Abstract
Background.
A 9-valent human papillomavirus (HPV) vaccine, licensed in 2014, prevents 4 HPV types targeted by the quadrivalent vaccine (6/11/16/18) and 5 additional high-risk (HR) types (31/33/45/52/58). Measuring seropositivity before vaccine introduction provides baseline data on exposure to types targeted by vaccines.
Methods.
We determined seroprevalence of HPV 6/11/16/18/31/33/45/52/58 among 4943 persons aged 14–59 years who participated in the National Health and Nutrition Examination Survey, 2005–2006.
Results.
Among females, seroprevalence was 40.5% for any of the 9 vaccine types, 30.0% for any 7 HR types (16/18/31/33/45/52/58), 19.0% for any 5 additional types (31/33/45/52/58), and 18.3% for 16/18. Compared with non-Hispanic whites, non-Hispanic blacks had higher seroprevalence of 31/33/45/52/58 (36.8% vs 15.9%) and 16/18 (30.1% vs 17.8%), while Mexican Americans had higher seroprevalence of 31/33/45/52/58 (23.6% vs 15.9%) (P < .05 for all). In multivariable analyses of data from females, race/ethnicity, number of sex partners, and age were associated with 16/18 and 31/33/45/52/58 seropositivity. Seropositivity was lower among males than among females (P < .001 for all type categories).
Conclusions.
In 2005–2006, about 40% of females and 20% of males had serological evidence of exposure to ≥1 of 9 HPV types. Seroprevalence of all type categories, especially HPV 31/33/45/52/58 among females, varied by race/ethnicity.
Keywords: human papillomavirus, seroprevalence, racial differences
Human papillomavirus (HPV) infection is one of the most common sexually transmitted infections [1]. It is estimated that at least 80% of the people in the United States will have an HPV infection during their lifetime [2]. While most infections are transient and do not cause disease, some infections can persist and lead to cancers. Of the approximately 40 HPV types that infect the genital tract, 12 are classified as high risk due to their carcinogenic potential [3, 4]. HPV is a necessary cause of cervical cancer; the majority of cancers at other anogenital sites (anus, penis, vulva, and vagina) and oropharyngeal cancer are attributable to HPV [5].The most common types detected in cervical cancer cases worldwide are HPV16, 18, 31, 33, 45, 52, and 58; with HPV 16/18 detected in approximately 70% of the cases and HPV 31/33/45/52/58 detected in about 20% of the cases [6, 7]. These 7 types are also among the most commonly detected types in HPV-associated cancers in the United States and in high-grade precancer cervical lesions [8, 9]. In the United States, almost 50% of high-grade cervical lesions, including cervical intraepithelial neoplasia grades 2 and 3 and adenocarcinoma in situ, have been found to be attributable to HPV 16/18 and approximately 25% to HPV 31/33/45/52/58 [9]. Racial differences in HPV types detected in precancer lesions, but not in cervical cancers, have been observed in the United States [10, 11].
Currently, 3 prophylactic HPV vaccines are licensed for use in the United States. The quadrivalent vaccine that protects against HPV 6/11/16/18 was licensed in 2006, the bivalent vaccine that protects against HPV 16/18 was licensed in 2009, and the 9-valent vaccine that targets HPV 6/11/16/18 as well as 5 additional high-risk (HR) types (31/33/45/52/58) was licensed in 2014. The Advisory Committee on Immunization Practices (ACIP) made its first recommendations regarding the HPV vaccine in 2006; the recommendations were updated several times, most recently in the February 2015 meeting to include the 9-valent vaccine [12, 13]. Currently, ACIP recommends routine vaccination for girls aged 11 or 12 years with any of the 3 vaccines and boys aged 11–12 years with the quadrivalent or the 9-valent vaccine [13]. All of the vaccines have been shown to have high efficacy in preventing infection and disease due to the targeted HPV types and to induce a high antibody response [14, 15].
Compared to vaccination, natural infection results in lower seroconversion rates and lower antibody titers, especially among men; a detectable serological response occurs in only 50%–70% of females and 4%–36% of males [16, 17]. Nevertheless, measuring seropositivity to vaccine-targeted types in prevaccine years provides baseline information on cumulative exposure through natural infection. Seroprevalence of HPV 6/11/16/18 in the United States in the prevaccine era has been reported previously [18, 19]. Our study describes the prevaccine era seroprevalence of the 5 additional HR types, the 7 HR types, and all 9 types included in the 9-valent HPV vaccine, and examines the racial differences in seroprevalence.
METHODS
Data Source
National Health and Nutrition Examination Survey (NHANES) is a collection of continuous and ongoing cross-sectional surveys conducted by National Center for Health Statistics at the Centers for Disease Control and Prevention (CDC). Nationally representative participants are selected from among noninstitutionalized civilians from households in the United States using a complex, multistage sampling design. In the 2005–2006 cycle, people with low income, adolescents aged 12–19 years, adults aged >60 years, African Americans, and Mexican Americans were oversampled to provide adequate sample size for subgroup analyses. NHANES surveys are composed of household interviews and health examinations, including biological specimen collection, in mobile examination centers (MECs). Participants completed questionnaires on sensitive topics, such as sexual behaviors, using audio computer-assisted self-interviews in the MEC. Informed consent was obtained from all participants or their guardians. Data collection was approved by the CDC Institutional Review Board.
