Abstract
Background
This is an update of a Cochrane review first published in The Cochrane Library in Issue 4, 2006.
Otitis media with effusion (OME) or 'glue ear' is an accumulation of fluid in the middle ear, in the absence of acute inflammation or infection. It is the commonest cause of acquired hearing loss in childhood and the usual reason for insertion of 'grommets'. Potential treatments include decongestants, mucolytics, steroids, antihistamines and antibiotics. Autoinflation devices have been proposed as a simple mechanical means of improving 'glue ear'.
Objectives
To assess the effectiveness of autoinflation compared with no treatment in children and adults with otitis media with effusion.
Search methods
We searched the Cochrane Ear, Nose and Throat Disorders Group Trials Register; the Cochrane Central Register of Controlled Trials (CENTRAL); PubMed; EMBASE; CINAHL; Web of Science; BIOSIS Previews; Cambridge Scientific Abstracts; ICTRP and additional sources for published and unpublished trials. The date of the most recent search was 12 April 2013.
Selection criteria
We selected randomised controlled trials that compared any form of autoinflation to no autoinflation in individuals with 'glue ear'.
Data collection and analysis
Two review authors independently assessed studies for inclusion, assessed risk of bias and extracted data from included studies.
Main results
Eight studies, with a total of 702 participants, met the inclusion criteria. Overall, the studies were predominantly assessed as being at low or unclear risk of bias; unclear risk was mainly due lack of information. There was no evidence of selective reporting.
Pooled estimates favoured the intervention, but did not show a significant effect on tympanometry (type C2 and B) at less than one month, nor at more than one month. Similarly, there were no significant changes for discrete pure‐tone audiometry and non‐discrete audiometry. Pooled estimates favoured, but not significantly, the intervention for the composite measure of tympanogram or audiometry at less than one month; at more than one month the result became significant (RRI 1.74, 95% CI 1.22 to 2.50). Subgroup analysis based on the type of intervention showed a significant effect using a Politzer device under one month (RRI 7.07, 95% CI 3.70 to 13.51) and over one month (RRI 2.25, 95% CI 1.67 to 3.04).
None of the studies demonstrated a significant difference in the incidence of side effects between interventions.
Authors' conclusions
All of the studies were small, of limited treatment duration and had short follow‐up. However, because of the low cost and absence of adverse effects it is reasonable to consider autoinflation whilst awaiting natural resolution of otitis media with effusion. Primary care could prove a beneficial place to evaluate such interventions and there is ongoing research in this area. Further research should also consider the duration of treatment, the long‐term impact on developmental outcomes in children and additional quality of life outcome measures for children and families.
Keywords: Adult, Child, Humans, Eustachian Tube, Acoustic Impedance Tests, Acoustic Impedance Tests/methods, Air, Hearing Loss, Hearing Loss/etiology, Hearing Loss/therapy, Insufflation, Insufflation/instrumentation, Insufflation/methods, Otitis Media with Effusion, Otitis Media with Effusion/complications, Otitis Media with Effusion/therapy, Pressure, Randomized Controlled Trials as Topic, Valsalva Maneuver
Plain language summary
Autoinflation for hearing loss associated with otitis media with effusion (glue ear)
Otitis media with effusion (OME) or 'glue ear' is very common in children and the hearing loss and discomfort, especially where the effusion is bilateral and long‐lasting, may lead to problems with language, development and behaviour. There are a number of treatment options including steroids, antibiotics, decongestants, antihistamines and surgery (the insertion of grommets (ventilation tubes)). Grommet insertion is one of the commonest operations of childhood. The best treatment strategy remains controversial, however, as glue ear often resolves spontaneously within a few months.
Autoinflation is a technique whereby the Eustachian tube (the tube that connects the middle ear and the back of the nose) is reopened by raising pressure in the nose. This can be achieved by forced exhalation with closed mouth and nose, blowing up a balloon through each nostril or using an anaesthetic mask. The aim is to introduce air into the middle ear, via the Eustachian tube, equalising the pressures and allowing better drainage of the fluid.
This review included eight randomised controlled trials of autoinflation for glue ear. All of the studies were small, of limited treatment duration and had short follow‐up.
The review authors used a combined outcome measure which included any outcome signifying improvement (as defined in the individual studies) and measured outcomes at the time points 'up to one month' and 'more than one month'. Improvement was demonstrated only in 'more than one month' analyses. Subgroup analysis based on the type of intervention showed a significant effect using a Politzer device at both under one month and over one month. None of the studies demonstrated a significant difference in the incidence of side effects between interventions.
The authors conclude that the evidence for the use of autoinflation in the short term appears favourable. Given the small number of studies and the lack of long‐term follow‐up, the long‐term effects associated with the use of these devices cannot be determined.
Background
This is an update of a Cochrane review first published in The Cochrane Library in Issue 4, 2006.
Otitis media with effusion (OME) or 'glue ear' is an accumulation of fluid in the middle ear, in the absence of acute inflammation or infection. It is the commonest cause of acquired hearing loss in childhood (Rovers 1999). It is common in children between the ages of one and three years and in seasons when there is a high incidence of the common cold, with a prevalence of OME between 10% and 30%. The cumulative incidence to the age of four years is 80% (Zielhuis 1990) and at age seven the prevalence is still 3% to 8% (Fiellau 1977; Fiellau 1983; Lous 1981; Teele 1989). The aetiology of 'glue ear' is uncertain, but low‐grade infection, poor Eustachian tube function and adenoidal infection or hypertrophy have all been implicated (Bluestone 1988). Otitis media with effusion often resolves spontaneously within a few months (Fiellau 1979).
Otitis media with effusion (OME) may be associated with ear ache (otalgia), infection and/or hearing loss. Hearing loss may be significant (20 dB to 30 dB), particularly when the disorder is bilateral and has lasted for more than a month (Fiellau 1983). However, some children have nearly normal hearing despite the presence of fluid within the middle ear. The hearing loss and discomfort may have linguistic, developmental, behavioural and other social consequences, particularly if the effusion is bilateral and of long duration (Lous 1995). Some studies have shown that children with unilateral or bilateral otitis media with effusion have balance problems (Golz 1998; Grace 1990).
For its diagnosis, tympanometry in combination with otomicroscopy or pneumatic otoscopy is the recommended technique (Bluestone 1988). Tympanometry is performed with a handheld device inserted in the ear canal and measures the eardrum responses to the sound and different pressures. Diagnostic tympanometry results in a flat curve (relative gradient less than 0.1, type B) or in a curve with a middle ear pressure between ‐399 to ‐200 daPa (C2 curve) (Jerger 1970; Zielhuis 1989) or when mobility of the tympanic membrane is absent or reduced, or fluid and/or air bubbles are evident behind the eardrum (Browning 2010). Type A and C1 tympanograms are considered not to represent otitis media with effusion. The presence of a significant (10 dB) air‐bone gap on pure‐tone audiometry correlates well with the presence of fluid in the middle ear.
Management options
OME usually resolves spontaneously (20% will resolve in one month and 40% at three months (Fiellau 1979)) and many children will require no specific treatment. The most common medical treatment options include the use of decongestants (Griffin 2011), mucolytics, steroids, antihistamines (Griffin 2011) and antibiotics (van Zon 2012). Of these options, only steroids have been shown effective and only for short‐term resolution (Simpson 2011). Surgical treatment is more widely used and better researched, with options including grommet insertion (Browning 2010), myringotomy tympanocentesis (surgical incision of the eardrum, with or without aspiration of fluid from the middle ear cavity) and adenoidectomy (van den Aardweg 2010). The optimal treatment strategy remains controversial, with wide international variability in clinical practice.
'Autoinflation' refers to the opening of, and forcing of air through, the Eustachian tube by the raising of intranasal pressure. This may be achieved by forced exhalation with closed mouth and nose, blowing up a balloon through each nostril, use of an anaesthetic mask or a Politzer device. The aim of these procedures is to introduce air into the middle ear, via the Eustachian tube, to equilibrate the pressures. This is most likely to help in 'Eustachian tube dysfunction' and has been proposed as a treatment for otitis media with effusion (Hunt‐Williams 1968). This systematic review aims to assess the effectiveness of autoinflation devices in children and adults with otitis media with effusion.
Objectives
To assess the effectiveness of autoinflation compared with no treatment in children and adults with otitis media with effusion.
Methods
Criteria for considering studies for this review
Types of studies
We included randomised controlled trials of an autoinflation device ‐ designed to force air into the middle ear ‐ in children or adults with clinically diagnosed serous otitis media.
Types of participants
Children and adults with unilateral or bilateral otitis media with effusion and a clinical diagnosis by primary care physicians or specialists using tympanometry (type B or C2), either alone or in combination with simple or pneumatic otoscopy or audiometry. The type of diagnosis used in each trial is presented in the table Characteristics of included studies.
Types of interventions
We included trials that studied any form of autoinflation (a technique to increase intranasal pressure repeatedly either through a nasal balloon or by other methods) compared to no autoinflation and which were unconfounded. By unconfounded, we mean studies where the two groups were treated equally, except for the provision of the intervention to one group. Other treatments (e.g. analgesia, decongestants or short courses of antibiotics) were permitted provided these were provided equally. Autoinflation interventions were classified into similar groups by an ENT surgeon, based on the description of the intervention whilst blinded to the outcomes of the study (see Appendix 1 for a brief description of the interventions).
Types of outcome measures
Primary outcomes
Improvement in tympanogram (to type C1 or A tympanogram)
Differences in hearing level on pure‐tone audiogram (average 10 dB improvement over the frequencies 250 Hz to 2000 Hz)
Improvement measured as a composite of change in tympanogram and/or audiometry (post hoc)
Secondary outcomes
Adverse effects of autoinflation
Presence or absence of fluid in middle ear cavity
Time points of outcome assessment
Up to and including one month from commencement of autoinflation
More than one month from commencement of autoinflation
We were unable to analyse the following secondary outcomes due to a lack of trial data:
Developmental test results
Behavioural test results
Language and speech development
Quality of life
Family function
Search methods for identification of studies
We conducted systematic searches for randomised controlled trials. There were no language, publication year or publication status restrictions. The date of the last search was 12 April 2013.
Electronic searches
We searched the following databases from their inception for published, unpublished and ongoing trials: the Cochrane Ear, Nose and Throat Disorders Group Trials Register; the Cochrane Central Register of Controlled Trials (CENTRAL, The Cochrane Library 2013, Issue 3); PubMed; EMBASE; CINAHL; LILACS; KoreaMed; IndMed; PakMediNet; CAB Abstracts; Web of Science; ISRCTN; ClinicalTrials.gov; ICTRP; Google Scholar and Google.
We modelled subject strategies for databases on the search strategy designed for CENTRAL. Where appropriate, we combined subject strategies with adaptations of the highly sensitive search strategy designed by The Cochrane Collaboration for identifying randomised controlled trials and controlled clinical trials (as described in the Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0, Box 6.4.b. (Handbook 2011)). Search strategies for major databases including CENTRAL are provided in Appendix 2.
Searching other resources
We scanned the reference lists of identified publications for additional trials and contacted trial authors where necessary. In addition, we searched PubMed, TRIPdatabase, The Cochrane Library and Google to retrieve existing systematic reviews relevant to this systematic review, so that we could scan their reference lists for additional trials. We searched for conference abstracts using the Cochrane Ear, Nose and Throat Disorders Group Trials Register.
Data collection and analysis
Selection of studies
The Trials Search Co‐ordinator of the Cochrane Ear, Nose and Throat Disorders Group assessed abstracts of all studies identified by the initial search and excluded clearly irrelevant studies. One review author assessed abstracts for relevance and we obtained full texts for these. Two review authors independently assessed all full‐text articles. Differences in opinion about inclusion of studies were resolved by discussion with a third review author. The review authors were not blind to the journal of origin, the authors, the institutions or the magnitude of results.
