Abstract
Venetoclax, a selective BCL-2 inhibitor, has shown superior efficacy in the treatment of AML. Nevertheless, some AML patients with AML1-ETO respond poorly to venetoclax treatment. In this report, a relapsed/refractory (R/R) venetoclax resistant AML1-ETO positive AML patient showed rapid tumor regression after combination therapy with gilteritinib and venetoclax. Additional laboratory findings indicated that the combined impact of the two drugs may be associated with the induction of the integrated stress response. This case presents a novel therapeutic approach for the treatment of FLT3 wild-type RR, AML1-ETO AML patients who have primary resistance to venetoclax.
Keywords: Refractory/relapse, Acute myeloid leukemia, Venetoclax, Gilteritinib, Case report
1. Introduction
Acute myeloid leukemia (AML) is a clonal malignant hematological disorder that originates from hematopoietic stem cells. The prognosis for most AML subtypes is poor, with long-term survival rates below 50 % [1,2]. Approximately 55 % of adult AML cases display non-random clonal chromosomal aberrations. Notably, the abnormal fusion gene AML1-ETO, a consequence of the t (8; 21) (q22; q22) chromosomal rearrangement, is identified in approximately 8 % of AML cases [3]. AML1-ETO fusion genes are associated with a relatively better prognosis in AML patients. AML1-ETO-positive AML is a highly heterogeneous disease that harbors a high rate of C-KIT mutations, which was believed to have a strong adverse effect on the relapse and survival of AML patients [4]. However, other studies indicated that C-KIT mutations may have no significant impact on the prognosis of the disease [5].
Venetoclax combined with hypomethylating agents (HMA) or low-dose cytarabine has received approval as a frontline therapy for elderly or unfit AML patients, marking a significant advancement in AML management [6]. Nonetheless, the clinical landscape is complicated by issues of resistance and relapse associated with BCL-2 inhibitor therapy, largely attributed to the upregulation of MCL-1 and BCL-XL. AML1-ETO has been identified as a significant risk factor for venetoclax resistance [7]. The elderly AML patients with AML1-ETO displayed a favorable prognosis in the intensive chemotherapy group, but not in the HMA or HMA/venetoclax group [8], suggesting that venetoclax treatment may provide less benefit in such patients. However, treatment options for older patients are limited. Therefore, rational combination approaches may be needed to improve the efficacy of ABT-199 in AML patients with AML1-ETO, thus potentially increasing their choice.
FLT3 mutations are one of the most prevalent genetic alterations in AML, presenting a valuable therapeutic target for relapsed/refractory (RR) cases [9]. Gilteritinib, a second-generation type I FLT3 inhibitor, has been recognized for its safety profile, and has recently received the US FDA approval for use in RR AML with FLT3 mutations [10]. Studies in preclinical FLT3 wild-type AML models indicated that the gilteritinib-venetoclax combination exhibits enhanced anti-AML synergy by reducing MCL-1 expression, though corresponding clinical research is pending [11].
In this report, we presented the case of a AML1-ETO (+), FLT3 wild-type AML patient who experienced primary resistance to AZA/VEN (azacitidine + venetoclax), but showed a rapid and positive response to a combination treatment regimen of gilteritinib and AZA/VEN. To explore the possible mechanism, further in vitro study was performed.