Overall, 77.8% of females and 76.9% of males screened participated in both the household interview and MEC portions of the 2005–2006 NHANES. Of the 5129 people aged 14–59 years interviewed, 4943 (96.5%) participated in the health examination portion. Serum samples from all female and male participants aged 14–59 years who participated in the health examinations were eligible for HPV serological analysis. Laboratory testing methods were described in detail previously [20, 21]. Serum samples were collected using a standard venipuncture procedure and stored at <70°C. Antibodies specific to HPV types 6, 11, 16, 18, 31, 33, 45, 52, and 58 were measured using a proprietary 9-plex, competitive Luminex Immunoassay (cLIA) (Merck & Co, Inc) at Pharmaceutical Product Development (PPD), Inc. Samples were considered seropositive if the quantity of type-specific antibodies detected was equal or above the threshold value established for that type; the threshold values for each type were listed in the lab manual [20]. Antibody quantity was measured in milliMerck units/mL. Sociodemographic and behavioral information were self-reported. Age was categorized into 5-year age groups for those aged 14–19 and 10-year age groups for those aged 20–59. Birth years (which were derived from subtracting reported age from 2006) that corresponded to the age groups were: 1987–1992, 1977–1986, 1967–1976, 1957–1966, and 1947–1956. Race/ethnicity was categorized as non-Hispanic white, non-Hispanic black, Mexican American, and other. Poverty income ratio (PIR) was calculated based on self-reported income, family size, and the federal poverty guidelines for 2005–2006. Participants with PIR ≤1 were classified as at or below poverty. The total number of lifetime sex partners includes both same-sex and opposite-sex partners, and was categorized into the following groups: 1 partner, 2–4 partners, 5–9 partners, and ≥10 partners. Those with a serum cotinine level of ≥10 ng/mL were considered current smokers, while those with <10 ng/mL were considered to be not current smokers [22].
Statistical Analysis
Female and male serological data were analyzed separately. We estimated seropositivity to all of the types included in the 9-valent HPV vaccine. Overall seropositivity and age-group-and race/ethnicity-specific seropositivity to ≥1 type were examined for different combinations of vaccine types: 6/11, 16/18, 5 types not previously targeted (31/33/45/52/58), 7 HR types (16/18/31/33/45/52/58), and all 9 types (6/11/16/18/31/33/45/52/58). We also examined seropositivity to individual types by race/ethnicity. Estimates by race/ethnicity were presented for non-Hispanic whites, non-Hispanic blacks, and Mexican Americans, but overall estimates included everyone in our sample. Within each HPV type category, differences by age group and race/ethnicity were tested using Wald χ2 tests with the F statistic; a P value of <.05 was considered statistically significant. Statistically significant racial/ethnic differences were further tested using Wald χ2 to compare non-Hispanic blacks and Mexican Americans to non-Hispanic whites.
To determine and compare risk factors for oncogenic type seropositivity, we used multivariable logistic regression models to estimate adjusted prevalence ratios (aPR) among sexually active females, separately for HPV 16/18 and for HPV 31/33/45/52/58. This analysis was restricted to females due to low seroprevalence among males. Variables included in the multivariable models were age group, race/ethnicity, lifetime partners, current smoking status, and poverty level. The complex survey sampling design was accounted for in all analyses. Data management and statistical analysis were conducted in SAS 9.3 (SAS Institute, Cary, North Carolina) and SAS-Callable SUDAAN 11.0 (RTI International, Research Triangle Park, North Carolina).
RESULTS
Females
Among females, overall seroprevalence was 40.5% for any 9 types, 30.0% for any 7 HR types, 19.0% for HPV 31/33/45/52/58, 18.3% for HPV 16/18, and 22.7% for HPV 6/11 (Table 1). Estimates for seroprevalence of individual HPV types 6/11/16/18 were 19.6%, 6.2%, 14.0%, and 6.7%, respectively; seroprevalence of the individual 5 additional types in the 9-valent vaccine ranged from 1.4% for HPV45 to 7.5% for HPV31 (Table 2). Of females, 23.0% were seropositive to only 1 type and 17.5% were seropositive to ≥2 types (data not shown).
Table 1.