Data extraction and management
Two review authors independently extracted study data using standardised forms. We entered data into RevMan 5 (RevMan 2012) and a second review author checked the data. Data extracted and presented included: author, publication year, journal, participants (numbers, duration of illness, demographics, characteristics of the disease, etc.), intervention (type of intervention and duration) and results (outcome measures, effect size, statistical significance, adverse effects). We contacted trial authors for missing or incomplete data.
Assessment of risk of bias in included studies
Two review authors independently assessed the risk of bias in all included trials. Differences were resolved by discussion with a third review author. We assessed risk of bias using the Cochrane 'Risk of bias' tool where types of bias are rated as low, high or unclear.
Randomisation/selection bias: selection generation
Randomisation/selection bias: allocation concealment
Detection bias: blinding of outcome assessment
Attrition bias: incomplete outcome data, losses to follow‐up and whether analysis was intention‐to‐treat
Blinding of outcome assessors
Selective reporting
Other bias: we also used this section to assess whether the groups were comparable at the start of the study for prognostic characteristics
We assessed the quality of the studies separately:
that the groups were equally provided of care, except for the treatment; and
adherence to study procedure.
Data synthesis
We carried out analysis on the basis of intention‐to‐treat. Where possible we combined data to give a summary measure of effect.
When outcome data obtained were binary ‐ recovered or improved glue ear or audiometry improvement ‐ we used relative risks of improvement to compare interventions. The data reported in the trials mainly used 'ears' as their analysis unit despite using individuals as randomisation units. To correct for this and to give adequate weight to the studies we adjusted the outcomes using the 'design effect', which takes into consideration the association found in using both ears from the same individual (Deeks 2005).
We estimated the 'design effects' from the Blanshard data (full data set provided by author) at one month and three months for participants with either types B or C2 tympanograms or only type B tympanograms. Minor differences in correlation between intervention and control groups (smaller at three months) were found, but an average was used, as differences could be due to sampling variation. For participants with type B or C2 tympanograms the design effect at one month was 1.25, while at three months it was 1.51. For participants with only type B tympanograms the design effect was 1.3 for both one and three months follow‐up.
We used the appropriate design effect (for less than one month or more than one month by tympanogram type at entry) to obtain adjusted risk ratios (RR) for all studies that provided data for the outcome. We calculated and then entered the log(RR) and the SE(log(RR)) into RevMan 5 (RevMan 2012). As the focus of the review is on recovery/improvement we emphasise the use of a positive outcome and refer to the RR obtained as the 'relative risk of improvement' or RRI; instead of using the term risk ratio which is normally associated with a negative outcome (e.g. death).
When data were continuous, we used mean differences to compare interventions. We used the GIV (generic inverse variance) method with a random‐effects model to pool studies together and examined heterogeneity using the Chi2 test and I2 statistic. Funnel plots were not used as the number of studies found was too small for these to provide relevant information. We carried out analyses using RevMan 5.
Subgroup analyses
Subgroup analyses had been planned on the basis of the diagnosis of otitis media with effusion and the extent of the hearing loss measured on audiometry. However, insufficient studies were identified to allow these analyses. We also planned subgroups according to age (under three years, three to 12 years and over 12 years). However, we could not identify any papers of sufficient quality including children aged under three years and only one paper included children over 12 years (Lesinskas 2003).
Results
Description of studies
Results of the search
From the 2013 update searches, we retrieved a total of 401 references. Following first‐level screening (i.e. removal of duplicates and clearly irrelevant references) by the Cochrane ENT Group Trials Search Co‐ordinator we were left with 127 references for further consideration. Following sifting by the authors two more duplicates were removed and 105 discarded following screening of titles and abstracts, leaving 20 reports for consideration. Two were studies which we have included in the updated review (De Nobili 2008; Ercan 2005). Sixteen further studies were excluded from the review, with reasons (Costantino 2010; Dalchow 2011; Ducla‐Soares 2007; El Hachem 2012; Hidir 2011; Jumah 2010; Karahatay 2008; Kawase 2008; Laina 2006; Leach 2008; Pau 2009; Poe 2011; Prabhakar 2007; Shim 2010; Talmon 2010; Toros 2010). Two were identified as ongoing studies (Tel‐Aviv Med Center 2006; Williamson 2011).
From the original searches (2006 publication of this review), 418 abstracts were obtained from the search, with 62 selected as possibly relevant. These were screened for relevance and we obtained 24 full‐text articles. Of these full‐text articles two review authors (JH and RP) filtered out 16 as duplicates or rejected them for not fulfilling the inclusion criteria. Two further reports were awaiting assessment in the original review and remain so (Niebuhr‐Jorgensen 1996; Scadding 2002) (see Characteristics of studies awaiting classification). Limited available information has meant that these studies cannot yet be excluded from meeting the review criteria. There have been no published data following on from either of the abstracts for these studies. Contact with one of the authors has indicated that one of the studies may be published in the future (Scadding 2002), whilst the other author has not been contactable (Niebuhr‐Jorgensen 1996). Six studies were included in the original (2006) version of the review.
It is hoped that the two ongoing studies will be able to contribute their results to this review in the future. The Tel‐Aviv Med Center 2006 study commenced in October 2006. The authors confirmed in 2010 that their results would be released shortly; unfortunately this has been delayed and a new date has not been announced. The Williamson 2011 study started in September 2011 and it is hoped that results will be available during winter of 2013‐14. (See Characteristics of ongoing studies).
A total of eight papers therefore met the eligibility criteria for inclusion in the current 2012 update of the review (Arick 2005; Blanshard 1993; Brooker 1992; De Nobili 2008; Ercan 2005; Fraser 1977; Lesinskas 2003; Stangerup 1992).
Included studies
See Characteristics of included studies table.
Participants
Two trials included only participants with bilateral OME confirmed by tympanometry (Blanshard 1993; Fraser 1977). Six trials (Arick 2005; Brooker 1992; De Nobili 2008; Ercan 2005; Lesinskas 2003; Stangerup 1992) included participants with both unilateral and bilateral OME. Diagnosis of OME was confirmed by tympanometry alone in two studies (De Nobili 2008; Stangerup 1992), tympanometry and pure‐tone audiometry in one study (Lesinskas 2003), tympanometry and otoscopy in one study (Ercan 2005), tympanometry, otoscopy and audiometry in one study (Brooker 1992) and audiometry and otoscopy in one study (Arick 2005). Stangerup and Blanshard only included participants with a diagnosis of bilateral OME of at least three months duration. The diagnosis of OME by tympanogram varied from study to study, with some defining types B and C2 as OME (Blanshard 1993; Stangerup 1992) while others only included type B (Brooker 1992; Ercan 2005).
Seven of the eight trials studied children aged between three and 16 years (Arick 2005; Blanshard 1993; Brooker 1992; De Nobili 2008; Ercan 2005; Fraser 1977; Stangerup 1992). One trial included participants aged between 16 and 75 years (Lesinskas 2003).
Interventions
The included studies used a range of interventions and outcome measures. Of the eight studies included, three (Blanshard 1993; Ercan 2005; Stangerup 1992) used a classic Otovent®, two (Brooker 1992; Fraser 1977) used a carnival blower + balloon and three (Arick 2005; De Nobili 2008; Lesinskas 2003) used Politzer devices. Seven out of the eight studies used observation without treatment as their control; the exception was Ercan who maintained nasal saline irrigation in the control. The duration of the intervention was variable, with a minimum of 10 days (Lesinskas 2003) to a maximum of seven weeks (Arick 2005). The number of times per day the autoinflation procedure was designed to be carried out was relatively consistent: three times per day (Blanshard 1993; Brooker 1992; Ercan 2005; Stangerup 1992) or two times per day (Arick 2005; Lesinskas 2003). Duration and intensity of intervention specific to each trial can be obtained from the Characteristics of included studies table. One study (Fraser 1977) used a three‐factorial design to compare autoinflation, ephedrine drops and Dimotapp® elixir to treat OME. In one study (Lesinskas 2003) the intervention was carried out by a physician, not the patient or a carer.
Outcomes and follow‐up
Tympanometry was the most commonly used outcome and was reported in four trials, as a dichotomous variable (improvement yes/no) (Blanshard 1993; De Nobili 2008; Stangerup 1992) and as a continuous variable (difference in mean middle ear compliance) (Fraser 1977). Three trials (Brooker 1992; Ercan 2005; Lesinskas 2003) reported dichotomous, composite measures obtained from tympanometry combined with other outcomes. Lesinskas reported a total score that combined pneumotoscopic appearance, tympanometry, patient complaint and audiometry. Brooker reported improvement in both tympanometry and audiometry as a single, combined outcome measure. Audiometry was reported as a separate outcome in three trials. One of these (Blanshard 1993) reported a dichotomous change in pure‐tone audiometry threshold at frequencies between 250 Hz and 4000 Hz, while the other two trials reported it as a continuous outcome (mean change in pure‐tone audiometry thresholds at frequencies between 500 Hz to 4000 Hz (Arick 2005) or 500 Hz to 2000 Hz (Fraser 1977)). Other outcomes reported were tympanic membrane mobility on otoscopy (Arick 2005) and clearance of fluid from the middle ear (Blanshard 1993). Six studies recorded outcomes at the end of treatment (Blanshard 1993; Brooker 1992; De Nobili 2008; Fraser 1977; Lesinskas 2003; Stangerup 1992). One trial (Arick 2005) recorded outcomes four weeks after the end of seven weeks of treatment. Three trials (Ercan 2005; Lesinskas 2003; Stangerup 1992) reported longer follow‐up times after treatment had finished.
Excluded studies
See Characteristics of excluded studies table.
A total of 30 studies were excluded for the following reasons:
18 were not randomised trials (Alper 1999; Arick 2000; Blanshard 1995; Costantino 2010; Gottschalk 1966; Gottschalk 1991; Havas 1995; Kaneko 1997; Karahatay 2008; Kutácová 2005; Laina 2006; Leunig 1995; Luntz 1991; Ogawa 2003; Poe 2011; Prabhakar 2007; Talmon 2010; Yu 2003);
six studies did not have participants with otitis media with effusion (Dalchow 2011; Ducla‐Soares 2007; Hidir 2011; Jumah 2010; Kawase 2008; Pau 2009);
four studies did not assess autoinflation as the intervention (El Hachem 2012; Kouwen 2005; Leach 2008; Shim 2010; Toros 2010);
one study did not assess hearing loss by either tympanometry and/or audiometry (Chan 1989); and
one study considered two different autoinflation techniques, but not against a control (Hanner 1997).
Risk of bias in included studies
We assessed studies for risk of bias in four areas: random sequence generation, allocation concealment, blinding of outcome assessment, incomplete outcome data and selective reporting. We also assessed the quality of the intervention in terms of equal provision of care for all groups and treatment adherence. We deemed none of the included studies to be of high quality (see individual entries in the Characteristics of included studies table, Table 1 and Figure 1).
