Abstract
Background
Plasma glial fibrillary acidic protein (GFAP), an astrocytic biomarker, has previously been linked with Alzheimer's disease (AD) status, amyloid levels, and memory performance in older adults. The neuroanatomical pathways by which astrogliosis/astrocyte reactivity might impact cognitive outcomes remains unclear. We evaluated whether plasma GFAP and amyloid levels had a synergistic effect on fornix structure, which is critically involved in AD-associated cholinergic pathways. We also examined whether fornix structure mediates associations between GFAP and verbal memory.
Methods
In a cohort of both asymptomatic and symptomatic older adults (total n = 99), we assessed plasma GFAP, amyloid-β42 (Aβ42), other AD-related proteins, and vascular markers, and we conducted comprehensive memory testing. Tractography-based methods were used to assess fornix structure with whole brain diffusion metrics to control for diffuse alterations in brain white matter.
Results
In individuals in the low plasma amyloid-β42 (Aβ42) group, higher plasma GFAP was associated with lower fractional anisotropy (FA; p = 0.007), higher mean diffusivity (MD; p < 0.001), higher radial diffusivity (RD; p < 0.001), and higher axial diffusivity (DA; p = 0.001) in the left fornix. These associations were independent of APOE gene status, plasma levels of total tau and neurofilament light, plasma vascular biomarkers, and whole brain diffusion metrics. In a sub-analysis of participants in the low plasma Aβ42 group (n = 33), fornix structure mediated the association between higher plasma GFAP levels and lower verbal memory performance.
Discussion
Higher plasma GFAP was associated with altered fornix microstructure in the setting of greater amyloid deposition. We also expanded on our prior GFAP-verbal memory findings by demonstrating that in the low plasma Aβ42 group, left fornix integrity may be a primary white matter conduit for the negative associations between GFAP and verbal memory performance. Overall, these findings suggest that astrogliosis/astrocyte reactivity may play an early, pivotal role in AD pathogenesis, and further demonstrate that high GFAP and low Aβ42 in plasma may reflect a particularly detrimental synergistic role in forniceal-memory pathways.
Keywords: GFAP, Astrogliosis, Biomarkers, Alzheimer's disease, Vascular, Verbal memory, Fornix
Highlights
-
•
Higher blood GFAP is associated with altered fornix structure in the setting of greater amyloid.
-
•
High GFAP and low blood Aβ42 may reflect a detrimental synergistic role in fornix-memory pathways.
-
•
GFAP is an early indicator of amyloidogenic processes and may be integral to prognostic models.
1. Introduction
Heterogeneity in late life cognitive decline is a cardinal feature of pathological aging (Boyle et al., 2021) and may be driven by a combination of factors, including abnormal protein deposition (e.g., amyloid and tau) and immune dysregulation. Although systemic inflammation has long been studied as a metric for “inflammaging” and as a risk factor for Alzheimer's disease (AD) (Franceschi et al., 2017; Walker et al., 2019), increasing evidence suggests that disruption of glial pathways may presage and/or exacerbate initial memory decline in late life (Chatterjee et al., 2022). Blood glial fibrillary acidic protein (GFAP), an astrocytic protein that is often conceptualized as a proxy for astrocyte reactivity or astrogliosis, increases with age, is predictive of future dementia status, and is associated with worse cognitive performance in both asymptomatic older adults (i.e., community dwelling older adults with no symptoms of AD) and older adults with AD dementia (Chatterjee et al., 2022; Kumar et al., 2023; Oeckl et al., 2019). Moreover, our group previously demonstrated that higher plasma GFAP was related to worse verbal memory performance and white matter diffusivity indicators (i.e., fractional anisotropy, FA) in AD vulnerable regions in late life (Bettcher et al., 2021). Although compelling evidence suggests that astrogliosis/astrocyte reactivity are related to both white matter integrity and verbal memory function in late life, comprehensive assessments of the relationships between astrogliosis, immunovascular markers, and white matter tracts that potentially underlie verbal memory dysfunction have not been conducted.