Seroprevalence of Human Papillomavirus Type Categories Among Females and Males, by Age and Race/Ethnicity, National Health and Nutrition Examination Surveys, 2005–2006
| HPV Type Categories, % (95% CI) | ||||||
|---|---|---|---|---|---|---|
|
|
||||||
| N | Any 9a | Any 7 HRb | 31/33/45/52/58 | 16/18 | 6/11 | |
|
| ||||||
| Female | ||||||
| Overallc | 2603 | 40.5 (37.0–44.1) | 30.0 (27.3–32.9) | 19.0 (16.7–21.5) | 18.3 (16.7–20.1) | 22.7 (19.5–26.2) |
| Age groups (y) | P < .001 | P < .001 | P < .001 | P < .001 | P < .001 | |
| 14–19 | 840 | 16.2 (13.1–19.8) | 10.7 (8.7–13.2) | 7.5 (6.2–9.1) | 5.3 (3.6–7.8) | 8.7 (5.9–12.5) |
| 20–29 | 611 | 44.6 (36.1–53.4) | 33.6 (27.4–40.5) | 21.4 (17.2–26.4) | 20.0 (15.7–25.1) | 25.0 (19.9–30.8) |
| 30–39 | 425 | 52.4 (46.5–58.3) | 40.5 (36.2–44.8) | 23.6 (19.4–28.2) | 27.8 (24.7–31.2) | 30.1 (23.8–37.4) |
| 40–49 | 412 | 45.5 (40.4–50.6) | 35.1 (30.3–40.2) | 22.4 (18.5–26.9) | 21.1 (17.1–25.6) | 23.6 (18.8–29.2) |
| 50–59 | 315 | 32.9 (27.1–39.3) | 21.5 (17.0–26.8) | 14.7 (11.1–19.0) | 11.5 (8.2–15.9) | 20.0 (14.4–27.1) |
| Race/ethnicity | P < .001 | P < .001 | P < .001 | P = .001 | P < .001 | |
| Non-Hispanic white | 1003 | 38.1 (34.1–42.3) | 27.9 (24.7–31.4) | 15.9 (13.4–18.9) | 17.8 (15.7–20.0) | 20.7 (17.1–24.7) |
| Non-Hispanic black | 702 | 59.3 (54.6–63.9) | 49.0 (44.4–53.7) | 36.8 (33.8–39.9) | 30.1 (26.6–33.9) | 39.3 (35.0–43.7) |
| Mexican American | 662 | 41.8 (37.9–45.9) | 34.2 (29.2–39.7) | 23.6 (19.6–28.1) | 17.2 (13.3–22.0) | 17.6 (15.1–20.3) |
| Male | ||||||
| Overallc | 2340 | 19.4 (16.6–22.6) | 11.9 (9.4–14.9) | 6.6 (4.8–9.0) | 6.6 (5.0–8.7) | 10.5 (8.8–12.6) |
| Age groups (y) | P < .001 | P < .001 | P < .001 | P < .001 | P < .001 | |
| 14–19 | 818 | 5.9 (3.9–8.7) | 4.1 (2.3–7.0) | 2.5 (1.5–4.3) | 1.6 (.5–4.7)d,e | 2.3 (1.2–4.1) |
| 20–29 | 410 | 15.6 (10.6–22.3) | 6.8 (4.3–10.6) | 4.5 (2.6–7.7) | 2.9 (1.5–5.6)d | 9.5 (6.2–14.3) |
| 30–39 | 393 | 23.6 (18.7–29.4) | 14.9 (11.7–18.9) | 8.7 (5.9–12.7) | 7.5 (5.4–10.3) | 11.9 (9.0–15.5) |
| 40–49 | 403 | 27.4 (21.1–34.7) | 19.4 (13.3–27.3) | 8.9 (5.3–14.6) | 13.4 (8.8–20.0) | 13.7 (9.6–19.3) |
| 50–59 | 316 | 18.4 (12.5–26.4) | 10.1 (5.7–17.4) | 6.5 (3.6–11.3) | 4.7 (2.2–10.1)d | 11.8 (7.4–18.2) |
| Race/ethnicity | P = .035 | P = .081 | P = .007 | P = .024 | P = .148 | |
| Non-Hispanic white | 923 | 17.9 (14.6–21.6) | 11.1 (8.4–14.6) | 5.4 (3.5–8.3) | 6.7 (4.8–9.3) | 9.6 (7.4–12.3) |
| Non-Hispanic black | 644 | 25.5 (20.0–32.0) | 16.7 (13.1–21.1) | 10.0 (7.2–13.9) | 10.4 (7.7–14.0) | 13.5 (10.1–17.7) |
| Mexican American | 598 | 21.4 (17.4–26.0) | 12.6 (10.1–15.7) | 9.3 (7.2–12.1) | 4.2 (2.4–7.1) | 11.6 (9.0–14.7) |
P values are obtained by Wald χ2 tests, using the F statistic.
Abbreviations: CI, confidence interval; HPV, human papillomavirus; HR, high-risk.
The 9 types are 6, 11, 16, 18, 31, 33, 45, 52, and 58.
The 7 HR types are 16, 18, 31, 33, 45, 52, and 58.
Overall estimates include persons in other race categories not shown in the table.
Relative standard error of >30%.
Percentage corresponds to fewer than 10 cases.
Table 2.