2. Case presentation
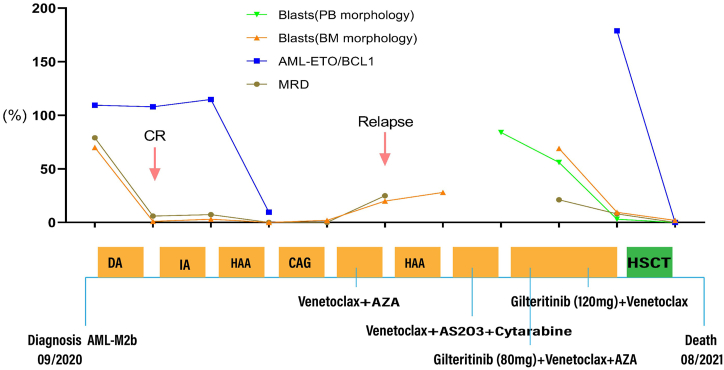
In 2020, a 53-year-old female with no significant past medical history was diagnosed with AML-M2b, characterized by 70 % blasts and a chromosomal profile of 45,X,-X,t (8; 21) (q22; q22), with AML1-ETO positivity at 109.45 %, cKIT mutation in D816V with a 43.4 % variant allele frequency, while the FLT3-mutation and other mutation was negative by a 150 myeloid-focused gene panel. The patient received daunorubicin and cytarabine (DA) as induction chemotherapy in September 2020. After three weeks, the bone marrow (BM) aspirate showed morphological complete response (CR). Subsequently, the patient received consolidation treatments with one cycle of IA (idarubicin + cytarabine), HAA (homoharringtonine + cytarabine + aclacinomycin), and CAG (cytarabine + aclacinomycin + granulocyte colony stimulating factor). However, next-generation sequencing (NGS) showed positive AML1-ETO fusion gene with a level of 9.61 % at the end of the last consolidation treatment, although the minimal residual disease (MRD) by flow cytometry (FCM) was negative (Fig. 1). In March 2021, the patient experienced morphological relapse during the treatment with a combination of venetoclax (100 mg on Day 1, 200 mg on Day 2, and 400 mg from Days 3–28) and azacitidine (75 mg/m2) for seven days. She was subsequently retreated with HAA regimen but showed no response, accompanied by an increase in BM blasts to 28 %. Thereafter, the patient received a combination treatment with venetoclax (100 mg on Day 1, 200 mg starting from Day 2), AS2O3 and chidamide, but did not achieve remission. Finally, the patient was treated with a combination of gilteritinib (at an initial dose of 80 mg), venetoclax and azacitidine. The blasts in peripheral blood decreased from 84 % to 56 %. Afterwards, the patient was administered a combination treatment of higher dosage gileritinib (120 mg) and venetoclax, and the proportion of the blasts in peripheral blood decreased to 3.0 % compared to 9.5 % in BM. Thereafter, the patient underwent salvage haploidentical hematopoietic stem cell transplantation (haplo-HSCT) in July 2021 (Fig. 1). Neutrophil engraftment was confirmed on day 15 after HSCT, with a peak absolute neutrophil count (ANC) of 2.7 × 109/L. Short tandem repeat (STR) testing indicated complete chimerism, with donor cells constituting 98.65 % of peripheral blood cells. Simultaneously, blasts in peripheral blood were cleared while BM aspirate revealed 2 % blasts. MRD by FCM was negative, and the AML1-ETO fusion gene levels decreased to 0.11 %. However, the patient developed secondary graft failure due to severe mixed bacterial and fungal infections, and grade II, acute gut graft versus host disease (GVHD), and her ANC dropped below 0.5 × 109/L after one month of haplo-HSCT. Severe infection ensued, and the patient died in a state of agranulocytosis.
Fig. 1.
Treatment process and effect evaluation of the patient.
3. Exploring the underlying molecular mechanism
This case report details a FLT3 wild-type refractory AML patient with AML1-ETO who demonstrated primary resistance to venetoclax (ABT-199). Our therapeutic strategy effectively highlighted the benefits of combining gilteritinib with venetoclax in treating FLT3 wild-type, AML1-ETO-positive, RR AML, facilitating a successful transition to HSCT, although the patient eventually died due to a severe infection. To further understand the synergistic effects and molecular mechanisms of this drug combination in FLT3 wild-type, AML1-ETO-positive, RR AML, we conducted in vitro experiments using relevant cell lines.
