):623‐9. [DOI] [PubMed] [Google Scholar]
Donner 2002
- Donner A, Klar N. Issues in the meta‐analysis of cluster randomized trials. Statistics in Medicine 2002;21(19):2971‐80. [DOI] [PubMed] [Google Scholar]
Egger 1997
- Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta‐analysis detected by a simple, graphical test. BMJ 1997;315(7109):629‐34. [DOI] [PMC free article] [PubMed] [Google Scholar]
Elbourne 2002
- Elbourne D, Altman DG, Higgins JPT, Curtina F, Worthingtond HV, Vaile A. Meta‐analyses involving cross‐over trials: methodological issues. International Journal of Epidemiology 2002;31(1):140‐9. [DOI] [PubMed] [Google Scholar]
Fritz 1995
- Fritz J, Tilmann E. Pirenzepine for clozapine‐induced hypersalivation. Lancet 1995;346:1034. [DOI] [PubMed] [Google Scholar]
Furukawa 2006
- Furukawa TA, Barbui C, Cipriani A, Brambilla P, Watanabe N. Imputing missing standard deviations in meta‐analyses can provide accurate results. Journal of Clinical Epidemiology 2006;59(1):7‐10. [DOI] [PubMed] [Google Scholar]
Gulliford 1999
- Gulliford MC. Components of variance and intraclass correlations for the design of community‐based surveys and intervention studies: data from the Health Survey for England 1994. American Journal of Epidemiology 1999;149(9):876‐83. [DOI] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ 2003;327(7414):557‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.2 [updated March 2011]. The Cochrane Collaboration, 2011. www.cochrane‐handbook.org.
Hori 2006
- Hori T, Makabe K, Nemoto K, Asada T. Hypersalivation induced by olanzapine with fluvoxamine. Progress in Neuro‐Psychopharmacology and Biological Psychiatry 2006;30(4):758‐60. [DOI] [PubMed] [Google Scholar]
Kay 1986
- Kay SR, Opler LA, Fiszbein A. Positive and Negative Syndrome Scale (PANSS) Manual. North Tonawanda, NY: Multi‐Health Systems, 1986. [Google Scholar]
Leucht 2005
- Leucht S, Kane JM, Kissling W, Hamann J, Etschel E, Engel RR. What does the PANSS mean?. Schizophrenia Research 2005;79(2‐3):231‐8. [PUBMED: 15982856] [DOI] [PubMed] [Google Scholar]
Leucht 2005a
- Leucht S, Kane JM, Kissling W, Hamann J, Etschel E, Engel R. Clinical implications of Brief Psychiatric Rating Scale scores. British Journal of Psychiatry 2005;187:366‐71. [PUBMED: 16199797] [DOI] [PubMed] [Google Scholar]
Leucht 2007
- Leucht S, Engel RR, Bauml J, Davis JM. Is the superior efficacy of new generation antipsychotics an artifact of LOCF?. Schizophrenia Bulletin 2007;33(1):183‐91. [PUBMED: 16905632] [DOI] [PMC free article] [PubMed] [Google Scholar]
Mandel 1975
- Mandel J, Zengo A, Katz R, Wotman S. Effects of adrenergic agents on salivary composition. Journal of Dental Research 1975;54:B27‐B33. [DOI] [PubMed] [Google Scholar]
Marshall 2000
- Marshall M, Lockwood A, Bradley C, Adams C, Joy C, Fenton M. Unpublished rating scales: a major source of bias in randomised controlled trials of treatments for schizophrenia. British Journal of Psychiatry 2000;176:249‐52. [DOI] [PubMed] [Google Scholar]
Miyamoto 2005
- Miyamoto S, Duncan GE, Marx CE, Lieberman JA. Treatments for schizophrenia: a critical review of pharmacology and mechanisms of action of antipsychotic drugs. Molecular Psychiatry 2005;10:79‐104. [DOI] [PubMed] [Google Scholar]
Overall 1962
- Overall JE, Gorham DR. The Brief Psychiatric Rating Scale. Psychological Reports 1962;10:799‐812. [Google Scholar]
Ozbilen 2009
- Ozbilen M, Adams CE. Systematic overview of Cochrane reviews for anticholinergic effects of antipsychotic drugs. Journal of Clinical Psychopharmacology 2009;29(2):141‐6. [DOI] [PubMed] [Google Scholar]
Safferman 1991
- Safferman A, Liberman J, Kane M, Szymanski S, Kinon B. Update on the clinical efficacy and side effects of clozapine. Schizophrenia Bulletin 1991;17:247‐61. [DOI] [PubMed] [Google Scholar]
Schünemann 2008
- Schünemann HJ, Oxman AD, Vist GE, Higgins JPT, Deeks JJ, Glasziou P, et al. Chapter 12: Interpreting results and drawing conclusions. In: Higgins JPT, Green S editor(s). Cochrane Handbook for Systematic Reviews of Interventions. The Cochrane Collaboration, 2008:359‐83. [Google Scholar]
Spivak 1997
- Spivak B, Adlersberg S, Rosen L, Gonen N, Mester R, Weizman A. Trihexyphenidyl treatment of clozapine induced hypersalivation. International Clinical Psychopharmacology 1997;12:213‐15. [DOI] [PubMed] [Google Scholar]
Syed 2008
- Syed R, Au K, Cahill C, Duggan L, He Y, Udu V, et al. Pharmacological interventions for clozapine‐induced hypersalivation. Cochrane Database of Systematic Reviews 2008, Issue 3. [DOI: 10.1002/14651858.CD005579.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ukai 1989
- Ukai Y, Taniguchi T, Kimura K. Muscarinic supersensitivity and subsensitivity induced by chronic treatment with atropine and disopylfluorophosphate in rat submaxillary glands. Archives of International Pharmacodynamic Therapy 1989;6:148‐57. [PubMed] [Google Scholar]
Ukoumunne 1999
- Ukoumunne OC, Gulliford MC, Chinn S, Sterne JAC, Burney PG. Methods for evaluating area‐wide and organisation‐based intervention in health and health care: a systematic review. Health Technology Assessment 1999;3(5):iii‐92. [PubMed] [Google Scholar]
Xia 2009
- Xia J, Adams CE, Bhagat N, Bhagat V, Bhoopathi P, El‐Sayeh H. Loss to outcomes stakeholder survey: the LOSS study. Psychiatric Bulletin 2009;33(7):254‐7. [Google Scholar]
Yaffe 2012
- Yaffe J, Montgomery P, Hopewell S, Shepard LD. Empty reviews: a description and consideration of Cochrane systematic reviews with no included studies. PLoS ONE 2012;7(5):e36626. [DOI: 10.1371/journal.pone.0036626] [DOI] [PMC free article] [PubMed] [Google Scholar]
Zorn 1994
- Zorn S, Jones S, Ward K, Liston D. Clozapine is a potent and selective muscarinic M4 receptor agonist. European Journal of Pharmacology 1994;269:R1‐2. [DOI] [PubMed] [Google Scholar]