Seroprevalence of Individual Human Papillomavirus Types 6, 11, 16, 18, 31, 33, 45, 52, and 58 by Sex and Race/Ethnicity Among Persons Aged 14–59 Years, National Health and Nutrition Examination Surveys, 2005–2006
| HPV Type, % (95% CI) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
|
||||||||||
| N | HPV 6 | HPV 11 | HPV 16 | HPV 18 | HPV 31 | HPV 33 | HPV 45 | HPV 52 | HPV 58 | |
|
| ||||||||||
| Female | ||||||||||
| Overalla | 2603 | 19.6 (16.8–22.7) | 6.2 (4.8–8.1) | 14.0 (12.4–15.8) | 6.7 (5.1–8.7) | 7.5 (6.3–9.0) | 4.2 (3.1–5.6) | 1.4 (.8–2.5) | 5.5 (4.2–7.1) | 5.8 (4.6–7.1) |
| Race/ethnicity | P < .001 | P = .004 | P = .011 | P = .012 | P =.023 | P = .007 | P = .111 | P < .001 | P = .002 | |
| Non-Hispanic white | 1003 | 17.4 (14.4–20.9) | 5.2 (3.5–7.6) | 14.1 (12.0–16.5) | 5.8 (3.9–8.6) | 6.7 (4.9–9.0) | 4.3 (3.0–6.2) | 1.1 (.5–2.4)b | 3.5 (2.4–5.0) | 3.9 (2.8–5.5) |
| Non-Hispanic black | 702 | 35.5 (32.5–38.7) | 14.0 (10.8–18.1) | 21.6 (17.1–26.8) | 13.7 (10.6–17.5) | 12.8 (9.7–16.6) | 6.3 (5.0–8.0) | 4.1 (2.1–7.7)b | 18.3 (14.6–22.8) | 13.1 (10.1–16.7) |
| Mexican American | 662 | 16.0 (12.8–19.7) | 4.9 (3.0–7.9) | 12.8 (9.3–17.4) | 6.2 (4.6–8.4) | 10.4 (8.1–13.3) | 2.7 (1.4–5.4)b | 1.5 (.4–6.2)b,c | 4.5 (2.9–6.8) | 7.6 (5.1–11.2) |
| Male | ||||||||||
| Overalla | 2340 | 9.4 (8.0–11.0) | 3.0 (1.8–4.9) | 4.9 (3.6–6.4) | 2.4 (1.4–4.0) | 2.3 (1.5–3.3) | 1.6 (.9–2.7) | 0.7 (.5–1.2) | 1.3 (.6–2.7)b | 1.5 (.9–2.8) |
| Race/ethnicity | P =.112 | P = .662 | P = .536 | P = .002 | P = .117 | P = .066 | P = .082 | P = .022 | P = .011 | |
| Non-Hispanic white | 923 | 8.5 (6.7–10.6) | 3.0 (1.5–5.7)b | 5.0 (3.6–7.0) | 2.4 (1.3–4.5) | 1.9 (1.0–3.7)b | 1.2 (.5–2.8)b,c | 0.7 (.3–1.3)b,c | 0.9 (.3–2.6)b,c | 1.1 (.4–2.6)b,c |
| Non-Hispanic black | 644 | 12.4 (9.0–17.0) | 3.1 (2.0–4.9) | 5.7 (3.5–9.1) | 5.5 (3.8–7.9) | 3.7 (2.2–6.0) | 1.3 (.6–2.9)b,c | 1.9 (.9–4.1)b,c | 4.0 (2.4–6.6) | 3.1 (1.5–6.2)b |
| Mexican American | 598 | 9.9 (7.8–12.5) | 4.0 (2.4–6.7) | 3.7 (2.1–6.3) | 0.9 (.3–2.8)b,c | 3.8 (2.6–5.4) | 3.2 (2.0–5.2) | 0.2 (0–1.8)b,c | 1.2 (.6–2.6)b,c | 2.5 (1.1–5.6)b,c |
P values are obtained by Wald χ2 tests, using the F statistic.
Abbreviations: CI, confidence interval; HPV, human papillomavirus.
Overall estimates include persons in other race categories not shown on the table.
Relative standard error of >30%.
Percentage corresponds to fewer than 10 cases.
Seroprevalence by age group generally followed a similar pattern for all of the HPV type categories. Seroprevalence increased sharply between age 14–19 and 20–29, was highest at age 30–39, and then declined after age 30–39 (Figure 1A). However, seroprevalence of HPV 31/33/45/52/58 was similar between ages 20–29 and 40–49. At age 30–39, seroprevalence of any 9 types, any 7 HR types, HPV 31/33/45/52/58, and HPV 16/18 was 52.4%, 40.5%, 23.6%, and 27.8%, respectively.
Figure 1.
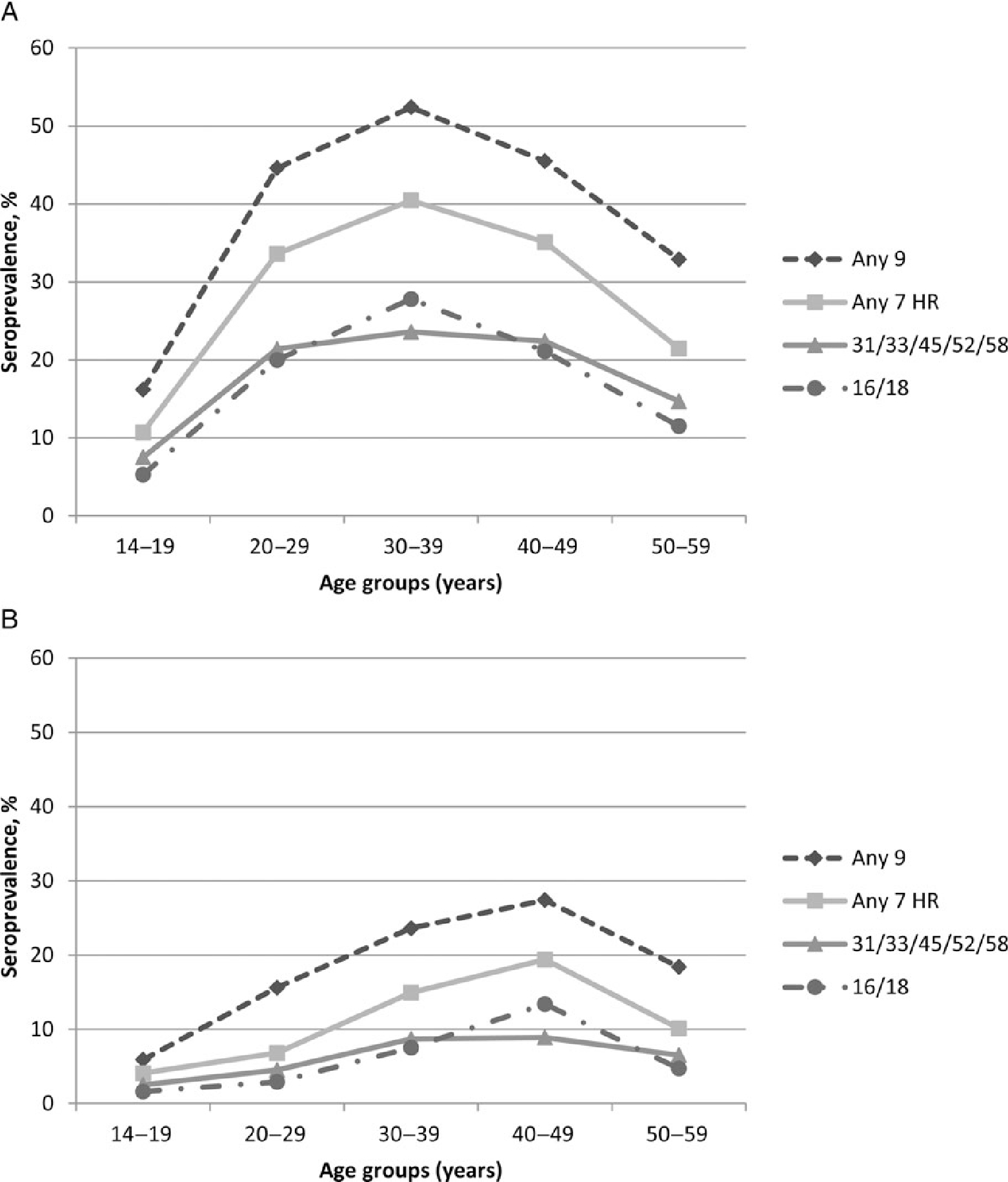
Seroprevalence for any of the 9 types (6/11/16/18/31/33/45/52/58), any of the 7 HR types (16/18/31/33/45/52/58), HPV 16/18, and any of the 5 additional types (31/33/45/52/58) in the 9-valent vaccine, by age group, for females (A) and males (B). Abbreviations: HPV, human papillomavirus; HR, high-risk.
