Abstract
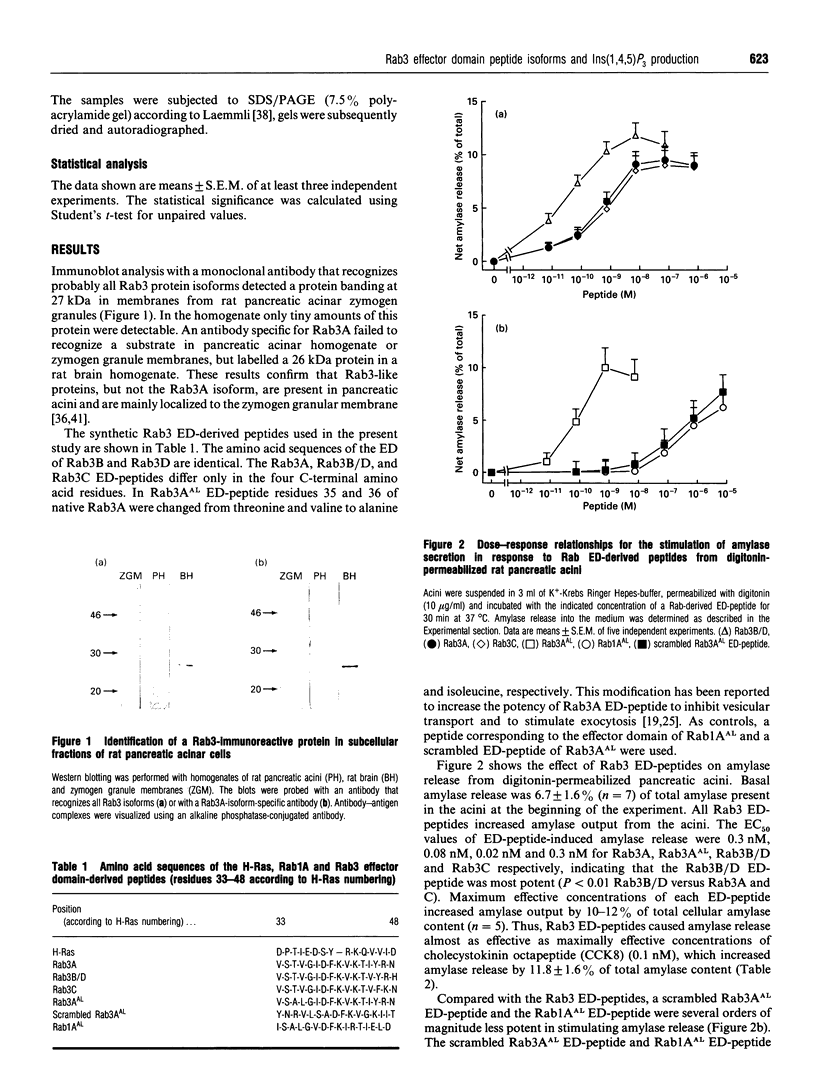
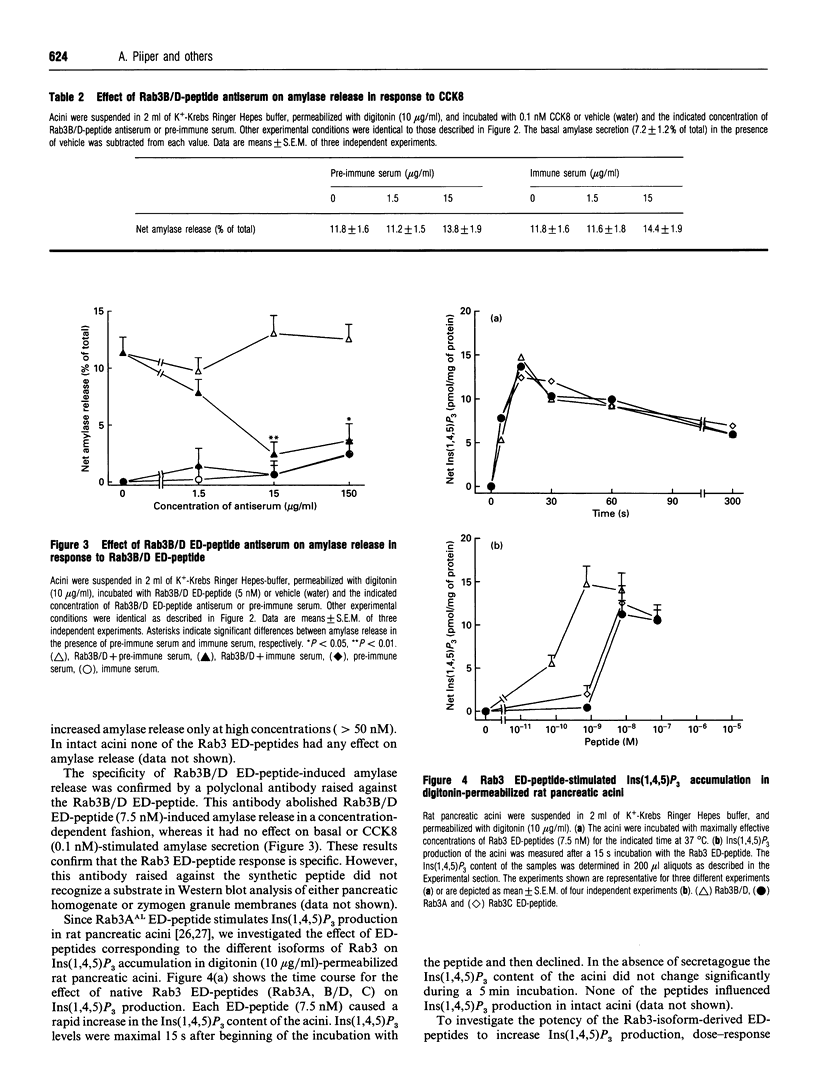
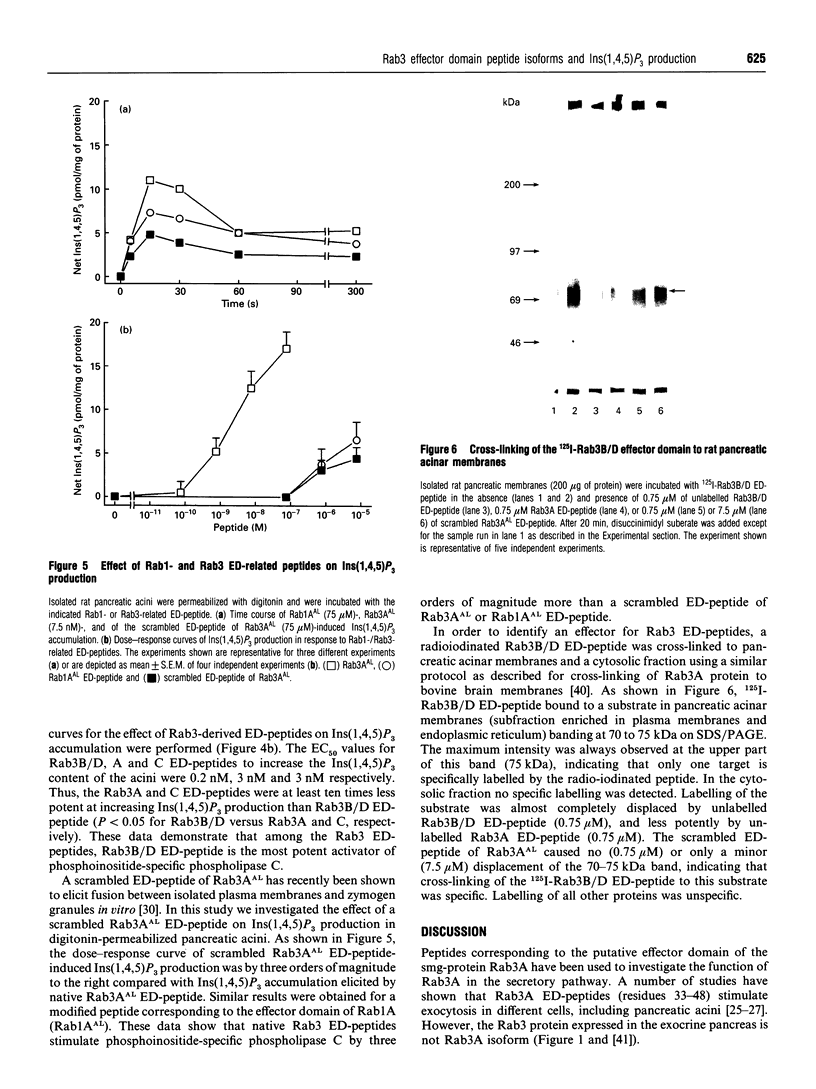
Rab3 proteins are localized on secretory vesicles and appear to be involved in regulated exocytosis. We have previously shown that a modified peptide corresponding to the effector domain of the small molecular mass GTP-binding protein Rab3A, Rab3AAL, stimulates inositol 1,4,5-trisphosphate [Ins(1,4,5)P3] production and amylase release in digitonin-permeabilized pancreatic acini. Experiments using monoclonal antibodies reveal that the Rab3-like protein present in pancreatic acini is not the Rab3A isoform. However, since the putative effector domains of the four as yet known Rab3 proteins (A, B, C and D) differ only in the C-terminal four amino acid residues, Rab3A effector domain peptide could mimic the action of the pancreas-specific Rab3 isoform. In the present study we report that peptides corresponding to the different Rab3 isoforms stimulate both Ins(1,4,5)P3 production and amylase secretion with an order of potency Rab3B/D > Rab3AAL > Rab3A = Rab3C. For Rab3A, B/D and C effector domain peptides the concentrations causing half-maximal response (EC50) were 3, 0.2 and 3 nM for Ins(1,4,5)P3 accumulation and 0.3, 0.02 and 0.3 nM for amylase release, respectively. A Rab1A effector domain peptide, Rab1AAL, and a scrambled peptide of Rab3AAL were less potent by several orders of magnitude in eliciting these responses compared with native Rab3 effector domain peptides. None of the peptides influenced Ins(1,4,5)P3 production and amylase release in intact acini. Cross-linking of 125I-Rab3B/D peptide to pancreatic acinar membranes showed a band at 70 to 75 kDa with maximum intensity at 75 kDa. Radiolabelling of the substrates could be displaced by unlabelled Rab3B/D peptide, and to a lesser extend by Rab3A peptide, whereas the scrambled peptide of Rab3AAL had no effect. These data suggest that phospholipase C and exocytosis might be regulated by Rab3B-or Rab3D-like proteins in pancreatic acinar cells. A 75 kDa protein that preferentially cross-linked to 125I-Rab3B/D effector domain peptide is a potential candidate as an effector protein of Rab3 effector domain peptides.