1. Side effects and compliance.
| Trial | Side effects | Compliance |
| Arick 2005 | As part of 1‐year follow‐up 2 children with MEE (hearing restored) 3 children had either grommets inserted and/or enlarged adenoid | Compliance of 97.9% (46/47) |
| Blanshard 1993 | Stratified by compliance level: Attacks of OM: 44% control, 36% HC, 30% LC URTI: 61% LC, 32% HC, 23% control Tonsillitis: 22% LC, 5% HC, 13% control Antibiotics: 43% LC, 21% HC, 33% control "No complications arose from using the treatment" | 45% high compliance (HC), 43% low compliance (LC), 12% unable to use device Some children with OME found doing the Valsalva manoeuvre painful |
| Brooker 1992 | None reported | Parents reported good compliance |
| De Nobili 2008 | None reported | Not reported |
| Ercan 2005 | None reported | "satisfactory" |
| Fraser 1977 | Some children developed further symptoms and abnormal signs while receiving treatments (do not mention which group) | Not reported |
| Lesinskas 2003 | Treatment (middle ear inflation) stopped for 1 adult patient due to painfulness of procedure | 100% follow‐up and compliance (done by physician) |
| Stangerup 1992 | "We have not observed an increase incidence in middle ear infection or eardrum perforation in connection with autoinflation" No comment given on baseline incidence level | Compliance at 2 weeks: 33/51 (65%) high compliance 10/51 (20%) low compliance 3/51 (6%) no compliance No data given on the other 5 children |
HC: high compliance LC: low compliance MEE: middle ear effusion OM: otitis media OME: otitis media with effusion URTI: upper respiratory tract infection
1.
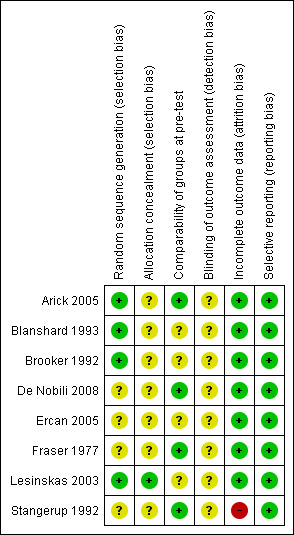
'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.
Randomisation (sequence generation) was reported and deemed low risk in three of the trials (Arick 2005; Blanshard 1993; Lesinskas 2003); the remaining trials were unclear risk.
Only one study fully commented on allocation concealment and we classed this as low risk (Lesinskas 2003). The remaining studies made no comment and so we classified them as unclear risk.
Four studies presented data showing the different arms of the study to be comparable on prognostic characteristics (Arick 2005; De Nobili 2008; Fraser 1977; Stangerup 1992). One study described distribution of age and sex as being similar between arms at baseline (Ercan 2005). One study (Blanshard 1993) commented that groups showed significance only in age distribution and exposure to smoking, while two studies (Brooker 1992; Lesinskas 2003) described as randomised controlled trials did not present data on the baseline characteristics. Confounding was not an issue in any trial.
Only one trial (Arick 2005) reported that the participating doctors and the outcome assessors were blinded, although with patients not blinded it is likely the patient's status could have become known to the assessors.
Follow‐up was predominantly low risk: four studies had no attrition (Arick 2005; Brooker 1992; De Nobili 2008; Lesinskas 2003) and three further studies had less than 15% attrition (Blanshard 1993; Ercan 2005; Fraser 1977). We assessed only one study (Stangerup 1992) as high risk, with 42% attrition in the experimental arm and 67% attrition in the control arm.
No trials showed any evidence of selective reporting.
Reported adherence to the use of autoinflation was variable; one trial reported 98% compliance (Arick 2005) while another reported 45% of participants as having high compliance, 43% low compliance and 12% being unable to use the autoinflation device (Blanshard 1993).
Effects of interventions
Eight studies met the inclusion criteria for this review and compared autoinflation with control.
Combining data proved difficult due to a lack of consistent reporting of a common single outcome measure. Hence, post hoc, we also analysed a composite measure of any outcome signifying improvement (as defined in the individual studies). We analysed the data in two time frames: up to one month and more than one month.
Improvement in tympanogram (to type C1 or A tympanogram)
Of the five studies that included tympanometry as a discrete outcome at less than one month, three favoured treatment (Blanshard 1993; Ercan 2005; Stangerup 1992) and two favoured control (Brooker 1992; De Nobili 2008). Within these five studies, at less than one month, autoinflation showed a non‐significant improvement in tympanometry as defined by type C2 and B tympanograms (relative risk of improvement (RRI) 1.47, 95% confidence interval (CI) 0.69 to 3.13), with substantial heterogeneity (I2 = 89%). See Analysis 1.1.1. Subgroup analyses suggested that autoinflation is beneficial in children with either initial type B tympanogram, relative to a type C1 or A tympanogram (RRI 2.15, 95% CI 1.41 to 3.28, I2 = 0%), or type C2 tympanograms (RRI 3.84, 95% CI 1.94 to 7.59, I2 = 0%) (Analysis 1.1). This anomaly was due to the fact that the Brooker 1992 trial did not report the baseline tympanometry level of the participants in the study, while De Nobili 2008 reported these as percentages at different stages (not tracked by ear) and therefore neither could be included in the subgroup analyses. As these are the two trials where the effect of the intervention is negative, their exclusion from the subgroup analyses changed the size of the estimated effect.
1.1. Analysis.
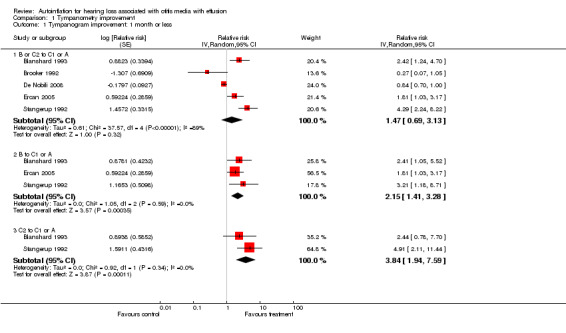
Comparison 1 Tympanometry improvement, Outcome 1 Tympanogram improvement: 1 month or less.
A sixth study (Fraser 1977) used mean change in middle ear pressure as the tympanometry outcome and was not included in the pooled estimates. Fraser found no significant difference between control and intervention groups.
Four studies followed participants for longer than a month (Blanshard 1993; De Nobili 2008; Ercan 2005; Stangerup 1992). In these studies autoinflation produced a non‐significant change in tympanometry compared to control (RRI 1.22, 95% CI 1.00 to 1.49, I2 = 0%).
Differences in hearing level on pure‐tone audiogram (average 10 dB improvement over the frequencies 250 Hz to 2000 Hz)
Two studies included an average improvement of 10 dB or more in the threshold for pure‐tone audiometry in a range of frequencies from 250 Hz to 2000 Hz as a discrete outcome measure (Blanshard 1993; Brooker 1992). The estimated recovery was worse in the autoinflation group (RRI 0.80, 95% CI 0.22 to 2.88, I2 = 63%) (Analysis 2.1). Two studies reported audiometry improvement as continuous (Arick 2005; Fraser 1977), with the pooled estimate showing a non‐significant improvement in the autoinflation group (mean difference (MD) 7.02 dB, 95% CI ‐6.92 to 20.96, I2 = 95%) (Analysis 2.2). In both cases significant heterogeneity was found in the combined studies.
2.1. Analysis.

Comparison 2 Audiometry improvement, Outcome 1 Average improvement >= 10 dB in pure‐tone audiogram (250 Hz to 2000 Hz).
2.2. Analysis.
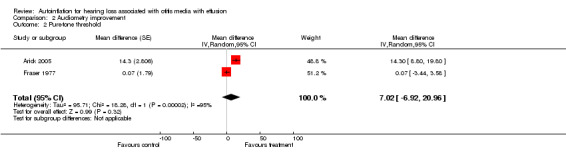
Comparison 2 Audiometry improvement, Outcome 2 Pure‐tone threshold.
Improvement measured as a composite of change in tympanogram and/or audiometry (post hoc)
We combined seven studies using a dichotomous outcome of 'improvement' in either tympanogram or audiometry (less than one month: Blanshard 1993; Brooker 1992; De Nobili 2008; Ercan 2005; Lesinskas 2003; Stangerup 1992; and over one month: Arick 2005; Blanshard 1993; De Nobili 2008; Ercan 2005; Lesinskas 2003; Stangerup 1992). The relative risk of improvement was 1.89 (95% CI 0.83 to 4.31, I2 = 93%, Analysis 3.1) at less than one month and 1.74 (95% CI 1.22 to 2.50, I2 = 69%, Analysis 3.2) at more than one month. The type of intervention was categorised by an independent rater and information on two types was obtained: a) Politzerisation and b) Otovent® or carnival blower + balloon.
3.1. Analysis.

Comparison 3 Improvement (tympanogram or composite), Outcome 1 Improvement composite: 1 month or less.
3.2. Analysis.
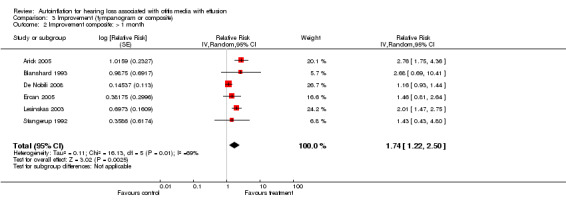
Comparison 3 Improvement (tympanogram or composite), Outcome 2 Improvement composite: > 1 month.
Subgroup analysis: type of intervention
We carried out a subgroup analysis based on the type of intervention judged by blinded assessment. Five studies (Blanshard 1993; Brooker 1992; De Nobili 2008; Ercan 2005; Stangerup 1992) provided information on type A interventions (classic Otovent® or carnival blower with balloon) and two (Arick 2005; Lesinskas 2003) provided information on type C interventions (Politzer and modified Politzer). Analyses showed a positive, statistically significant effect using a Politzer device under one month (RRI 7.07, 95% CI 3.70 to 13.51) (Analysis 3.3) and over one month (RRI 2.25, 95% CI 1.67 to 3.04, I2 = 21.1%) (Analysis 3.4). A non‐significant effect was found when using Otovent® or carnival blower + balloon under one month (RRI 1.47, 95% CI 0.69 to 3.13 I2 = 89%) (Analysis 3.3) and over one month (RRI 1.22, 95% CI 1.00 to 1.49, I2 = 0%) (Analysis 3.4).
3.3. Analysis.
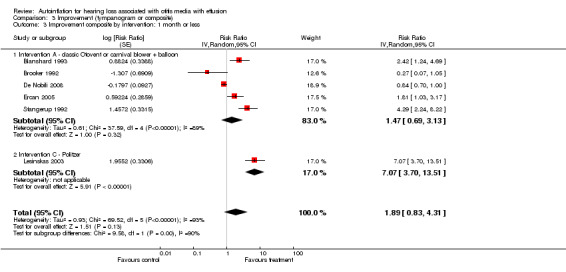
Comparison 3 Improvement (tympanogram or composite), Outcome 3 Improvement composite by intervention: 1 month or less.
3.4. Analysis.
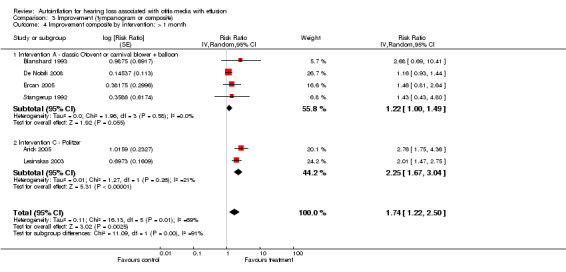
Comparison 3 Improvement (tympanogram or composite), Outcome 4 Improvement composite by intervention: > 1 month.
Adverse effects of autoinflation and compliance
None of the studies demonstrated a significant difference in the incidence of side effects between the control or intervention groups. The side effects/complications studied included incidence of middle ear infection, eardrum perforation, attacks of otitis media, upper respiratory tract infections, tonsillitis, use of antibiotics, middle ear effusion, insertion of grommets and enlarged adenoids. One study (Lesinskas 2003) reported that one patient stopped the treatment due to the pain caused by the procedure.
Six of the eight trials reported compliance. Four studies assessed the adherence to the intervention as 'high compliance' (as defined by the study authors) ranging from 45% to 100%. Two studies reported 'good' or 'satisfactory' compliance. One study commented that "some children with OME found doing the Valsalva manoeuvre painful" (Blanshard 1993), but other than Lesinskas 2003 no other study made any comment.