White matter degeneration in limbic tracts has been shown to precede grey matter atrophy in the hippocampus (Fletcher et al., 2014), with the fornix being a white matter tract whose degeneration is strongly linked to early dysfunction of memory systems and Mild Cognitive Impairment (MCI) (Benear et al., 2020; Lee et al., 2012). The fornix has both efferent and afferent connections with the hippocampus and serves as the primary conduit for the limbic circuit to transmit acetylcholine signaling from the basal forebrain to the hippocampus (Senova et al., 2020). AD is characterized by a deficit in cholinergic neurons and cholinergic signaling, with mounting evidence suggesting that this deficit is associated with degeneration in basal forebrain-fornix-hippocampal pathways. Importantly, forniceal transmission of acetylcholine signaling to the hippocampus results in the hippocampus changing from a consolidation state to an active encoding state (Benear et al., 2020), and damage to the fornix causes a diminution in episodic memory encoding processes. Furthermore, the fornix-hippocampal pathway, along with the vagus nerve, have been termed the cholinergic anti-inflammatory pathway (CAP) as they provide an important system check on inflammation and glial activation (Gamage et al., 2020; Teles-Grilo et al., 2013). Astrocytes are part of the neurovascular unit, and they encapsulate cholinergic neurons and associated cell processes; activation of astrocyte acetylcholine receptors (a7nAChRs) induces this anti-inflammatory effect through several mechanisms (including inhibition of nuclear factor-kB pathways) via fornix-hippocampal connections (Gamage et al., 2020). Astrocyte regulation of forniceal pathways is well established in animal models, but the mechanisms by which astrocyte dysregulation may impact fornix integrity and the comorbid factors that may influence this pathway in humans are less understood.
Plasma GFAP is strongly linked to amyloid deposition, with levels of GFAP increasing in amyloid positive individuals years prior to significant tau accumulation (Pereira et al., 2021). This suggests that astrocyte reactivity or dysregulation in tandem with amyloid deposition may be a critical early propagator of pathways leading to memory dysregulation in late life. Collectively, evidence suggests that a) astrocyte regulation, fornix integrity, and verbal memory are impacted early in AD pathogenesis; b) astrocytic proteins are associated with white matter microstructure disruption in AD vulnerable regions and worse verbal memory performance; and c) the fornix-hippocampal pathway is critical for the anti-inflammatory transmission of acetylcholine in normal verbal memory function. It is unclear, however, whether there is a synergistic role of astrogliosis/astrocyte reactivity (as measured by GFAP) and amyloid on fornix structure; in addition, it is also unclear if, in the setting of greater amyloid deposition, disruption of fornix microstructure is a partial mediator of the pathway between elevated plasma GFAP and verbal memory performance in late life.
The overarching goal of this study was to examine the association of astrogliosis/astrocyte reactivity with fornix integrity and elucidate the role of AD-related pathology in modifying these associations. Note that throughout, we will refer to GFAP as a biomarker of astrogliosis/astrocyte reactivity; however, we also acknowledge that the GFAP may reflect a host of other astrocyte-related processes. Our primary hypothesis was that in individuals with higher amyloid burden (i.e., lower plasma amyloid-β42 levels), higher levels of an astrocytic marker (GFAP) would be associated with altered diffusion metrics in the fornix. Given that astrocyte dysregulation may be particularly detrimental in the setting of amyloid deposition (Beyer et al., 2023), we first appraised whether amyloid-β42 levels (delineated by tertiles) moderated associations between plasma GFAP and fornix structure using tractography methodology. Second, as vascular plasma biomarkers are also a critical consideration given that the fornix is vulnerable to vascular dysfunction (Bettcher et al., 2013; Bateman et al., 2019), these plasma biomarkers were adjusted for in secondary models of GFAP and fornix structure. Finally, to elucidate potential neuroanatomic pathways for the previously documented link between astrogliosis/astrocyte reactivity and verbal memory, we tested in an exploratory analysis whether fornix integrity mediated associations between plasma GFAP and verbal memory.