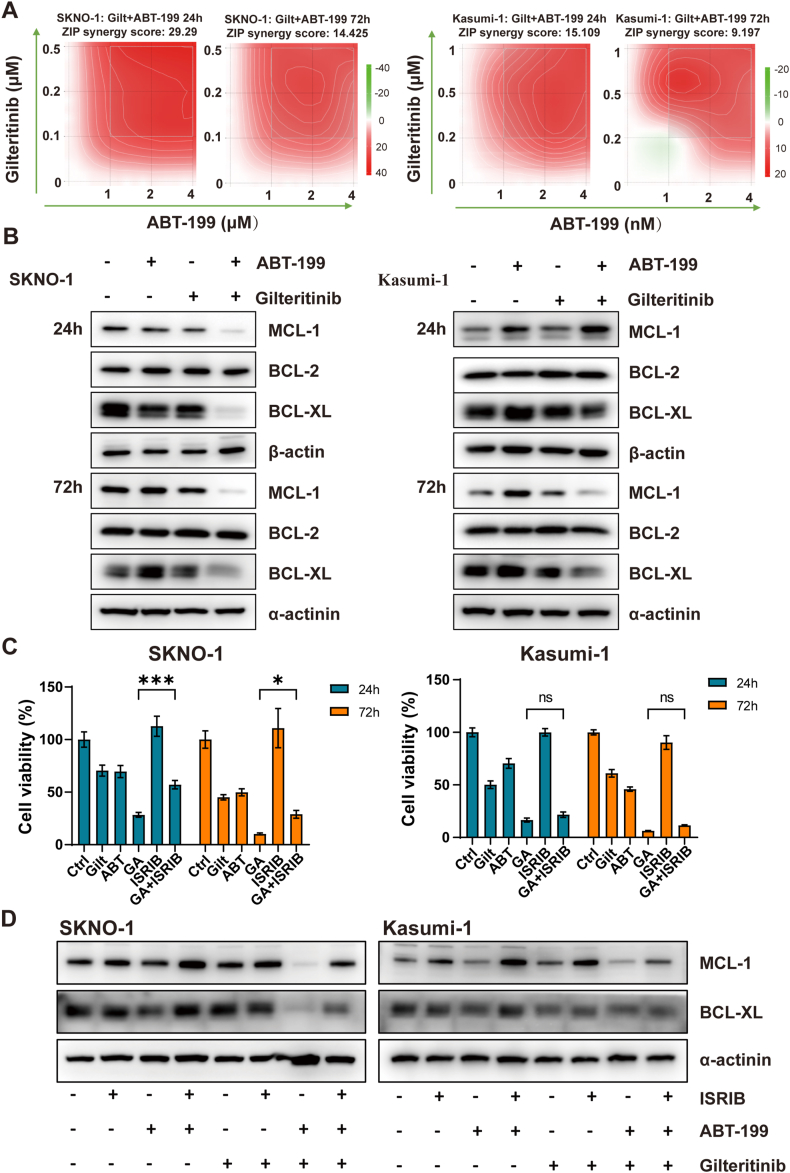
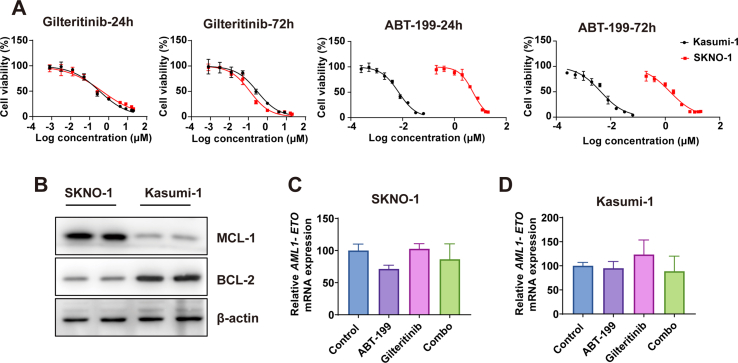
We utilized two cell lines, Kasumi-1 and SKNO-1, both originating from AML patients with AML1-ETO. Intriguingly, while both cell lines showed similar sensitivity to gilteritinib, their responses to ABT-199, a BCL-2 inhibitor, varied significantly. Notably, the IC50 value for ABT-199 at 72 hours post-administration in SKNO-1 was a thousand-fold that in Kasumi-1 (Supplemental Fig. 1A). Western blot analysis revealed that MCL-1 expression in SKNO-1 exceeded that in Kasumi-1, whereas BCL-2 expression was considerably lower (Supplemental Fig. 1B). This disparity might partially account for the different sensitivities to ABT-199 observed between the two cell lines. In both Kasumi-1 and SKNO-1 cells, the gilteritinib and ABT-199 combination showed a potent synergistic effect, effectively reducing the protein expression levels of MCL-1 and BCL-XL (Fig. 2A and B). This synergistic impact was more pronounced in SKNO-1 cells, evidenced by a higher synergy coefficient and a notable decrease in MCL-1 and BCL-XL protein expression as early as Day 1. However, the combination of gilteritinib with ABT-199 failed to reduce AML1-ETO mRNA expression in Kasumi-1 and SKNO-1 cells (Supplemental Figs. 1C and 1D). The observed reduction in the AML1-ETO fusion gene in this case is likely attributed to the pre-transplant conditioning regimen received by the patient.
Fig. 2.