Data on reported side effects and compliance for each individual trial can be found in the table of 'Side effects and compliance' (Table 1).
Presence or absence of fluid in middle ear cavity
One study reported otoscopy findings (Blanshard 1993) and demonstrated significantly higher clearance of free fluid from the middle ear at one and two, but not at three months, in the intervention group compared to the control group (P < 0.05).
Other outcomes
Lesinskas 2003 presented outcomes as a pooled score of pneumotoscopy, tympanometry, patient complaint and audiometry. At day 10 and day 60 a significant overall improvement was demonstrated in the autoinflation group compared to the control group (P < 0.001). We were unable to obtain data on individual outcomes. Blanshard 1993 analysed low compliance and high compliance subgroups within the intervention. Arick 2005 reported improvement in tympanometric peak pressure (TPP) and hearing recovery (used as primary outcome of improvement in the pooled estimate).
None of the studies reported developmental test results, behavioural test results, language and speech development, quality of life or family function.
Discussion
Overall autoinflation appears to have a beneficial effect on the resolution of glue ear, with a low incidence of adverse side effects (Table 1). Six of the eight studies showed beneficial effects at least in the short term (maximum duration of follow‐up in the included studies was three months), while one of the two that reported a negative effect in the short term reported a positive effect in the long term (two months). This is in keeping with a previous systematic review on the use of autoinflation (Reidpath 1999). This review concludes that the evidence for the use of autoinflation in the treatment of glue ear in children is conflicting but suggests that it may be of clinical benefit in the short term.
There are several limitations in the collection and interpretation of the findings. The type of device used may be critical in determining the benefits of autoinflation. Pooled effect estimates suggest Politzerisation to be more effective than the classic Otovent® device or carnival blower with a balloon attached. However, both papers that used Politzerisation as the method of autoinflation had slightly different entry criteria to the rest. They were deemed suitable for inclusion in this review as they both used multiple criteria to make the diagnosis of otitis media with effusion (OME) (hearing loss, tympanometry and, in one paper, otoscopy). Information in this area is sparse. A study (Hanner 1997, not part of this review) comparing two different methods of autoinflation (Valsalva versus Otovent®) did not identify a difference between them, however a reanalysis of their data showed a significant difference in favour of Otovent® (relative risk of improvement (RRI) 1.70, 95% confidence interval (CI) 1.08 to 2.69).
Duration of treatment and follow‐up also appear to be factors in determining the effects of autoinflation. Autoinflation seems more beneficial in the short term (one month or less of treatment) than in the longer term. Paradoxically the meta‐analysis of composite outcomes shows the relative risk of improvement at one month or less to be non‐significant (RRI 1.89, 95% CI 0.83 to 4.31) whilst the relative risk of improvement at more than one month is significant (RRI 1.74, 95% CI 1.22 to 2.50). The results are affected by the two studies that found autoinflation to be markedly worse than control at least in the short term (Brooker 1992; De Nobili 2008), leading to significant heterogeneity (I2 = 89%). The study by Brooker 1992 did not continue follow‐up for longer than one month, therefore it was not included in the composite outcomes at longer time frames. The study by De Nobili 2008 reported percentage of ears at different tympanogram levels (B, C2, C1 and A) at baseline, 12 days and two months. For our calculations we have assumed that all ears in the category B and C2 are included and ears in categories A and C1 will not revert to worse tympanograms. De Nobili 2008 reports a positive difference (in favour of autoinflation) for the two‐month follow‐up. A final important difference is that for this study most OMEs are resolved within the two months of follow‐up; five out of 60 ears remain as C2 or B. Several differences were noted in the duration of the intervention (range 10 days to three months) and also in the duration of follow‐up, ranging from cessation of the intervention to two months after the intervention. Only three studies continued to follow up participants after the intervention had ceased (Arick 2005; Lesinskas 2003; Stangerup 1992). For these reasons it is difficult to determine whether the benefits of autoinflation are sustained after treatment.
The combined effects show a mixed pattern of autoinflation, with both statistical and clinical heterogeneity. Considerable heterogeneity may be due to the populations, the intervention or the outcome definitions. One study (Brooker 1992) found autoinflation to be worse than control; another (De Nobili 2008) showed it to be worse in the short term (12 days) but better in the long term (two months). The children in Brooker's study are comparable to those in other studies in terms of age, but there are few data with which to compare the prognostic characteristics of the control and intervention groups in this study with the other studies. For De Nobili 2008 it is difficult to assess if the assumption made about the change in tympanogram for ears is potentially affecting the results (no data on improvement at ear or individual level are provided).
Brooker's populations may be different to those of other studies in that the proportion of controls recovering in this trial was much higher than in any other studies. A possible reason for this is the outcome reported, which is a composite of improvement in pure‐tone audiogram and tympanogram. Another difference is that it is suggested in the paper that only children with type B tympanograms were included, but this was not clearly stated. Hence, heterogeneity could be explained if autoinflation is helpful to children with type C2 but not type B tympanograms. Subgroup analyses of the Blanshard 1993 and Stangerup 1992 papers suggest that this might be the case (although the effect size is not statistically significant in either subgroup). Finally, Brooker did not use a standardised Otovent® device, instead using a carnival blower and balloon. This may be a less effective or even harmful method of autoinflation, however the independent rater classified this intervention to be equivalent to Otovent®.
The studies mainly gave information on tympanometry and composite outcomes. Autoinflation does not appear to improve pure‐tone audiometry thresholds. Only one study reported otoscopy findings so it is difficult to comment on this outcome. Reduction of hearing loss is an important outcome for children and parents, but there was little evidence within the included studies to address the impact of hearing loss. The development of a quality of life questionnaire specifically for children with hearing loss means that the impact of hearing loss could be assessed in future studies (Umansky 2011).
However, it appeared that autoinflation was beneficial at one and two months. A large primary care study is in progress (Williamson 2011) which will report on one and three‐month outcomes.
Adverse events do not appear to differ significantly between intervention and control groups. Data for compliance were variable. There is some anecdotal evidence that some children, possibly in selected groups, do get pain when undertaking autoinflation and that continued enthusiasm for the procedure over time can wane. Blanshard 1993 comments that "significant improvement was seen only in those who used the treatment with a high compliance for the duration of the study". More details on adverse events and compliance are needed from trials, along with evidence on how to achieve good compliance.
Limitations
The heterogeneity of trial designs, inclusion criteria, interventions, outcome reporting and time scales makes it difficult to draw robust conclusions. Another factor that affects validity is the variation in the level of data analysis. Most studies used ears as their data analysis unit, but some used patients. To overcome this problem we estimated the within‐patient correlation. Furthermore, some studies reported their outcomes as continuous variables and some as dichotomous variables. Definitions of improvement varied in the studies that reported dichotomous outcomes. The quality of the trials was variable, in particular none of the studies used blinding of participants or assessors and some studies used inferior methods of randomisation.
One of the major concerns in children with glue ear is possible developmental sequelae; none of the included papers looked at this issue. Currently there are no proven treatments that affect speech and language development, including grommets (Browning 2010). Therefore, it is not possible to say whether the statistically significant improvements in tympanometry translate into clinically significant benefits. This is an important consideration when reviewing treatments for a self limiting condition such as otitis media with effusion; there is a real possibility that watchful waiting without intervention may have similar long‐term outcomes to other interventions.
Authors' conclusions
Implications for practice.
Although this analysis does not enable us to make any firm conclusions, the evidence appears favourable at least in the short term. Therefore, it may be reasonable to consider autoinflation whilst awaiting natural resolution of otitis media with effusion (OME). One particular concern is the level of adherence: are the child and parent willing to tolerate the inconvenience of the procedure? A recent Cochrane review has concluded that the benefits of grommets for OME compared to watchful waiting are small and then diminish over time (Browning 2010). Currently most autoinflation interventions are instigated in secondary care. With increased awareness and the ability to instruct and educate parents and children in their use, this intervention would be well suited to primary care and there is ongoing research in this area (Williamson 2011). The use of a Politzer device appears promising, however due to the limited amount of information available it is difficult to make any definitive recommendation. None of these trials provided data on long‐term sequelae such as language development. However, the trials of ventilation tubes suggest there is little long‐term impact achieved through treatment of otitis media with effusion (Browning 2010).
Implications for research.
All of the studies we included were small and of limited duration of treatment and follow‐up. Further studies with larger numbers of participants and longer duration of intervention, completed in primary care, are much needed. Studies need to provided further evidence on adverse events and treatment compliance. Further studies should also consider the impact of autoinflation on both developmental and hearing loss outcomes in children with otitis media with effusion. The heterogeneity suggests that the method of autoinflation, including the device used, may be important and this merits further investigation. Studies looking at factors predicting which children may benefit from autoinflation would help in identifying subgroups of children for whom autoinflation could be recommended. Future work could also include the possibility of investigating whether autoinflation is effective in preventing disease relapses in children prone to OME.
What's new
| Date | Event | Description |
|---|---|---|
| 24 April 2013 | New citation required but conclusions have not changed | Two new studies were included in the review (De Nobili 2008; Ercan 2005). Sixteen further studies were identified and excluded, with reasons. Two further ongoing studies were identified. There was no substantive change to the review conclusions. |
| 15 April 2013 | New search has been performed | New searches run. |
History
Review first published: Issue 4, 2006
| Date | Event | Description |
|---|---|---|
| 20 October 2008 | Amended | Converted to new review format. |
Acknowledgements
We are grateful to all authors who supplied us with additional details regarding their studies. We would like to thank Carolyn Dorée for creating the search strategy, running the literature search and extracting the relevant articles. Also, thanks to Martin Burton who provided expert advice throughout the review, particularly in the classification of interventions studied in this review. We would like to thank Daniel Reidpath and Chris Del Mar for allowing us to update and extend their systematic review. We are particularly grateful to Jonathan Blanshard for allowing us access to his original data. Thank you to Gemma Sandberg for running the literature searches for the 2013 update and to Jenny Bellorini for all her advice and support. Thank you also to Jayne Haynes, co‐author of the original (2006) version of the review.
Appendices
Appendix 1. Otovent® and a home Politzerization device (EarPopper™)
Both interventions described here involve inflating the middle ear by blowing air up the nose during the act of swallowing. The main difference is that one intervention is active with the individual (child or adult) inflating a balloon (Otovent®) and then allowing it to deflate; while the other one is passive with air being delivered as a constant flow into the nose (Politzer device). The patient swallows while air is flowing in. During the swallow the air is diverted up the Eustachian tube ventilating the middle ear. There are (at least) two commercial products available, Otovent® (Figure 10) and a handheld, battery‐operated device called the EarPopper™ (Figure 11) that allows Politzerisation to be performed at home.
2.

Child demonstrating the use of the Otovent® device
3.