Racial differences in seroprevalence were observed for all HPV type categories; non-Hispanic black females had the highest seroprevalence in all categories. Compared with non-Hispanic white females, non-Hispanic black females had higher seroprevalence of HPV 31/33/45/52/58 (36.8% vs 15.9%; P < .001), HPV16/18 (30.1% vs 17.8%; P < .001), and HPV 6/11 (39.3% vs 20.7%; P < .001) (Table 1). Compared to non-Hispanic white females, Mexican American females had similar seroprevalence of HPV 16/18 and HPV 6/11, but higher seroprevalence of HPV 31/33/45/52/58 (23.6% vs 15.9%; P = .014). Seroprevalence of the individual types HPV 31/33/52/58 varied by race/ethnicity; non-Hispanic black females had the highest seroprevalence for each of the types compared to non-Hispanic white and Mexican American females (P < .05 for all 4 types) (Table 2). Overall seroprevalence of HPV 45 was low and race/ethnicity-specific estimates were unstable.
We compared HPV 31/33/45/52/58 seroprevalence to HPV 16/18 seroprevalence within each race/ethnicity group. The ratio of HPV 31/33/45/52/58 seroprevalence to HPV 16/18 seroprevalence was 0.9 (95% confidence interval [CI], .7–1.0) among non-Hispanic white females, 1.2 (95% CI, 1.1–1.3) among non-Hispanic black females, and 1.4 (95% CI, .9–1.8) among Mexican American females (data not shown).
In multivariable analysis, age group, race/ethnicity, number of lifetime partners, and poverty were significantly associated with HPV16/18 seropositivity (Table 3). Non-Hispanic black females (aPR = 1.51; 95% CI, 1.23–1.86), but not Mexican American females, had higher seroprevalence compared to non-Hispanic white females. The seroprevalence of HPV 16/18 increased with the number of lifetime partners; those reporting ≥10 lifetime partners had the highest seroprevalence compared to those reporting 1 lifetime partner (aPR = 3.93; 95% CI, 2.32–6.66).
Table 3.
Factors Associated With Seropositivity to Human Papillomavirus (HPV) Types 16/18 or Any of the 5 Additional HPV Types in the 9-Valent HPV Vaccine Among Sexually Active Females
| HPV 16/18 aPR (95% CI) | HPV 31/33/45/52/58 aPR (95% CI) | |
|---|---|---|
|
| ||
| Age group (y) | P = .001 | P = .011 |
| 14–19 | Reference | Reference |
| 20–29 | 1.75 (1.15–2.67) | 1.48 (1.15–1.91) |
| 30–39 | 2.44 (1.62–3.69) | 1.68 (1.32–2.12) |
| 40–49 | 1.74 (1.10–2.77) | 1.73 (1.29–2.31) |
| 50–59 | 1.13 (.62–2.09) | 1.20 (.89–1.62) |
| Race/ethnicity | P = .003 | P < .001 |
| Non-Hispanic white | Reference | Reference |
| Non-Hispanic black | 1.51 (1.23–1.86) | 2.09 (1.64–2.67) |
| Mexican American | 1.17 (.82–1.65) | 1.95 (1.48–2.59) |
| Lifetime sex partners | P < .001 | P < .001 |
| 1 | Reference | Reference |
| 2–4 | 1.94 (1.07–3.54) | 3.34 (1.62–6.89) |
| 5–9 | 3.18 (1.72–5.89) | 4.50 (2.69–7.52) |
| ≥10 | 3.93 (2.32–6.66) | 6.20 (3.84–10.01) |
| Current smoking | P = .515 | P = .623 |
| No | Reference | Reference |
| Yes | 1.07 (.81–1.41) | 0.92 (.68–1.26) |
| Poverty | P = .026 | P = .085 |
| Above | Reference | Reference |
| At or below | 1.43 (1.08–1.89) | 1.30 (.95–1.78) |
The aPRs are adjusted for all variables listed on the table; P values were obtained by contrasting all levels of a variable to the reference level.