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baldini G., Hohl T., Lin H. Y., Lodish H. F. Cloning of a Rab3 isotype predominantly expressed in adipocytes. Proc Natl Acad Sci U S A. 1992 Jun 1;89(11):5049–5052. doi: 10.1073/pnas.89.11.5049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Darchen F., Zahraoui A., Hammel F., Monteils M. P., Tavitian A., Scherman D. Association of the GTP-binding protein Rab3A with bovine adrenal chromaffin granules. Proc Natl Acad Sci U S A. 1990 Aug;87(15):5692–5696. doi: 10.1073/pnas.87.15.5692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwardson J. M., MacLean C. M., Law G. J. Synthetic peptides of the rab3 effector domain stimulate a membrane fusion event involved in regulated exocytosis. FEBS Lett. 1993 Mar 29;320(1):52–56. doi: 10.1016/0014-5793(93)81656-k. [DOI] [PubMed] [Google Scholar]
- Elferink L. A., Anzai K., Scheller R. H. rab15, a novel low molecular weight GTP-binding protein specifically expressed in rat brain. J Biol Chem. 1992 Mar 25;267(9):5768–5775. [PubMed] [Google Scholar]
- Fischer von Mollard G., Mignery G. A., Baumert M., Perin M. S., Hanson T. J., Burger P. M., Jahn R., Südhof T. C. rab3 is a small GTP-binding protein exclusively localized to synaptic vesicles. Proc Natl Acad Sci U S A. 1990 Mar;87(5):1988–1992. doi: 10.1073/pnas.87.5.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer von Mollard G., Stahl B., Khokhlatchev A., Südhof T. C., Jahn R. Rab3C is a synaptic vesicle protein that dissociates from synaptic vesicles after stimulation of exocytosis. J Biol Chem. 1994 Apr 15;269(15):10971–10974. [PubMed] [Google Scholar]
- Fischer von Mollard G., Stahl B., Li C., Südhof T. C., Jahn R. Rab proteins in regulated exocytosis. Trends Biochem Sci. 1994 Apr;19(4):164–168. doi: 10.1016/0968-0004(94)90278-x. [DOI] [PubMed] [Google Scholar]
- Fischer von Mollard G., Südhof T. C., Jahn R. A small GTP-binding protein dissociates from synaptic vesicles during exocytosis. Nature. 1991 Jan 3;349(6304):79–81. doi: 10.1038/349079a0. [DOI] [PubMed] [Google Scholar]