Possible mechanisms of ABT-199 synergy with gilteritinib in AML1-ETO-positive AML. (A) The ZIP scores of SKNO-1 and Kasumi-1 were subjected to treatment with ABT-199 and gilteritinib for 24h and 72h. (B) The protein expression of BCL-2, MCL-1, BCL-XL, and β-actin in SKNO-1 and Kasumi-1 cell lines treated with ABT-199 and/or gilteritinib for 24h and 72h. (C) Viability of SKNO-1 and Kasumi-1 after treatment with DMSO, ABT-199 (ABT), gilteritinib (Gilt), or a combination of the two drugs (GA) in the absence or presence of ISRIB for 24h and 72h. (D) MCL-1, BCL-XL, and α-actinin protein expression of SKNO-1 and Kasumi-1 after treatment with DMSO, ABT-199, gilteritinib, or a combination of the two drugs in the absence or presence of ISRIB for 72h. ABT: ABT-199; Gilt: gilteritinib; GA: ABT-199 + gilteritinib.
Recent studies have highlighted that targeting the integrated stress response (ISR) pathway, in conjunction with ABT-199, can synergistically produce anti-leukemic effects. To ascertain whether ISR activation is essential for the synergistic efficacy of gilteritinib and ABT-199, we utilized ISRIB, a chemical inhibitor of ISR, to impede the downstream consequences of eIF2α phosphorylation. ISRIB treatment successfully restored the viability of SKNO-1 cells and mitigated the reduction in MCL-1 protein levels after the combined administration of gilteritinib and ABT-199. However, these effects were not observed in Kasumi-1 cells (Fig. 2C and D).
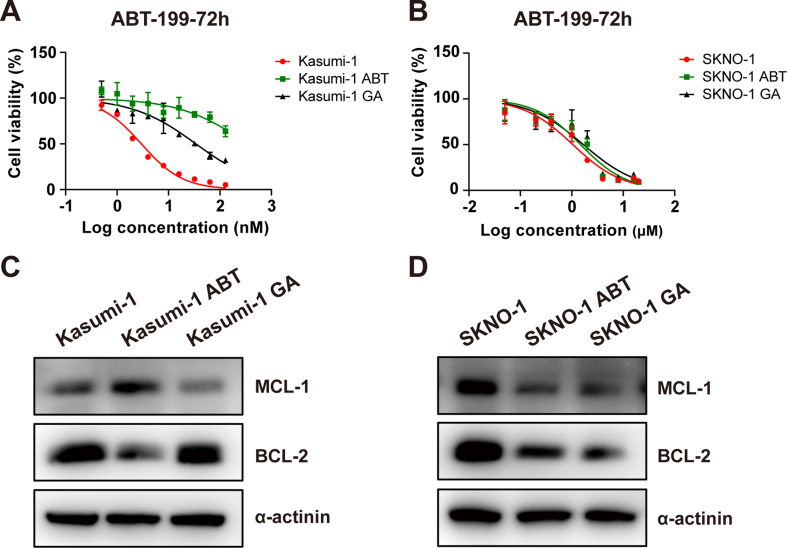
Given the increased risk of resistance with extended combination therapy, we exposed Kasumi-1 and SKNO-1 cells to increasing concentration of ABT-199 alone (Kasumi-1 ABT, SKNO-1 ABT) or with the combination of gilteritinib (Kasumi-1 GA, SKNO-1 GA) for 30 days to evaluate shifts in ABT-199 sensitivity (Supplemental Figs. 2A and 2B). The findings revealed that in Kasumi-1 cell line, sequential administration of ABT-199 therapy swiftly led to resistance development, marked by an upsurge in MCL-1 protein and a decline in BCL-2 protein levels (Supplemental Fig. 2C). Conversely, the 30-day combination treatment of gilteritinib and ABT-199 delayed the emergence of ABT-199 resistance in Kasumi-1 cells. This effect was not observed in the SKNO-1 cell line, perhaps due to its inherently lower sensitivity to ABT-199 and the insufficiency of a 30-day period to foster a heightened level of resistance (Supplemental Fig. 2D).