Abbreviations: aPR, adjusted prevalence ratio; CI, confidence interval; HPV, human papillomavirus.
Age group, race/ethnicity, and the number of lifetime partners were significantly associated with HPV 31/33/45/52/58 seropositivity in a multivariable model (Table 3). Both non-Hispanic black females (aPR = 2.09; 95% CI, 1.64–2.67) and Mexican American females (aPR = 1.95; 95% CI, 1.48–2.59) had higher HPV 31/33/45/52/58 seroprevalence than non-Hispanic white females. Seroprevalence increased with the number of lifetime partners; females who had ≥10 lifetime partners were 6.20 times (95% CI, 3.84–10.01) more likely to be seropositive for HPV 31/33/45/52/58 than those who had 1 lifetime partner.
Males
Among males, overall seropositivity to any 9 types, any 7 HR types, HPV 31/33/45/52/58, HPV16/18, and HPV 6/11 was 19.4%, 11.9%, 6.6%, 6.6%, and 10.5%, respectively (Table 1). Seropositivity for all HPV type categories was significantly lower than among females (P < .001 for all categories). Estimates for seroprevalence of individual types HPV 6/11/16/18 ranged from 2.4% for HPV 18 to 9.4% for HPV 6, and individual estimates for the 5 additional types ranged from 0.7% for HPV 45 to 2.3% for HPV 31 (Table 2). Seroprevalence for any 9 types, any 7 HR types, HPV 31/33/45/52/58, and HPV 16/18 was highest among 40–49-year-olds at 27.4%, 19.4%, 8.9%, and 13.4%, respectively (Table 1, Figure 1B). Due to low seropositivity among males, some age-group-specific estimates had a relative standard error of >30%. Of males, 14.9% were seropositive to only 1 type and 4.5% were seropositive to ≥2 types (data not shown).
Racial differences in seroprevalence were observed for some categories of HPV types, including any 9 types, HPV 16/18, and HPV 31/33/45/52/58. Seroprevalence of any 9 types was highest among non-Hispanic black males at 25.5% compared with non-Hispanic white males (17.9%) and Mexican American males (21.4%) (P = .035). Compared to non-Hispanic white males, non-Hispanic black males had higher seroprevalence of any 9 types (25.5% vs 17.9%; P = .011), HPV 16/18 (10.4% vs 6.7%; P = .014), and HPV 31/33/45/52/58 (10.0% vs 5.4%; P = .006). Compared to non-Hispanic white males, Mexican American males had higher seroprevalence of HPV 31/33/45/52/58 (9.3% vs 5.4%; P = .001). Seroprevalence of the individual HPV 31/33/45/52/58 types were low among males (Table 2). Although seroprevalence for HPV 52 and 58 varied by race/ethnicity, most race/ethnicity-specific estimates for these 2 types were unstable. There was no significant racial/ethnic difference for the other types.
DISCUSSION
This report includes the first analysis of the seroprevalence of the 5 additional HPV types (31/33/45/52/58) and of all HPV types (6/11/16/18/31/33/45/52/58) in the 9-valent vaccine prior to the introduction of the HPV vaccination program in the United States. In our analysis of 2005–2006 NHANES data, we found that seroprevalence of the 5 additional types among females ranged from 1.4% for HPV 45 to 7.5% for HPV 31, and the combined seroprevalence of HPV 31/33/45/52/58 was similar to HPV 16/18. Seroprevalence was lower among males. The seroprevalence of all HPV type categories varied by age and race/ethnicity in both sexes, although HPV 31/33/45/52/58 seroprevalence showed less variation by age than HPV 16/18.
As HPV seropositivity is an indicator of cumulative exposure, it is expected that seroprevalence would increase with age. The sharp increase in seroprevalence of all HPV type categories between age 14–19 and age 20–29 among females reflects the increase in the number of sexually active adolescents and young adults over those age groups. Among females, seroprevalence of any 9 types reached a high of 52% at age 30–39 years. The decline of HPV seroprevalence among older adults could be explained by several factors, such as having fewer lifetime sex partners in earlier birth cohorts or the decline of detectable antibodies over time [23, 24]. As reported previously for types in the quadrivalent HPV vaccine, the age-specific seroprevalence varied by sex for most HPV type categories, increasing through age 30–39 for females and age 40–49 for males [18, 19].
Racial/ethnic differences in seroprevalence of some HPV types have also been reported in previous NHANES studies. Analyses of 2003–2004 and 2003–2006 NHANES data showed that non-Hispanic black females and males had the highest seroprevalence of HPV 16/18 compared with other race/ethnic groups [18, 19]. We extended these findings to show that seroprevalence of HPV 31/33/45/52/58 is also higher in non-Hispanic black females and Mexican American females compared to non-Hispanic white females. Even after adjusting for sexual behaviors and other factors, non-Hispanic black and Mexican American females were significantly more likely to be seropositive for HPV31/33/45/52/58 compared to non-Hispanic white females; however, only non-Hispanic black females were significantly more likely to be seropositive for HPV 16/18 than non-Hispanic whites. Furthermore, comparing seroprevalence within each race/ethnicity group, we found that non-Hispanic black females had higher seroprevalence of HPV 31/33/45/52/58 than HPV 16/18, while there was no such difference among non-Hispanic white females.