- Goud B., Salminen A., Walworth N. C., Novick P. J. A GTP-binding protein required for secretion rapidly associates with secretory vesicles and the plasma membrane in yeast. Cell. 1988 Jun 3;53(5):753–768. doi: 10.1016/0092-8674(88)90093-1. [DOI] [PubMed] [Google Scholar]
- Higashijima T., Ross E. M. Mapping of the mastoparan-binding site on G proteins. Cross-linking of [125I-Tyr3,Cys11]mastoparan to Go. J Biol Chem. 1991 Jul 5;266(19):12655–12661. [PubMed] [Google Scholar]
- Holz R. W., Brondyk W. H., Senter R. A., Kuizon L., Macara I. G. Evidence for the involvement of Rab3A in Ca(2+)-dependent exocytosis from adrenal chromaffin cells. J Biol Chem. 1994 Apr 8;269(14):10229–10234. [PubMed] [Google Scholar]
- Jena B. P., Gumkowski F. D., Konieczko E. M., von Mollard G. F., Jahn R., Jamieson J. D. Redistribution of a rab3-like GTP-binding protein from secretory granules to the Golgi complex in pancreatic acinar cells during regulated exocytosis. J Cell Biol. 1994 Jan;124(1-2):43–53. doi: 10.1083/jcb.124.1.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johannes L., Lledo P. M., Roa M., Vincent J. D., Henry J. P., Darchen F. The GTPase Rab3a negatively controls calcium-dependent exocytosis in neuroendocrine cells. EMBO J. 1994 May 1;13(9):2029–2037. doi: 10.1002/j.1460-2075.1994.tb06476.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karniguian A., Zahraoui A., Tavitian A. Identification of small GTP-binding rab proteins in human platelets: thrombin-induced phosphorylation of rab3B, rab6, and rab8 proteins. Proc Natl Acad Sci U S A. 1993 Aug 15;90(16):7647–7651. doi: 10.1073/pnas.90.16.7647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Law G. J., Northrop A. J., Mason W. T. rab3-peptide stimulates exocytosis from mast cells via a pertussis toxin-sensitive mechanism. FEBS Lett. 1993 Oct 25;333(1-2):56–60. doi: 10.1016/0014-5793(93)80374-4. [DOI] [PubMed] [Google Scholar]
- Li G., Regazzi R., Balch W. E., Wollheim C. B. Stimulation of insulin release from permeabilized HIT-T15 cells by a synthetic peptide corresponding to the effector domain of the small GTP-binding protein rab3. FEBS Lett. 1993 Jul 26;327(2):145–149. doi: 10.1016/0014-5793(93)80159-r. [DOI] [PubMed] [Google Scholar]