Young woman demonstrating use of modified Politzer device (EarPopper™)
Appendix 2. Search strategies
| CENTRAL | PubMed | EMBASE (Ovid) | CINAHL (EBSCO) |
| 1. OTITIS‐MEDIA (MeSH, explode all trees) 2. (otitis NEXT media) OR (glue NEXT ear) 3. (serous NEAR otitis) OR (secretory NEAR otitis) 4. SOM OR OME 5. 1 OR 2 OR 3 OR 4 6. EAR‐DISEASES (MeSH, explode all trees) 7. otitis OR inflam* OR effusion* OR infect* OR suppurat* OR secret* OR pressure 8. 6 OR 7 9. EAR‐MIDDLE (MeSH, explode all trees) 10. (middle NEAR ear*) OR (eustachian NEXT tube*) 11. 9 OR 10 12. 8 AND 11 13. 5 OR 12 14. MIDDLE‐EAR‐VENTILATION (MeSH, explode all trees) 15. INSUFFLATION (MeSH) 16. VALSALVA‐MANEUVER (MeSH) 17. autoinflat* OR (auto NEXT inflat*) OR (ear* NEAR inflat*) OR (ear* NEAR aerat*) OR (ME NEXT inflat*) OR (air NEXT inflat*) 18. (nose OR nasal) AND balloon 19. valsalva OR politzer OR popper 20. open* NEAR eustachian 21. insufflat* 22. 14 OR 15 OR 16 OR 17 OR 18 OR 19 OR 20 OR 21 23. 13 AND 22 | #1 "Otitis Media"[Mesh] #2 "otitis media" [tiab] OR "glue ear" [tiab] #3 (serous [tiab] OR secretory [tiab]) AND otitis [tiab] #4 SOM [tiab] OR OME [tiab] #5 #1 OR #2 OR #3 OR #4 #6 "Ear Diseases"[Mesh] #7 otitis [tiab] OR inflam* [tiab] OR effusion* [tiab] OR infect* [tiab] OR suppurat* [tiab] OR secret* [tiab] or pressure [tiab] #8 #6 OR #7 #9 "Ear, Middle"[Mesh] #10 (middle [tiab] AND ear* [tiab]) OR (eustachian [tiab] AND tube* [tiab]) #11 #9 OR #10 #12 #8 AND #11 #13 #5 OR #12 #14 "Middle Ear Ventilation"[Mesh] #15 "Insufflation"[Mesh] #16 "VALSALVA MANEUVER"[Mesh] #17 autoinflat* [tiab] OR ((auto [tiab] OR ear* [tiab] OR ME [tiab] OR air [tiab]) AND (inflat* [tiab] OR aerat* [tiab])) #18 (nose [tiab] OR nasal [tiab]) AND balloon [tiab] #19 valsalva [tiab] OR politzer [tiab] #20 open* [tiab] AND eustachian* [tiab] #21 insufflat* [tiab] OR popper [tiab] #22 #14 OR #15 OR #16 OR #17 OR #18 OR #19 OR #20 OR #21 #23 #13 AND #22 | 1 exp *Otitis Media/ 2 ((otitis adj media) or (glue adj ear)).tw. 3 ((secretory or serous) adj otitis).tw. 4 (SOM or OME).tw. 5 exp Ear Disease/ 6 (otitis or inflam* or effusion* or infect* or suppurat* or secret* or pressure).tw. 7 6 or 5 8 exp Middle Ear/ 9 ((middle and ear*) or (eustachian and tube*)).tw. 10 8 or 9 11 7 and 10 12 11 or 4 or 1 or 3 or 2 13 middle ear ventilation/ 14 aeration/ 15 valsalva maneuver/ 16 (autoinflat* or ((auto or ear* or ME or air) and (inflat* or aerat*))).tw. 17 ((nose or nasal) and balloon).tw. 18 (valsalva or politzer).tw. 19 (open* and eustachian*).tw. 20 (insufflat* OR popper).tw. 21 17 or 20 or 15 or 14 or 18 or 13 or 16 or 19 22 21 and 12 | S1 (MH "Otitis Media+") S2 TX otitis AND media S3 TX glue AND ear S4 TX SOM OR OME S5 TX "secretory otitis" OR "serous otitis" S6 otitis OR inflam* OR effusion* OR infect* OR suppurat* OR secret* or pressure S7 (MH "Ear Diseases+") S8 s6 OR s7 S9 (MH "Ear, Middle+") S10 TX "middle ear" OR "eustachian tube" S11 S9 OR s10 S12 s8 and s11 S13 S1 OR s2 OR s3 OR s4 OR s5 OR s12 S14 (MH "Middle Ear Ventilation") S15 (MH "Insufflation") S16 (MH "Valsalva's Maneuver") S17 TX autoinflat* S18 TX auto OR ear OR ME OR air S19 TX inflat* OR aerat* S20 TX insufflat* S21 TX "nose balloon" OR "nasal balloon" S22 TX valsalva OR politzer OR popper S23 TX open AND eustachian s24 s14 OR s15 OR s16 OR s17 OR s18 OR s19 OR s20 OR s21 OR s22 OR s23 s25 s13 AND s24 |
| Cochrane Ear, Nose and Throat Disorders Group Trials Register | Web of Science/BIOSIS Previews (Web of Knowledge) | CAB Abstracts (Ovid) | ICTRP |
| ((otitis OR ear*) AND (autoinflat* OR insufflat* OR aeration OR valsalva OR inflat* OR popper OR politzer* OR balloon)) | TI=((otitis OR ear*) AND (autoinflat* OR insufflat* OR aeration OR valsalva OR inflat* OR popper OR politzer*)) NB the last search on BIOSIS Previews was conducted in June 2012. |
1 exp *Otitis Media/ 2 ((otitis adj media) or (glue adj ear)).tw. 3 ((secretory or serous) adj otitis).tw. 4 (SOM or OME).tw. 5 exp Ear Disease/ 6 (otitis or inflam* or effusion* or infect* or suppurat* or secret* or pressure).tw. 7 6 or 5 8 exp Middle Ear/ 9 ((middle and ear*) or (eustachian and tube*)).tw. 10 8 or 9 11 7 and 10 12 11 or 4 or 1 or 3 or 2 13 middle ear ventilation/ 14 aeration/ 15 valsalva maneuver/ 16 (autoinflat* or ((auto or ear* or ME or air) and (inflat* or aerat*))).tw. 17 ((nose or nasal) and balloon).tw. 18 (valsalva or politzer).tw. 19 (open* and eustachian*).tw. 20 (insufflat* OR popper).tw. 21 17 or 20 or 15 or 14 or 18 or 13 or 16 or 19 22 21 and 12 | ear AND inflation OR ear AND autoinflation OR ear AND insufflation OR ear AND popper OR ear AND politzer OR ear AND balloon OR ear AND valsalva otitis AND inflation OR otitis AND autoinflation OR otitis AND insufflation OR otitis AND popper OR otitis AND politzer OR otitis AND balloon OR otitis AND valsalva |
Data and analyses
Comparison 1. Tympanometry improvement.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Tympanogram improvement: 1 month or less | 5 | Relative risk (Random, 95% CI) | Subtotals only | |
| 1.1 B or C2 to C1 or A | 5 | Relative risk (Random, 95% CI) | 1.47 [0.69, 3.13] | |
| 1.2 B to C1 or A | 3 | Relative risk (Random, 95% CI) | 2.15 [1.41, 3.28] | |
| 1.3 C2 to C1 or A | 2 | Relative risk (Random, 95% CI) | 3.84 [1.94, 7.59] | |
| 2 Tympanogram improvement: > 1 month | 4 | Relative Risk (Random, 95% CI) | Subtotals only | |
| 2.1 B or C2 to C1 or A | 4 | Relative Risk (Random, 95% CI) | 1.22 [1.00, 1.49] |
1.2. Analysis.
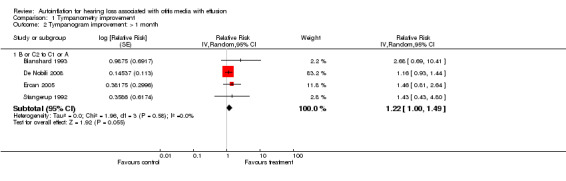
Comparison 1 Tympanometry improvement, Outcome 2 Tympanogram improvement: > 1 month.
Comparison 2. Audiometry improvement.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Average improvement >= 10 dB in pure‐tone audiogram (250 Hz to 2000 Hz) | 2 | Relative Risk (Random, 95% CI) | 0.80 [0.22, 2.88] | |
| 2 Pure‐tone threshold | 2 | Mean difference (Random, 95% CI) | 7.02 [‐6.92, 20.96] |
Comparison 3. Improvement (tympanogram or composite).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Improvement composite: 1 month or less | 6 | Relative Risk (Random, 95% CI) | 1.89 [0.83, 4.31] | |
| 2 Improvement composite: > 1 month | 6 | Relative Risk (Random, 95% CI) | 1.74 [1.22, 2.50] | |
| 3 Improvement composite by intervention: 1 month or less | 6 | Risk Ratio (Random, 95% CI) | 1.89 [0.83, 4.31] | |
| 3.1 Intervention A ‐ classic Otovent or carnival blower + balloon | 5 | Risk Ratio (Random, 95% CI) | 1.47 [0.69, 3.13] | |
| 3.2 Intervention C ‐ Politzer | 1 | Risk Ratio (Random, 95% CI) | 7.07 [3.70, 13.51] | |
| 4 Improvement composite by intervention: > 1 month | 6 | Risk Ratio (Random, 95% CI) | 1.74 [1.22, 2.50] | |
| 4.1 Intervention A ‐ classic Otovent or carnival blower + balloon | 4 | Risk Ratio (Random, 95% CI) | 1.22 [1.00, 1.49] | |
| 4.2 Intervention C ‐ Politzer | 2 | Risk Ratio (Random, 95% CI) | 2.25 [1.67, 3.04] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Arick 2005.
| Methods | Randomised controlled trial | |
| Participants | Bilateral or unilateral OME on audiometry and otoscopy 94 children Inclusion criteria: 1) Aged 4 to 11 years 2) At least 2‐month history of MEE and associated hearing loss documented by physician 3) Pure‐tone conduction thresholds of 20 dB HL or more (500 Hz to 4000 Hz) 4) A tympanometric peak pressure (TPP) of ‐100 daPa or less 5) Otologic diagnosis of MEE at pretest 6) Absence of enlarged adenoids, acute otitis media or other ear abnormalities at pretest |
|
| Interventions | Modified Politzer device for 7 weeks; parent administered twice daily, alternating nostrils Control group received equal care except for the intervention |
|
| Outcomes | Air conduction thresholds for each ear (500 Hz to 4000 Hz), TPP, hearing recovery (by patients and by ears) Data on outcome were obtained for all individuals Adherence was reported in 97.9% of the intervention group |
|
| Notes | Results reported as continuous for all outcomes except hearing recovery | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | 4 pieces of paper (2 experimental, 2 control) in a bag, one selected by parent (email from author 9 March 2006) |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Comparability of groups at pre‐test | Low risk | "the mean pretest air‐conduction thresholds for the experimental and control groups were similar"; "the mean pure‐tone average in both ears in both groups were symmetrical to within 3.3 dB"; "the mean pretest tympanometric peak pressure in both ears in both groups were symmetrical to within 40.3 daPa" |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Audiologists and otolaryngologists blinded to each patient's disease status; patients not blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No attrition; 100% follow‐up |
| Selective reporting (reporting bias) | Low risk | No evidence of selective reporting |
Blanshard 1993.
| Methods | Randomised controlled trial | |
| Participants | Bilateral OME on tympanometry 85 children 3 to 10 years on waiting list for ventilation tubes |
|
| Interventions | Otovent 3 times a day or no treatment for 3 months Control group received equal care except for the intervention |
|
| Outcomes | Tympanometry (1, 2 and 3 months) Pure‐tone audiometry (3 months) Clearance of fluid on otoscopy (1, 2 and 3 months) Adverse effects (3 months) Between 93.4% and 95.8% follow‐up; 45% had high compliance, 43% low and 12% were unable to use device |
|
| Notes | Results reported as ears Intervention group subdivided into low compliance and high compliance Original data obtained by JH and RP Groups were comparable at outset, except for smoke exposure |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random numbers |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Comparability of groups at pre‐test | Unclear risk | "...significance only in the age distribution and exposure to smoking. Those in low compliance group were younger than those in the control group (p=0.04) and younger than those in the high compliance group (p=0.03). There was not difference between the high compliance and control groups." |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Over 90% follow‐up at all outcome points for both intervention and control arms (by ears) |
| Selective reporting (reporting bias) | Low risk | No evidence of selective reporting |
Brooker 1992.