Studies in the United States have found that while the majority of cervical intraepithelial neoplasia grades 2 and 3 and adenocarcinoma in situ (CIN2+) are attributable to HPV 16/18 in all race/ethnicity groups, non-Hispanic black females have a higher proportion of CIN2+ attributable to HPV 31/33/45/52/58 compared to non-Hispanic white females [9]. Potential reasons for this could include differences in the distribution of types circulating in sexual networks, sexual behaviors, antibody response, and host–virus interaction. Our findings suggest that greater cumulative infection with HPV 31/33/45/52/58 relative to HPV 16/18 among non-Hispanic blacks and Mexican Americans may explain some of the racial/ethnic differences in HPV type attribution in CIN2+. Differences in host–virus interaction have been suggested by previous studies. A study of incident HPV31 infections found that African American women were more likely to be infected with HPV31 C variants than other variants, and that C-variant infections persisted longer among African American women than white women [25]. It is important to note that while differences in seroprevalence and the percentage of precancer lesions attributable to these types by race/ethnicity have been observed, over 66% of cervical cancers in the United States are attributable to HPV 16/18 and there are no appreciable differences by race/ethnicity [11].This is likely due to the higher likelihood of precancer lesions caused by HPV 16/18 progressing to cancer [26].
Interpretations of the seroprevalence estimates for males in our analysis are limited due to low seroprevalence, as was observed previously in NHANES [18, 19]. Previous studies have shown that males have significantly lower seroconversion rate after natural infection than females, even though HPV infection rates among males are similar to that of females [17, 27]. The differences between female and male serologic response could be explained by the difference in infection sites between the 2 sexes; infections on the mucous membranes in the female genital tract may be more likely to induce an antibody response than infections on the keratinized epithelial tissues of the male genital tract [28].
Our study has several limitations. We only had seroprevalence data for all 9 types from 2005–2006, thus sample sizes were too small for some subgroup or individual-type analyses, especially among males. Even when grouped, age- and race/ethnicity-specific estimates for some HPV types had a relative standard error of >30% and were unstable. Seropositivity in our sample was measured by the 9-plex cLIA and may not be comparable to estimates using other assays. The number of lifetime partners may be under- or overreported due to social desirability and recall bias. However, using audio computer-assisted self-interviews for the sexual behavior questionnaire may have reduced social desirability bias.
In summary, using nationally representative data from the prevaccine era, we found that about 40% of females had serological evidence of exposure to at least 1 of the 9 HPV types targeted by the 9-valent vaccine, with the prevalence exceeding 50% among females aged 30–39 years. There were considerable differences by race/ethnicity in the seroprevalence of all HPV type categories examined in our study, but the differences were especially prominent in the seroprevalence of HPV 31/33/45/52/58 among females. Our data suggest that some of the racial/ethnic differences observed in the proportion of cervical precancer lesions attributable to HPV 31/33/45/52/58 may be due to relative differences in cumulative infection with HPV16/18 and HPV 31/33/45/52/58. While seroprevalence data provide information on cumulative infection, neither seroprevalence or prevalence of HPV types in the general population reflects the contribution of specific HPV types to the burden of HPV-associated cancers. HPV 16/18 account for the majority of cervical cancers, and HPV 16 accounts for the majority of other HPV-attributable cancers [11]. All 3 available vaccines will reduce the burden of infection and disease due to HPV 16/18, and the 9-valent vaccine will provide further protection against HPV 31/33/45/52/58.
Financial support.
This work was supported in part by an appointment to the Research Participation Program at the CDC administered by the Oak Ridge Institute for Science and Education (ORISE) through an interagency agreement between the U.S. Department of Energy and CDC. However, ORISE had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript.
Footnotes
Potential conflicts of interest. All authors: No reported conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Disclaimer. The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention (CDC).