4. Discussion
This case provides preliminary evidence for the clinical effectiveness of combining gilteritinib with BCL-2 inhibitors (BCL-2i) in the treatment of FLT3 wild-type RR AML patients with AML1-ETO. However, to substantiate these findings, large-scale clinical trials are essential. Furthermore, given the heterogeneity of AML, it is important to understand whether the gilteritinib and BCL-2i combination yields a synergistic killing effect across all AML variants. In our in vitro experiments, gilteritinib and venetoclax demonstrated a potent synergistic effect in AML1-ETO-positive cell lines, notably reducing MCL-1 and BCL-XL expression. Yet, the underlying mechanisms of their synergistic interaction appear to differ, potentially due to the distinct genetic backgrounds of the cell lines, their variable sensitivities to venetoclax, and alterations in signaling pathways induced by the drug combination. These factors warrant further experimental investigation for a better understanding. Furthermore, there were several limitations in our study. The generalizability of this study is limited because it is a single case report. Additionally, we merely performed the in vitro experiment to investigate the effect and the possible underlying mechanisms of combining gilteritinib and venetoclax, the in vivo as well as clinical experiment will be performed in future experiments. Finally, our findings suggest that patients exhibiting lower sensitivity to venetoclax may derive greater benefit from the combined use of gilteritinib and venetoclax. While this case enhances the decision-making process for clinical treatment plans in RR AML with AML1-ETO, further research is necessary to develop more effective, personalized treatment modalities.
Ethics statement
Written informed consent was obtained from the patient's family, agreeing to publish the relevant clinical image/data included in the manuscript.
Funding
The present study was supported by the Zhejiang Provincial Natural Science Foundation (No. LY21H290003), National Natural Science Foundation of China (No. 81503296), Zhejiang Scientific Research Fund of Traditional Chinese Medicine (NO. 2020ZB085), Specific Program of Scientific Research of Zhejiang Chinese Medicine University for Affiliated Hospital (NO.2023FSYYZZ04), Project of Academic Inheritance Studio of Famous and Aged Chinese Medicine Experts in Zhejiang Province (No. GZS2021022) and Science and Technological Innovation Project for College Students in Zhejiang Province (Xinmiao Talent Plan) (No. 2023R410003).
Ethics approval and consent to participate
The in vitro study of cell lines was approved by the ethical committee of the First Affiliated Hospital of Zhejiang Chinese Medical University (NO.2022-KL-159-02). All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Data availability statement
In this study, all relevant data supporting the findings of this research are included within the article. Additional data associated with this study has not been deposited into a publicly available repository, but it will be made available upon reasonable request.
CRediT authorship contribution statement
Man Li: Writing – original draft, Project administration, Investigation. Xiawan Yang: Writing – original draft, Investigation, Data curation. Yaonan Hong: Investigation, Data curation. Qi Liu: Methodology, Investigation. Yingying Shen: Investigation, Data curation. Tonglin Hu: Validation, Investigation, Data curation. Yiping Shen: Resources, Investigation. Guoyin Kai: Writing – review & editing, Resources, Methodology, Investigation. Dijiong Wu: Writing – review & editing, Resources, Project administration, Methodology, Investigation, Funding acquisition, Conceptualization.
Declaration of competing interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: Corresponding Author “Dijiong Wu” is an associate editor of the journal of Heliyon Oncology. If there are other authors, they declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
We appreciate the patient's family for agreeing to publish the relevant clinical data.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.heliyon.2024.e35847.
Contributor Information
Guoyin Kai, Email: kaiguoyin@163.com.
Dijiong Wu, Email: wudijiong@zcmu.edu.cn.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
figs1.
figs2.