| Methods | Randomised controlled trial (ears) | |
| Participants | Unilateral or bilateral OME diagnosed by otoscopy, audiometry and tympanometry 40 children Aged 3 to 10 referred to ENT |
|
| Interventions | Carnival balloon 3 times a day or no treatment for 3 weeks Both groups had equal care except for the intervention |
|
| Outcomes | Pure‐tone audiometry
Tympanometry
(Both at 3 weeks) Parents reported "good compliance" |
|
| Notes | Ears Children unable to use the carnival blower excluded prior to randomisation |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation by selection of envelopes (e‐mail confirmation from author 13 November 2012) |
| Allocation concealment (selection bias) | Unclear risk | Sealed envelopes |
| Comparability of groups at pre‐test | Unclear risk | Not stated |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No attrition; 100% follow‐up |
| Selective reporting (reporting bias) | Low risk | No evidence of selective reporting |
De Nobili 2008.
| Methods | Randomised controlled trial | |
| Participants | Bilateral or unilateral OME and tubaric dysfunction 40 children Aged 4 to 10 At least 3 episodes in the last year Tympanogram type B, C1 or C2 |
|
| Interventions | Intervention group and control group received, for 12 days, inhalation, crenotherapeutic Politzer and aerosol with sulphurous calcic‐magnesiac water therapy Intervention group then received a further home therapy of autoinflation (Otovent) 3 times per day for 1 week for 2 consecutive months |
|
| Outcomes | Tympanometry at 12 days and 2 months following intervention Data on outcome were obtained for all individuals, presented as the proportion of ears with tympanograms type B, C2, C1 or A |
|
| Notes | No significant statistical difference at 12 days | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No method of randomisation described |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Comparability of groups at pre‐test | Low risk | Similar stage of illness, age and gender with 2 groups |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No attrition; 100% follow‐up |
| Selective reporting (reporting bias) | Low risk | No evidence of selective reporting |
Ercan 2005.
| Methods | Randomised controlled trial | |
| Participants | Bilateral or unilateral chronic OME 60 children: 30 in intervention group (48 ears: 12 unilateral, 18 bilateral), 30 in control (45 ears: 15 unilateral, 15 bilateral) Age 6.2 years (mean) Free of otitis media for 4 weeks. All children treated with antibiotics in 3 months prior to randomisation. Excluded if they had one of a large number of pre‐existing conditions. Chronic OME identified by visible symptoms, otoscopic examination and type B tympanogram |
|
| Interventions | Intervention: autoinflation 3 times per day for 6 weeks (Otovent) and nasal saline irrigations 3 times per day for 6 weeks Control: only nasal saline irrigation for 6 weeks |
|
| Outcomes | Pneumatic otoscopy and tympanogram every 2 weeks for the first 2 months, and then once a month for 7 months Outcome measure: clear of OME or ventilation tubes fitted Results reported by ears Data recorded at 6th week, 3rd, 6th and 9th month |
|
| Notes | "In the case of upper respiratory tract infection the patient was advised not to autoinflate" Adherence of intervention group was not measured, although described as "satisfactory" |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of randomisation not described |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Comparability of groups at pre‐test | Unclear risk | "Distribution of age and sex of these groups were similar"; full baseline data not stated |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Attrition at 9 months (only reported by ears): intervention group = 12.5%, control group = 11.1% |
| Selective reporting (reporting bias) | Low risk | No evidence of selective reporting |
Fraser 1977.
| Methods | 3‐factorial randomised controlled trial | |
| Participants | Bilateral OME on tympanometry 85 children Aged 3 to 12 |
|
| Interventions | Carnival blower 2 times a day or no treatment for 6 weeks Other arms (factorial design) received equal care except for the intervention |
|
| Outcomes | Pure‐tone audiometry
Tympanometry
(Both at 6 weeks) Adherence not reported |
|
| Notes | Also randomised to one, both or neither of Dimotapp elixir or 0.5% ephedrine nose drops | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No method of randomisation stated |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Comparability of groups at pre‐test | Low risk | "The comparability of patients in the groups which were to be compared after treatment was examined and no great differences were found between the patients in any of the groups" |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 97% follow‐up |
| Selective reporting (reporting bias) | Low risk | No evidence of selective reporting |
Lesinskas 2003.
| Methods | Randomised controlled trial | |
| Participants | Unilateral or bilateral OME diagnosed by tympanometry and PTA 198 adults aged 16 to 75 |
|
| Interventions | Politzer inflation 2 times a day for 10 days, with or without oral antibiotics, or no treatment Control group received equal care except for the intervention |
|
| Outcomes | Pooled score combining pneumotoscopic appearance, tympanometry, patient complaint and audiometry (days 3 to 5, 10 and 60) 100% follow‐up and adherence (intervention done by ENT doctor) |
|
| Notes | Results reported by ears Outcomes only presented as pooled data |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Randomisation was completed by an independent person. The envelopes were sealed, opaque, not numbered" E‐mail from author 14 November 2012 |
| Allocation concealment (selection bias) | Low risk | Sealed, opaque envelopes |
| Comparability of groups at pre‐test | Unclear risk | Not stated |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No attrition; 100% follow‐up |
| Selective reporting (reporting bias) | Low risk | No evidence of selective reporting |
Stangerup 1992.
| Methods | Randomised controlled trial | |
| Participants | Unilateral or bilateral OME for at least 3/12 diagnosed by tympanometry 100 children Aged 3 to 10 |
|
| Interventions | Otovent 3 times a day for 2 weeks, extended to 4 weeks in those with persistent OME, or no treatment Control group received equal care except for the intervention |
|
| Outcomes | Tympanometry Otitis media (days 14, 30, 60 and 90) | |
| Notes | Results reported as ears | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation described as "consecutively randomised" as individuals; no method of randomisation described |
| Allocation concealment (selection bias) | Unclear risk | No statement |
| Comparability of groups at pre‐test | Low risk | "No statistically significant differences were computed between the treated group and the control group with regard to nursing conditions, occurrence of acute otitis media, adenoidectomy, grommet insertion, or the use of antibiotics in the year preceding the study period" |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Experimental: 42% attrition based on 42 ears completing the study from an original randomised 73 (50 participants). 30% attrition at 2 weeks due to non‐adherence (22/73). Analysis based on 51 ears who maintained adherence to intervention in first 2 weeks. Control: 67% attrition based on 49 ears completing the study from an original randomised 73 ears (50 participants) Retention of participants in the study stated, but only for those that completed follow‐up at 2 weeks plus 2 of the 3 follow‐up points (experimental 92%, control 94%). Data not supplied for individuals completing full 90 days of study. |
| Selective reporting (reporting bias) | Low risk | No evidence of selective reporting |
ENT: ear, nose and throat MEE: middle ear effusion OME: otitis media with effusion PTA: pure‐tone audiogram TPP: tympanometric peak pressure
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Alper 1999 | ALLOCATION: case report only |
| Arick 2000 | ALLOCATION: not a randomised controlled trial |
| Blanshard 1995 | ALLOCATION: not a randomised controlled trial |
| Chan 1989 | ALLOCATION: participants were stratified according to their ability of tubal opening during autoinflation. Within each strata children were randomised to autoinflation or control using a set of random numbers. PARTICIPANTS: criteria for diagnosing OME was otoscopy alone; no tympanometry or audiometry |
| Costantino 2010 | ALLOCATION: not a randomised controlled trial |
| Dalchow 2011 | ALLOCATION: not stated PARTICIPANTS: "patients with a history of tubal dysfunction"; no OME |
| Ducla‐Soares 2007 | ALLOCATION: not stated PARTICIPANTS: no hearing loss associated with otitis media with effusion |
| El Hachem 2012 | ALLOCATION: not stated (it unlikely to be a RCT) PARTICIPANTS: 60 patients between 4 and 49 months affected by otitis media with or without effusion INTERVENTION: whilst passive opening of the Eustachian tube is described, there is no increasing of the intranasal pressure |
| Gottschalk 1966 | ALLOCATION: not a randomised controlled trial |
| Gottschalk 1991 | ALLOCATION: not a randomised controlled trial |
| Hanner 1997 | ALLOCATION: randomised controlled trial; no details given on randomisation PARTICIPANTS: 76 children with unilateral or bilateral negative middle ear pressure lower than ‐200 mm water (type C2 or B tympanometry) for at least 3 months, verified by tympanometry and older than 3 years INTERVENTION: both intervention groups (children) received an 'autoinflation' treatment: Otovent (28) versus Valsalva manoeuvre (27) |
| Havas 1995 | ALLOCATION: not a randomised controlled trial |
| Hidir 2011 | ALLOCATION: not stated, but unlikely as allocated according to Eustachian tube function PARTICIPANTS: no hearing loss associated with otitis media with effusion; in healthy adults |
| Jumah 2010 | ALLOCATION: "double blind study"; not clear if RCT PARTICIPANTS: no hearing loss associated with otitis media with effusion |
| Kaneko 1997 | ALLOCATION: not a randomised controlled trial |
| Karahatay 2008 | ALLOCATION: not a randomised controlled trial |
| Kawase 2008 | ALLOCATION: "retrospectively analysed"; unlikely to be RCT, but not stated PARTICIPANTS: no hearing loss associated with otitis media with effusion |
| Kouwen 2005 | ALLOCATION: randomised controlled trial; no information given on randomisation PARTICIPANTS: 32 children aged 2 to 5 years with bilateral OME diagnosed by combined and repeated tympanometry and otoscopy INTERVENTION: comparison is functional therapy (15) versus watchful waiting (17); no autoinflation intervention. Functional therapy consisted of therapy by a speech pathologist, combining hygiene and behavioural changes with specific motoric exercises once a week for a period of 3 months. |
| Kutácová 2005 | ALLOCATION: not a randomised controlled trial |
| Laina 2006 | ALLOCATION: not a randomised controlled trial |
| Leach 2008 | ALLOCATION: "double blind study", "randomised to receive" PARTICIPANTS: "infants with first detection of OME", no indication how diagnosed INTERVENTION: no autoinflation |
| Leunig 1995 | ALLOCATION: unable to determine if this was a randomised controlled trial PARTICIPANTS: 146 children above 4 years with unilateral or bilateral OME ascertained by audiometry and tympanometry INTERVENTION: comparison is autoinflation versus paracentesis, not autoinflation versus control |
| Luntz 1991 | ALLOCATION: not a randomised controlled trial |
| Ogawa 2003 | ALLOCATION: not a randomised controlled trial |
| Pau 2009 | ALLOCATION: not stated, though unlikely to be a RCT PARTICIPANTS: no hearing loss associated with otitis media with effusion INTERVENTION: no autoinflation |
| Poe 2011 | ALLOCATION: not a randomised controlled trial |
| Prabhakar 2007 | ALLOCATION: not a randomised controlled trial PARTICIPANTS: no hearing loss associated with otitis media with effusion |
| Shim 2010 | ALLOCATION: unlikely to be a RCT, but not stated PARTICIPANTS: "patients with chronic otitis media and who underwent middle ear surgery" INTERVENTION: no autoinflation |
| Talmon 2010 | ALLOCATION: not a randomised controlled trial |
| Toros 2010 | ALLOCATION: "retrospective study"; unlikely to be RCT, but not stated PARTICIPANTS: "patients who underwent surgical repair of tympanic membrane perforations due to chronic suppurative otitis media without cholesteatoma" INTERVENTION: no autoinflation |
| Yu 2003 | ALLOCATION: not a randomised controlled trial |
RCT: randomised controlled trial
Characteristics of studies awaiting assessment [ordered by study ID]
Niebuhr‐Jorgensen 1996.