- Lledo P. M., Vernier P., Vincent J. D., Mason W. T., Zorec R. Inhibition of Rab3B expression attenuates Ca(2+)-dependent exocytosis in rat anterior pituitary cells. Nature. 1993 Aug 5;364(6437):540–544. doi: 10.1038/364540a0. [DOI] [PubMed] [Google Scholar]
- MacLean C. M., Law G. J., Edwardson J. M. Stimulation of exocytotic membrane fusion by modified peptides of the rab3 effector domain: re-evaluation of the role of rab3 in regulated exocytosis. Biochem J. 1993 Sep 1;294(Pt 2):325–328. doi: 10.1042/bj2940325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsui Y., Kikuchi A., Kondo J., Hishida T., Teranishi Y., Takai Y. Nucleotide and deduced amino acid sequences of a GTP-binding protein family with molecular weights of 25,000 from bovine brain. J Biol Chem. 1988 Aug 15;263(23):11071–11074. [PubMed] [Google Scholar]
- Matteoli M., Takei K., Cameron R., Hurlbut P., Johnston P. A., Südhof T. C., Jahn R., De Camilli P. Association of Rab3A with synaptic vesicles at late stages of the secretory pathway. J Cell Biol. 1991 Nov;115(3):625–633. doi: 10.1083/jcb.115.3.625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKiernan C. J., Brondyk W. H., Macara I. G. The Rab3A GTPase interacts with multiple factors through the same effector domain. Mutational analysis of cross-linking of Rab3A to a putative target protein. J Biol Chem. 1993 Nov 15;268(32):24449–24452. [PubMed] [Google Scholar]
- Mizoguchi A., Kim S., Ueda T., Takai Y. Tissue distribution of smg p25A, a ras p21-like GTP-binding protein, studied by use of a specific monoclonal antibody. Biochem Biophys Res Commun. 1989 Aug 15;162(3):1438–1445. doi: 10.1016/0006-291x(89)90835-8. [DOI] [PubMed] [Google Scholar]
- Novick P., Brennwald P. Friends and family: the role of the Rab GTPases in vesicular traffic. Cell. 1993 Nov 19;75(4):597–601. doi: 10.1016/0092-8674(93)90478-9. [DOI] [PubMed] [Google Scholar]
- Oberhauser A. F., Balan V., Fernandez-Badilla C. L., Fernandez J. M. RT-PCR cloning of Rab3 isoforms expressed in peritoneal mast cells. FEBS Lett. 1994 Feb 14;339(1-2):171–174. doi: 10.1016/0014-5793(94)80409-5. [DOI] [PubMed] [Google Scholar]