References
- 1.Döhner H., Estey E., Grimwade D., Amadori S., Appelbaum F.R., Büchner T., Dombret H., Ebert B.L., Fenaux P., Larson R.A., Levine R.L., Lo-Coco F., Naoe T., Niederwieser D., Ossenkoppele G.J., Sanz M., Sierra J., Tallman M.S., Tien H.F., Wei A.H., Löwenberg B., Bloomfield C.D. Diagnosis and management of AML in adults: 2017 ELN recommendations from an international expert panel. Blood. 2017;129:424–447. doi: 10.1182/blood-2016-08-733196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Akgul C. Mcl-1 is a potential therapeutic target in multiple types of cancer. Cell. Mol. Life Sci. 2009;66:1326–1336. doi: 10.1007/s00018-008-8637-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rücker F.G., Bullinger L., Gribov A., Sill M., Schlenk R.F., Lichter P., Döhner H., Döhner K. Molecular characterization of AML with ins(21;8)(q22;q22q22) reveals similarity to t(8;21) AML. Genes Chromosomes Cancer. 2011;50:51–58. doi: 10.1002/gcc.20830. [DOI] [PubMed] [Google Scholar]
- 4.Rulina A.V., Spirin P.V., Prassolov V.S. Activated leukemic oncogenes AML1-ETO and c-kit: role in development of acute myeloid leukemia and current approaches for their inhibition. Biochemistry (Mosc). 2010;75:1650–1666. doi: 10.1134/s0006297910130092. [DOI] [PubMed] [Google Scholar]
- 5.Riera L., Marmont F., Toppino D., Frairia C., Sismondi F., Audisio E., Di Bello C., D'Ardia S., Di Celle P.F., Messa E., Inghirami G., Vitolo U., Pich A. Core binding factor acute myeloid leukaemia and c-KIT mutations. Oncol. Rep. 2013;29:1867–1872. doi: 10.3892/or.2013.2328. [DOI] [PubMed] [Google Scholar]
- 6.Guerra V.A., DiNardo C., Konopleva M. Venetoclax-based therapies for acute myeloid leukemia. Best Pract. Res. Clin. Haematol. 2019;32:145–153. doi: 10.1016/j.beha.2019.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zong L., Yin M., Kong J., Zhang J., Song B., Zhu J., Xue S., Wu X., Wu D., Bao X., Qiu H. Development of a scoring system for predicting primary resistance to venetoclax plus hypomethylating agents (HMAs) in acute myeloid leukemia patients. Mol. Carcinog. 2023;62:1572–1584. doi: 10.1002/mc.23600. [DOI] [PubMed] [Google Scholar]
- 8.Park S., Kim T.Y., Cho B.S., Kwag D., Lee J.M., Kim M., Kim Y., Koo J., Raman A., Kim T.K., Kim H.J. Prognostic value of European Leukemia Net 2022 criteria and genomic clusters using machine learning in older adults with acute myeloid leukemia. Haematologica. 2023 doi: 10.3324/haematol.2023.283606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Perl A.E., Altman J.K., Cortes J., Smith C., Litzow M., Baer M.R., Claxton D., Erba H.P., Gill S., Goldberg S., Jurcic J.G., Larson R.A., Liu C., Ritchie E., Schiller G., Spira A.I., Strickland S.A., Tibes R., Ustun C., Wang E.S., Stuart R., Röllig C., Neubauer A., Martinelli G., Bahceci E., Levis M. Selective inhibition of FLT3 by gilteritinib in relapsed or refractory acute myeloid leukaemia: a multicentre, first-in-human, open-label, phase 1-2 study. Lancet Oncol. 2017;18:1061–1075. doi: 10.1016/s1470-2045(17)30416-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Perl A.E., Martinelli G., Cortes J.E., Neubauer A., Berman E., Paolini S., Montesinos P., Baer M.R., Larson R.A., Ustun C., Fabbiano F., Erba H.P., Di Stasi A., Stuart R., Olin R., Kasner M., Ciceri F., Chou W.C., Podoltsev N., Recher C., Yokoyama H., Hosono N., Yoon S.S., Lee J.H., Pardee T., Fathi A.T., Liu C., Hasabou N., Liu X., Bahceci E., Levis M.J. Gilteritinib or chemotherapy for relapsed or refractory FLT3-mutated AML. N. Engl. J. Med. 2019;381:1728–1740. doi: 10.1056/NEJMoa1902688. [DOI] [PubMed] [Google Scholar]
- 11.Janssen M., Schmidt C., Bruch P.M., Blank M.F., Rohde C., Waclawiczek A., Heid D., Renders S., Göllner S., Vierbaum L., Besenbeck B., Herbst S.A., Knoll M., Kolb C., Przybylla A., Weidenauer K., Ludwig A.K., Fabre M., Gu M., Schlenk R.F., Stölzel F., Bornhäuser M., Röllig C., Platzbecker U., Baldus C., Serve H., Sauer T., Raffel S., Pabst C., Vassiliou G., Vick B., Jeremias I., Trumpp A., Krijgsveld J., Müller-Tidow C., Dietrich S. Venetoclax synergizes with gilteritinib in FLT3 wild-type high-risk acute myeloid leukemia by suppressing MCL-1. Blood. 2022;140:2594–2610. doi: 10.1182/blood.2021014241. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
In this study, all relevant data supporting the findings of this research are included within the article. Additional data associated with this study has not been deposited into a publicly available repository, but it will be made available upon reasonable request.