References
- 1.Satterwhite CL, Torrone E, Meites E, et al. Sexually transmitted infections among US women and men: prevalence and incidence estimates, 2008. Sex Transm Infect 2013; 40:187–93. [DOI] [PubMed] [Google Scholar]
- 2.Chesson HW, Dunne EF, Hariri S, Markowitz LE. The estimated lifetime probability of acquiring human papillomavirus in the United States. Sex Transm Infect 2014; 41:660–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Doorbar J, Quint W, Banks L, et al. The biology and life-cycle of human papillomaviruses. Vaccine 2012; 30(suppl 5):F55–70. [DOI] [PubMed] [Google Scholar]
- 4.IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. Biological agents. Volume 100 B. A review of human carcinogens. IARC monographs on the evaluation of carcinogenic risks to humans 2012/11/30 ed. Vol. 100, 2012:1–441. [PMC free article] [PubMed] [Google Scholar]
- 5.Forman D, de Martel C, Lacey CJ, et al. Global burden of human papillomavirus and related diseases. Vaccine 2012; 30(suppl 5):F12–23. [DOI] [PubMed] [Google Scholar]
- 6.de Sanjose S, Quint WG, Alemany L, et al. Human papillomavirus genotype attribution in invasive cervical cancer: a retrospective cross-sectional worldwide study. Lancet Oncol 2010; 11:1048–56. [DOI] [PubMed] [Google Scholar]
- 7.Serrano B, Alemany L, Tous S, et al. Potential impact of a nine-valent vaccine in human papillomavirus related cervical disease. Infect Agent Cancer 2012; 7:38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Guan P, Howell-Jones R, Li N, et al. Human papillomavirus types in 115,789 HPV-positive women: a meta-analysis from cervical infection to cancer. Int J Cancer 2012; 131:2349–59. [DOI] [PubMed] [Google Scholar]
- 9.Hariri S, Unger ER, Schafer S, et al. HPV type attribution in high grade cervical lesions: assessing the potential benefits of vaccines in a population-based evaluation in the United States. Cancer Epidemiol Biomarkers Prev 2015; 24:393–9. [DOI] [PubMed] [Google Scholar]
- 10.Hariri S, Unger ER, Powell SE, et al. Human papillomavirus genotypes in high-grade cervical lesions in the United States. J Infect Dis 2012; 206:1878–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Saraiya M, Unger ER, Thompson TD, et al. US assessment of HPV types in cancers: implications for current and 9-valent HPV vaccines. J Natl Cancer Inst 2015; 107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Markowitz LE, Dunne EF, Saraiya M, et al. Human papillomavirus vaccination: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Morb Mortal Wkly Rep 2014; 63:1–30. [PubMed] [Google Scholar]
- 13.Petrosky E, Bocchini JA, Hariri S, et al. Use of 9-valent human papillomavirus (HPV) vaccine: updated HPV vaccination recommendations of the advisory committee on immunization practices. MMWR Morb Mortal Wkly Rep 2015; 64:300–4. [PMC free article] [PubMed] [Google Scholar]
- 14.Schiller JT, Castellsague X, Garland SM. A review of clinical trials of human papillomavirus prophylactic vaccines. Vaccine 2012; 30(suppl 5):F123–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Joura EA, Guiliano AR, Iversen OE, et al. A 9-valent HPV vaccine against infection and intraepithelial neoplasia in women. N Engl J Med 2015; 372:711–23. [DOI] [PubMed] [Google Scholar]
- 16.Carter JJ, Koutsky LA, Hughes JP, et al. Comparison of human papillomavirus types 16, 18, and 6 capsid antibody responses following incident infection. J Infect Dis 2000; 181:1911–9. [DOI] [PubMed] [Google Scholar]
- 17.Edelstein ZR, Carter JJ, Garg R, et al. Serum antibody response following genital {alpha}9 human papillomavirus infection in young men. J Infect Dis 2011; 204:209–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Introcaso CE, Dunne EF, Hariri S, Panicker G, Unger ER, Markowitz LE. Prevaccine era human papillomavirus types 6, 11, 16 and 18 seropositivity in the USA, National Health and Nutrition Examination Surveys, 2003–2006. Sex Transm Infect 2014; 90:505–8. [DOI] [PubMed] [Google Scholar]
- 19.Markowitz LE, Sternberg M, Dunne EF, McQuillan G, Unger ER. Seroprevalence of human papillomavirus types 6, 11, 16, and 18 in the United States: National Health and Nutrition Examination Survey 2003–2004. J Infect Dis 2009; 200:1059–67. [DOI] [PubMed] [Google Scholar]
- 20.CDC. Laboratory Procedure Manual – Human Papillomavirus. http://www.cdc.gov/nchs/data/nhanes/nhanes_05_06/HPVSRM_D_met.pdf. Updated January 2015. Accessed 22 April 2015.
- 21.Roberts C, Green T, Hess E, et al. Development of a human papillomavirus competitive luminex immunoassay for 9 HPV types. Hum Vaccin Immunother 2014; 10:2168–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.CDC. Laboratory Procedure Manual—Cotinine. http://www.cdc.gov/NCHS/data/nhanes/nhanes_09_10/COT_F_met.pdf. Updated 10 September 2008. Accessed 2 February 2015.
- 23.Liu G, Hariri S, Bradley H, Gottlieb SL, Leichliter JS, Markowitz LE. Trends and patterns of sexual behaviors among adolescents and adults aged 14–59 years, United States. Sex Transm Dis 2015; 42:20–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mollers M, Vossen JM, Scherpenisse M, van der Klis FR, Meijer CJ, de Melker HE. Review: current knowledge on the role of HPV antibodies after natural infection and vaccination: implications for monitoring an HPV vaccination programme. J Med Virol 2013; 85:1379–85. [DOI] [PubMed] [Google Scholar]
- 25.Xi LF, Schiffman M, Koutsky LA, et al. Persistence of newly detected human papillomavirus type 31 infection, stratified by variant lineage. Int J Cancer 2013; 132:549–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen HC, Schiffman M, Lin CY, et al. Persistence of type-specific human papillomavirus infection and increased long-term risk of cervical cancer. J Natl Cancer Inst 2011; 103:1387–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Partridge JM, Hughes JP, Feng Q, et al. Genital human papillomavirus infection in men: incidence and risk factors in a cohort of university students. J Infect Dis 2007; 196:1128–36. [DOI] [PubMed] [Google Scholar]
- 28.Giuliano AR, Nielson CM, Flores R, et al. The optimal anatomic sites for sampling heterosexual men for human papillomavirus (HPV) detection: the HPV detection in men study. J Infect Dis 2007; 196:1146–52. [DOI] [PMC free article] [PubMed] [Google Scholar]