- Oberhauser A. F., Monck J. R., Balch W. E., Fernandez J. M. Exocytotic fusion is activated by Rab3a peptides. Nature. 1992 Nov 19;360(6401):270–273. doi: 10.1038/360270a0. [DOI] [PubMed] [Google Scholar]
- Olszewski S., Deeney J. T., Schuppin G. T., Williams K. P., Corkey B. E., Rhodes C. J. Rab3A effector domain peptides induce insulin exocytosis via a specific interaction with a cytosolic protein doublet. J Biol Chem. 1994 Nov 11;269(45):27987–27991. [PubMed] [Google Scholar]
- Padfield P. J., Balch W. E., Jamieson J. D. A synthetic peptide of the rab3a effector domain stimulates amylase release from permeabilized pancreatic acini. Proc Natl Acad Sci U S A. 1992 Mar 1;89(5):1656–1660. doi: 10.1073/pnas.89.5.1656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeffer S. R. GTP-binding proteins in intracellular transport. Trends Cell Biol. 1992 Feb;2(2):41–46. doi: 10.1016/0962-8924(92)90161-f. [DOI] [PubMed] [Google Scholar]
- Piiper A., Plusczyk T., Eckhardt L., Schulz I. Effects of cholecystokinin, cholecystokinin JMV-180 and GTP analogs on enzyme secretion from permeabilized acini and chloride conductance in isolated zymogen granules of the rat pancreas. Eur J Biochem. 1991 Apr 23;197(2):391–398. doi: 10.1111/j.1432-1033.1991.tb15923.x. [DOI] [PubMed] [Google Scholar]
- Piiper A., Stryjek-Kaminska D., Stein J., Caspary W. F., Zeuzem S. A synthetic peptide of the effector domain of rab3A stimulates inositol 1,4,5-trisphosphate production in digitonin-permeabilized pancreatic acini. Biochem Biophys Res Commun. 1993 May 14;192(3):1030–1036. doi: 10.1006/bbrc.1993.1520. [DOI] [PubMed] [Google Scholar]
- Piiper A., Stryjek-Kaminska D., Stein J., Caspary W. F., Zeuzem S. Tyrphostins inhibit secretagogue-induced 1,4,5-IP3 production and amylase release in pancreatic acini. Am J Physiol. 1994 Mar;266(3 Pt 1):G363–G371. doi: 10.1152/ajpgi.1994.266.3.G363. [DOI] [PubMed] [Google Scholar]