| Methods | Randomised (conference abstract) |
| Participants | 44 children Age 0.7 to 3.0 years C2 or B tympanogram with a flat curve |
| Interventions | Intervention: home Politzerisation 3 times a day for 2 weeks Control: observed without treatment |
| Outcomes | Follow‐up: 2 to 4 weeks Outcome measure: tympanometry and otomicroscopy |
| Notes | 31 children in follow‐up (30% attrition) No contact from author |
Scadding 2002.
| Methods | Conference abstract Double‐blind, placebo‐controlled study |
| Participants | 200 children OME persisting over 3 months |
| Interventions | Intervention 1: Flixonase alone (100 µg daily for 2 years) Intervention 2: Otovent alone (3 times daily until improvement in hearing, restarted after any upper respiratory infection) Intervention 3: Flixonase and Otovent Control: matching placebo |
| Outcomes | Primary outcome: treatment failure (hearing loss of more than 30 dB in both ears or any other need for grommet insertion) Follow‐up: every 3 months Assessment on follow‐up: height, weight, peak flow, ear examination, tympanometry and audiometry |
| Notes | Randomisation not confirmed Author confirmed still intending to publish (8 November 2012) |
OME: otitis media with effusion RCT: randomised controlled trial
Characteristics of ongoing studies [ordered by study ID]
Tel‐Aviv Med Center 2006.
| Trial name or title | The influence of the Ear Popper on serous otitis and on the accompanying conductive hearing loss in children |
| Methods | Study to check the effect of the use of the Ear Popper device Inclusion criteria: clinical serous otitis media for a duration of more than 3 months, a conductive hearing loss of at least 15 dB air‐bone gap and tympanometry type B or C |
| Participants | 30 children aged 3 to 18 |
| Interventions | 7 weeks use of Ear Popper |
| Outcomes | Primary outcome: audiometry and tympanometry test results, plus otoscopic findings at 7 weeks and 3 months from the beginning of the use of the Ear Popper Secondary outcome: hearing improvement at 7 weeks and 3 months from the beginning of the trial; rate of referrals for tympanostomy tube insertion at 3 months |
| Starting date | October 2006 |
| Contact information | Yael Oestreicher, MD (dkyo@barak‐online.net.il) |
| Notes |
Williamson 2011.
| Trial name or title | AIRS (An open randomised study of autoinflation in school age children (4‐11 years) with otitis media with effusion (OME)) |
| Methods | Randomised controlled trial (Southampton and Oxford areas) |
| Participants | 29 children Aged 4 to 11 years |
| Interventions | Autoinflation (blowing out through each nostril into a balloon (Otovent)) 3 times per day per nostril for up to 3 months plus standard care, versus standard care alone |
| Outcomes | Proportion of children showing evidence of resolution (cure) of at least one effusion (B type) using tympanometry at 1 month |
| Starting date | Pilot study in follow‐up Main trial commenced 1 September 2011 |
| Contact information | Dr Ian Williamson, Chief Investigator, University of Southampton (I.G.Williamson@soton.ac.uk) |
| Notes | Telephone randomised allocation Analysis by child Analysis on intention‐to‐treat basis http://www.phc.ox.ac.uk/research/clinical‐trials/column‐1/airs |
Differences between protocol and review
There has been one main change to the protocol since the original review. We have adopted the Cochrane 'Risk of bias' assessment tool which offers a clearer assessment of study bias across several domains and allows the reader to view how judgements were arrived at.
Contributions of authors
Original review: Rafael Perera: trial selection, data extraction, statistical analysis and writing review. Jayne Haynes: design of data extraction sheet, trial selection, data extraction and writing review. Paul Glasziou: basic protocol, compilation of trials, trial selection and writing review. Carl Heneghan: data extraction and writing review.
2013 update: Rafael Perera: data extraction, statistical analysis and updating review. Julie McLellan: trial selection, data extraction and updating review. Ian Williamson: trial selection, data extraction and updating review.
Sources of support
Internal sources
None, Not specified.
External sources
None, Not specified.
Declarations of interest
None known.
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
Arick 2005 {published data only}
- Arick D, Silman S. Nonsurgical home treatment of middle ear effusion and associated hearing loss in children. Part I: Clinical trial. Ear, Nose and Throat Journal 2005;84(9):567‐78. [PubMed] [Google Scholar]
Blanshard 1993 {published data only}
- Blanshard J, Maw A, Bawden R. Conservative treatment of otitis media with effusion by autoinflation of the middle ear. Clinical Otolaryngology and Allied Sciences 1993;18:188‐92. [DOI] [PubMed] [Google Scholar]
- Blanshard J, Maw A, Bawden R. The treatment of otitis media with effusion in children by autoinflation. 2nd European Congress of Oto‐Rhino‐Laryngology and Cervico‐facial surgery. 1992:31‐7.
Brooker 1992 {published data only}
- Brooker D, McNeice A. Autoinflation in the treatment of glue ear in children. Clinical Otolaryngology and Allied Sciences 1992;17:289‐90. [DOI] [PubMed] [Google Scholar]
De Nobili 2008 {published data only}
- Nobili E, Bellomo A. Comparative evaluation of efficacy of crenotherapeutic Politzer with sulphurous water versus crenotherapeutic Politzer and autoinflation (Otovent) in patients with tubaric dysfunction and secretory otitis media [Studio comparativo dell’efficacia del Politzer crenoterapico con acqua sulfurea versus Politzer crenoterapico e autoinsufflazione domiciliare (metodo Otovent®) in pazienti affetti da disfunzione tubarica e otite media secretiva]. Medicina Clinica e Termale 2008;20(64):30‐4. [Google Scholar]
Ercan 2005 {published data only}
- Ercan I, Burak Ömür C, Kayaoglu S, Turgut S, Cerrahisi K, Kliniği Ş. Long term effect of autoinflation in the treatment of otitis media with effusion. KBB Forum 2005;4(4):166‐70. [Google Scholar]
Fraser 1977 {published data only}
- Fraser J, Mehta M, Fraser P. The medical treatment of secretory otitis media. A clinical trial of three commonly used regimes. Journal of Laryngology and Otology 1977;91(9):757‐65. [DOI] [PubMed] [Google Scholar]
Lesinskas 2003 {published data only}
- Lesinskas E. Factors affecting the results of non‐surgical treatment of secretory otitis media in adults. Auris, Nasus, Larynx 2003;30:7‐14. [DOI] [PubMed] [Google Scholar]
Stangerup 1992 {published data only}
- Stangerup S, Sederberg‐Olsen J, Balle V. Autoinflation as a treatment of secretory otitis media: a randomized controlled study. Archives of Otolaryngology ‐ Head and Neck Surgery 1992;118:149‐52. [DOI] [PubMed] [Google Scholar]
- Stangerup S, Tos M. Autoinflation in the treatment of Eustachian tube dysfunction, secretory otitis media and its sequelae. Recent Advances in Otitis Media. Amsterdam/New York: Kugler Publications, 1994:759‐63.
References to studies excluded from this review
Alper 1999 {published data only}
- Alper CM, Swarts JD, Doyle W. Middle ear inflation for diagnosis and treatment of otitis media with effusion. Auris Nasus Larynx 1999;26:479‐86. [DOI] [PubMed] [Google Scholar]
Arick 2000 {published data only}
- Arick D, Silman S. Treatment of otitis media with effusion based on politzerization with an automated device. Ear, Nose and Throat Journal 2000;79(4):290‐8. [PubMed] [Google Scholar]
Blanshard 1995 {published data only}
- Blanshard J. Autoinflation for otitis media with effusion. Maternal and Child Health 1995;9:304‐7. [Google Scholar]
Chan 1989 {published data only}
- Chan K, Bluestone CD. Lack of efficacy of middle‐ear inflation: treatment of otitis media with effusion in children. Otolaryngology ‐ Head and Neck Surgery 1989;100(4):317‐23. [DOI] [PubMed] [Google Scholar]
Costantino 2010 {published data only}
- Costantino M, Mastursi C, Contaldi E, Capua M, Filippelli A. The role of SPA therapy with Politzer method in dysfunction of the eustachian tube. Gazzetta Medica Italiana Archivio per le Scienze Mediche 2010;169(4):131‐6. [Google Scholar]
Dalchow 2011 {published data only}
- Dalchow C, Muenscher A, Knecht R. Endoscopic dilatation of the eustachian tube. Otolaryngology ‐ Head and Neck Surgery 2011;145:93. [Google Scholar]
Ducla‐Soares 2007 {published data only}
- Ducla‐Soares JL, Santos‐Bento M, Laranjo S, Andrade A, Ducla‐Soares E, Boto JP, et al. Wavelet analysis of autonomic outflow of normal subjects on head‐up tilt, cold pressor test, Valsalva manoeuvre and deep breathing. Experimental Physiology 2007;92(4):677‐86. [DOI] [PubMed] [Google Scholar]
El Hachem 2012 {published data only}
- Hachem N, Halimi M. New technique for the passive opening of the Eustachian tube. Tympanometry assessment of the effect of a new manipulation technique on the opening of the Eustachian tube in children less than six years with otitis media with or without effusion [French]. Kinesitherapie 2012;132:25‐33. [Google Scholar]
Gottschalk 1966 {published data only}
- Gottschalk GH. Further experience with controlled middle ear inflation in the treatment of serous otitis. Eye, Ear, Nose and Throat Monthly 1966;45:49‐51. [PubMed] [Google Scholar]
Gottschalk 1991 {published data only}
- Gottschalk GH. Middle ear inflation: its place in the treatment of otitis media with effusion. In: Sadé J editor(s). The Eustachian Tube and Middle Ear Diseases: Clinical Aspects. The Hague: Kugler Publications, 1991:293‐5. [Google Scholar]
Hanner 1997 {published data only}
- Hanner P. Non surgical treatment of otitis media with effusion. Indian Journal of Otology 1997;3(3):101‐7. [Google Scholar]
Havas 1995 {published data only}
- Havas T, Koutsis A, Jacobson I. Nasal balloon auto‐inflation (Otovent) as a treatment of otitis media with effusion in children. Journal of the Oto‐laryngological Society of Australia 1995;2(1):37‐41. [Google Scholar]
Hidir 2011 {published data only}
- Hidir Y, Ulus S, Karahatay S, Satar B. A comparative study on efficiency of middle ear pressure equalization techniques in healthy volunteers. Auris Nasus Larynx 2011;38(4):450‐5. [DOI] [PubMed] [Google Scholar]
Jumah 2010 {published data only}
- Jumah MD, Schlachta M, Hoelzl M, Werner A, Sedlmaier B. Pressure regulating ear plug testing in a pressure chamber. Aviation Space and Environmental Medicine 2010;81(6):560‐5. [DOI] [PubMed] [Google Scholar]
Kaneko 1997 {published data only}
- Kaneko Y, Takasaka T, Sakuma M, Kambayashi J, Okitsu T. Middle ear inflation as a treatment for secretory otitis media in children. Acta Otolaryngologica (Stockholm) 1997;117:564‐8. [DOI] [PubMed] [Google Scholar]
Karahatay 2008 {published data only}