- Plutner H., Schwaninger R., Pind S., Balch W. E. Synthetic peptides of the Rab effector domain inhibit vesicular transport through the secretory pathway. EMBO J. 1990 Aug;9(8):2375–2383. doi: 10.1002/j.1460-2075.1990.tb07412.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richmond J., Haydon P. G. Rab effector domain peptides stimulate the release of neurotransmitter from cell cultured synapses. FEBS Lett. 1993 Jul 12;326(1-3):124–130. doi: 10.1016/0014-5793(93)81775-u. [DOI] [PubMed] [Google Scholar]
- Senyshyn J., Balch W. E., Holz R. W. Synthetic peptides of the effector-binding domain of rab enhance secretion from digitonin-permeabilized chromaffin cells. FEBS Lett. 1992 Aug 31;309(1):41–46. doi: 10.1016/0014-5793(92)80735-y. [DOI] [PubMed] [Google Scholar]
- Shirataki H., Kaibuchi K., Sakoda T., Kishida S., Yamaguchi T., Wada K., Miyazaki M., Takai Y. Rabphilin-3A, a putative target protein for smg p25A/rab3A p25 small GTP-binding protein related to synaptotagmin. Mol Cell Biol. 1993 Apr;13(4):2061–2068. doi: 10.1128/mcb.13.4.2061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirataki H., Kaibuchi K., Yamaguchi T., Wada K., Horiuchi H., Takai Y. A possible target protein for smg-25A/rab3A small GTP-binding protein. J Biol Chem. 1992 Jun 5;267(16):10946–10949. [PubMed] [Google Scholar]
- Weber E., Berta G., Tousson A., St John P., Green M. W., Gopalokrishnan U., Jilling T., Sorscher E. J., Elton T. S., Abrahamson D. R. Expression and polarized targeting of a rab3 isoform in epithelial cells. J Cell Biol. 1994 May;125(3):583–594. doi: 10.1083/jcb.125.3.583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeuzem S., Feick P., Zimmermann P., Haase W., Kahn R. A., Schulz I. Intravesicular acidification correlates with binding of ADP-ribosylation factor to microsomal membranes. Proc Natl Acad Sci U S A. 1992 Jul 15;89(14):6619–6623. doi: 10.1073/pnas.89.14.6619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeuzem S., Stryjek-Kaminska D., Caspary W. F., Stein J., Piiper A. Effect of a Rab3A effector domain-related peptide, CCK, and EGF in permeabilized pancreatic acini. Am J Physiol. 1994 Sep;267(3 Pt 1):G350–G356. doi: 10.1152/ajpgi.1994.267.3.G350. [DOI] [PubMed] [Google Scholar]