- Karahatay S, Yilmaz YF, Birkent H, Ay H, Satar B. Middle ear barotrauma with hyperbaric oxygen therapy: incidence and the predictive value of the nine‐step inflation/deflation test and otoscopy. Ear, Nose, and Throat Journal 2008;87(12):684‐8. [PubMed] [Google Scholar]
Kawase 2008 {published data only}
- Kawase T, Hori Y, Kikuchi T, Oshima T, Kobayashi T. The effects of mastoid aeration on autophony in patients with patulous Eustachian tube. European Archives of Oto‐Rhino‐Laryngology 2008;265(8):893‐7. [DOI] [PubMed] [Google Scholar]
Kouwen 2005 {published data only}
- Kouwen H, Balen FAM, Dejonckere PH. Functional tubal therapy for persistent otitis media with effusion in children: myth or evidence?. International Journal of Pediatric Otorhinolaryngology 2005;69:943‐51. [DOI] [PubMed] [Google Scholar]
Kutácová 2005 {published data only}
- Kutácová S, Komínek P, Horník PS. Autoinflation in curing up the middle ear inflammation. Otorinolaryngologie a Foniatrie 2005;54(1):52‐5. [Google Scholar]
Laina 2006 {published data only}
- Laina V, Pothier DD. Should we aspirate middle‐ear effusions prior to insertion of ventilation tubes?. Journal of Laryngology and Otology 2006;120(10):818‐21. [DOI] [PubMed] [Google Scholar]
Leach 2008 {published data only}
- Leach AJ, Morris PS, Mathews JD, Hayhurst B, Koops H, Melder A, et al. Compared to placebo, long‐term antibiotics resolve otitis media with effusion and prevent acute otitis media with perforation in a high‐risk population: a randomised controlled trial. BMC Pediatrics 2008;8:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
Leunig 1995 {published data only}
- Leunig A. Middle ear aeration with the Otovent‐latex membrane system. Laryno‐Rhino‐Otologie 1995;74(b):352‐4. [DOI] [PubMed] [Google Scholar]
Luntz 1991 {published data only}
- Luntz M, Sadé J. The diagnostic and therapeutic value of middle ear inflation. In: Sadé J editor(s). The Eustachian Tube, Basic Aspects. Amsterdam: Kugler and Ghedini, 1991:183‐5. [Google Scholar]
Ogawa 2003 {published data only}
- Ogawa I. Otitis media with effusion: treatment by autoinflation using a balloon. Journal of Otolaryngology of Japan 2003;106:685‐91. [DOI] [PubMed] [Google Scholar]
Pau 2009 {published data only}
- Pau HW, Sievert U, Just T, Sad J. Pressure changes in the human middle ear without opening the Eustachian tube. Acta Oto‐Laryngologica 2009;129(11):1182‐6. [DOI] [PubMed] [Google Scholar]
Poe 2011 {published data only}
- Poe DS, Silvola J, Pyykko I. Balloon dilation of the cartilaginous Eustachian tube. Otolaryngology ‐ Head and Neck Surgery 2011;144(4):563‐9. [DOI] [PubMed] [Google Scholar]
Prabhakar 2007 {published data only}
- Prabhakar H, Bithal PK, Suri A, Rath GP, Dash HH. Intracranial pressure changes during Valsalva manoeuvre in patients undergoing a neuroendoscopic procedure. Minimally Invasive Neurosurgery 2007;50(2):98‐101. [DOI] [PubMed] [Google Scholar]
Shim 2010 {published data only}
- Shim HJ, Choi AY, Yoon SW, Kwon KH, Yeo SG. The value of measuring Eustachian tube aeration on temporal bone CT in patients with chronic otitis media. Clinical and Experimental Otorhinolaryngology 2010;3(2):59‐64. [DOI] [PMC free article] [PubMed] [Google Scholar]
Talmon 2010 {published data only}
- Talmon Y. In reference to an easy technique to prevent post‐tympanoplasty ventilation tube blockage. Laryngoscope 2010;120(6):1282. [DOI] [PubMed] [Google Scholar]
Toros 2010 {published data only}
- Toros SZ, Habesoglu TE, Habesoglu M, Bolukbasi S, Naiboglu B, Karaca CT, et al. Do patients with sclerotic mastoids require aeration to improve success of tympanoplasty?. Acta Oto‐Laryngologica 2010;130(8):909‐12. [DOI] [PubMed] [Google Scholar]
Yu 2003 {published data only}
- Yu L, Ma X. Trans‐external ear canal blow oxygen method. Lin Chuang Er Bi Yan Hou Ke Zsa Zhi [Journal of Clinical Otorhinolaryngology] 2003;17(1):35‐6. [PubMed] [Google Scholar]
References to studies awaiting assessment
Niebuhr‐Jorgensen 1996 {published data only}
- Niebuhr‐Jorgensen M. Autoinflation as a treatment of otitis media in children younger than three years. Proceedings of the Sixth International Symposium: Recent Advances in Otitis Media with Effusion. 1996:212‐3.
Scadding 2002 {unpublished data only}
- Scadding G, Rajput K, Parikh A, Hilss S, Jansz JA, Darby YC, et al. Double blind, placebo‐controlled study of Flixonase with and without Otovent in the treatment of otitis media with effusion. 8th International Congress of Paediatric Otorhinolaryngology. 2002.
References to ongoing studies
Tel‐Aviv Med Center 2006 {unpublished data only}
- Tel‐Aviv Sourasky Medical Center. The influence of the Ear Popper on serous otitis media and the accompanying conductive hearing loss in children. Available from http://clinicaltrials.gov/ct2/show/NCT00393159. [NCT00393159]
Williamson 2011 {published data only}
- Williamson I, Benge S, Perera R, Turner D, Harnden H, Raftery J, et al. Study of autoinflation in 4‐11 year old school children with glue ear. Available from: http://www.controlled‐trials.com/ISRCTN55208702 2011. [ISRCTN55208702]
Additional references
Bluestone 1988
- Bluestone CD, Klein JO. Otitis Media in Infants and Children. Philadelphia: WB Saunders Company, 1988. [Google Scholar]
Browning 2010
- Browning GG, Rovers MM, Williamson I, Lous J, Burton MJ. Grommets (ventilation tubes) for hearing loss associated with otitis media with effusion in children. Cochrane Database of Systematic Reviews 2010, Issue 10. [DOI: 10.1002/14651858.CD001801.pub3] [DOI] [PubMed] [Google Scholar]
Deeks 2005
- Deeks JJ, Higgins, JPT, Altman DG. Section 8.11.2.2 Approximate analyses of cluster‐randomized trials for a meta‐analysis: effective sample sizes. In: Higgins JPT, Green S editor(s). Cochrane Handbook for Systematic Reviews of Interventions 4.2.5 [updated May 2005]. The Cochrane Collaboration, 2005. [Google Scholar]
Fiellau 1977
- Fiellau‐Nikolajsen M, Lous J, Pedersen SV, Schousboe HH. Tympanometry in three‐year‐old children. I. A regional prevalence study on the distribution of tympanometric results in a non‐selected population of three‐year‐old children. Scandinavian Audiology 1977;6(4):199‐204. [DOI] [PubMed] [Google Scholar]
Fiellau 1979
- Fiellau‐Nikolajsen M, Lous J. Prospective tympanometry in three‐year‐old children: a study of the spontaneous course of tympanometry types in a non‐selected population. Archives of Otolaryngology 1979;105(8):461‐6. [DOI] [PubMed] [Google Scholar]
Fiellau 1983
- Fiellau‐Nikolajsen M. Tympanometry and secretory otitis media. Observations on diagnosis, epidemiology, treatment, and prevention in prospective cohort studies of three‐year‐old children [thesis]. Acta Oto‐Laryngologica. Supplement 1983;394:1‐73. [PubMed] [Google Scholar]
Golz 1998
- Golz A, Netzer A, Angel‐Yeger B, Westerman ST, Gilbert LM, Joachims HZ. Effects of middle ear effusions on the vestibular system in children. Otolaryngology ‐ Head and Neck Surgery 1998;119(6):695‐9. [DOI] [PubMed] [Google Scholar]
Grace 1990
- Grace AR, Pfleiderer AG. Dysequilibrium and otitis media with effusion: what is the association?. Journal of Laryngology and Otology 1990;104(9):682‐4. [DOI] [PubMed] [Google Scholar]
Griffin 2011
- Griffin G, Flynn CA. Antihistamines and/or decongestants for otitis media with effusion (OME) in children. Cochrane Database of Systematic Reviews 2011, Issue 9. [DOI: 10.1002/14651858.CD003423.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Handbook 2011
- Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Hunt‐Williams 1968
- Hunt‐Williams R. A method of maintaining middle ear ventilation in children. Journal of Laryngology and Otology 1968;82:921. [DOI] [PubMed] [Google Scholar]
Jerger 1970
- Jerger J. Clinical experience with impedance audiometry. Archives of Otolaryngology 1970;92(4):311‐24. [DOI] [PubMed] [Google Scholar]
Lous 1981
- Lous J, Fiellau‐Nikolajsen M. Epidemiology of middle ear effusion and tubal dysfunction: a one‐year prospective study comprising monthly tympanometry in 387 non‐selected 7‐year‐old children. International Journal of Pediatric Otorhinolaryngology 1981;3(4):303‐17. [DOI] [PubMed] [Google Scholar]
Lous 1995
- Lous J. Secretory otitis media in schoolchildren. Is screening for secretory otitis media advisable?. Danish Medical Bulletin 1995;42(1):71‐99. [PubMed] [Google Scholar]
Reidpath 1999
- Reidpath DD, Glasziou PP, Mar CD. Systemic review of autoinflation for treatment of glue ear. BMJ 1999;318:1177‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
RevMan 2012 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.2. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2012.
Rovers 1999
- Rovers MM, Zielhuis GA, Straatman H, Ingels K, Wilt GJ, Kauffman‐de Boer M. Comparison of the CAPAS and Ewing tests for screening of hearing in infants. Journal of Medical Screening 1999;6(4):188‐92. [DOI] [PubMed] [Google Scholar]
Simpson 2011
- Simpson SA, Lewis R, Voort JH, Butler CC. Oral or topical nasal steroids for hearing loss associated with otitis media with effusion in children. Cochrane Database of Systematic Reviews 2011, Issue 5. [DOI: 10.1002/14651858.CD001935.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Teele 1989
- Teele DW, Klein JO, Rosner B. Epidemiology of otitis media during the first seven years of life in children in greater Boston: a prospective, cohort study. Journal of Infectious Diseases 1989;160(1):83‐94. [DOI] [PubMed] [Google Scholar]
Umansky 2011
- Umansky AM, Jeffe DB, Lieu JE. The HEAR‐QL: quality of life questionnaire for children with hearing loss. Journal of the American Academy of Audiology 2011;10:644‐53. [DOI] [PMC free article] [PubMed] [Google Scholar]
van den Aardweg 2010
- Aardweg MTA, Schilder AGM, Herkert E, Boonacker CWB, Rovers MM. Adenoidectomy for otitis media in children. Cochrane Database of Systematic Reviews 2010, Issue 1. [DOI: 10.1002/14651858.CD007810.pub2] [DOI] [PubMed] [Google Scholar]
van Zon 2012
- Zon A, Heijden GJ, Dongen TMA, Burton MJ, Schilder AGM. Antibiotics for otitis media with effusion in children. Cochrane Database of Systematic Reviews 2012, Issue 9. [DOI: 10.1002/14651858.CD009163.pub2] [DOI] [PubMed] [Google Scholar]
Zielhuis 1989
- Zielhuis GA, Rach GH, Broek P. Screening for otitis media with effusion in preschool children. Lancet 1989;1(8633):311‐4. [DOI] [PubMed] [Google Scholar]
Zielhuis 1990
- Zielhuis GA, Rach GH, van‐den‐Broek P. The occurrence of otitis media with effusion in Dutch pre‐school children. Clinical Otolaryngology and Allied Sciences 1990;15(2):147‐53. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Perera 2006
- Perera R, Haynes J, Glasziou PP, Heneghan CJ. Autoinflation for hearing loss associated with otitis media with effusion. Cochrane Database of Systematic Reviews 2006, Issue 4. [DOI: 10.1002/14651858.CD006285] [DOI] [PubMed] [Google Scholar]