2. Methods
Participants: Healthy older adults (henceforth referred to as asymptomatic to reflect a lack of clinical symptomology for AD (Jack et al., 2018; Jack et al., 2024)) and adults with symptomatic AD were selected from the Bio-AD study, which is a study of aging and AD that involves comprehensive cognitive testing, health history assessment, phlebotomy, neurological and physical examination, neuroimaging, and informant interview of all participants (i.e., Clinical Dementia Rating Scale, CDR) (described previously (Bettcher et al., 2021; Bateman et al., 2019)). Of the participants with plasma biomarkers available (n = 114), participants were included in the current analysis if they had available diffusion neuroimaging data (n = 99). Asymptomatic participants (n = 66) were defined as community dwelling older adults with no diagnosis of MCI or dementia, and no evidence of a neurodegenerative phenotype based on a neurological exam. Participants with symptomatic AD (n = 33) were defined as having adjudicated MCI due to possible AD or AD dementia based on NIA-AA clinical criteria (Albert et al., 2011; McKhann et al., 2011). Exclusions were based solely on the parent study's (Bio-AD) criteria; specifically, participants were excluded if they had a major psychiatric disorder, current non-AD neurological condition known to affect cognition (e.g., Parkinson's disease, frontotemporal dementia, multiple sclerosis, large vessel infarct, focal brain lesion in the past two years, current substance abuse/dependence, significant systemic medical illness or active neoplastic disease, significant sensory or motor deficits that would interfere with cognitive testing, or traumatic brain injury in the past 5 years with loss of consciousness longer than 5 min). Taken together, our final participant sample included 66 asymptomatic older adults and 33 older adults with symptomatic AD.
All participants were reviewed at an interdisciplinary consensus conference with a board-certified neuropsychologist, board-certified behavioral neurologists, advanced practice provider, and clinical research coordinators. All participants signed an informed consent approved by the University of Colorado (CU) Multiple Institutional Review Board. Study data were collected and managed using REDCap electronic data capture tools hosted at CU Anschutz Medical Campus (Harris et al., 2009).
Cognitive Assessment: Participants completed cognitive testing with the Montreal Cognitive Assessment [MoCA (Nasreddine et al., 2005)] and the Spanish English Neuropsychological Assessment Scales [SENAS (Mungas et al., 2004, 2005)]. Note that only English-speaking participants enrolled in and were included in this project. An informant-based interview was (Clinical Dementia Rating Scale [CDR]) used to assess and rate functional severity.
The SENAS battery was based on item response theory (IRT), and psychometrically matched measures were created across different scales, thus assuring reliability across the full ability continuum (Mungas et al., 2000, 2004, 2005, 2011). For the purposes of this study, IRT composite scores were used for the domains described below. These IRT scores do not have floor or ceiling effects and are normally distributed. IRT scores may be interpreted as unadjusted standard scores (Mean = 0; SD = 1) based on a demographically diverse sample of older adults aged 60+24. Our primary cognitive outcome was verbal episodic memory and was selected due to its mechanistic role in memory formation, which is impacted in early stages of AD (Ewers et al., 2010). It was also the primary cognitive outcome for the current analysis given our prior work indicating that elevated plasma GFAP was more strongly related to memory performance compared to other domains of cognition (Bettcher et al., 2021). The Verbal Memory IRT composite score was assessed with a multi-trial list-learning measure (5 learning trials; 15 items), incorporating all learning trials and also delayed recall.
Neuroimaging: Prior studies of white matter imaging with plasma GFAP have focused primarily on non-specific diffusion tensor imaging (DTI) methods using FA, which rely on atlases to extract FA from regions of interest in a skeletonized diffusion map. While these DTI methods provide important information regarding white matter integrity, these approaches are suboptimal for delineating microstructure of small and/or curved structures including the fornix (Rabin et al., 2019). Accordingly, we used a tractography-based approach to delineate the fornix bundle (as well as to obtain whole brain diffusion metrics) in native space for each participant to more comprehensively appraise alterations in this tract. Whole brain MRI scans were obtained on a 3.0 T S (Iselin, NJ) Skyra scanner equipped with a 20-channel head coil. Diffusion imaging data were acquired utilizing multi-shell 2D echo planar imaging sequence (56 slices; TR/TE = 8400/105 ms, acquisition matrix = 112 × 112; 2 × 2 × 2 mm3 isotropic spatial resolution; 64 x b = 2500 s/mm2 A > P direction; 32 x b = 700 s/mm2 P > A direction; 18 x b = 0 s/mm2). Diffusion data were first pre-processed to correct for susceptibility induced distortions using FMIRB Software Library (FSL) tools topup function, as well as eddy current distortions and motion artifacts using FSL's eddy function (Smith et al., 2004; Andersson and Sotiropoulos, 2016). The corrected diffusion data were imported into DSI studio (https://dsi-studio.labsolver.org) to create the diffusion maps and perform tractography analysis.
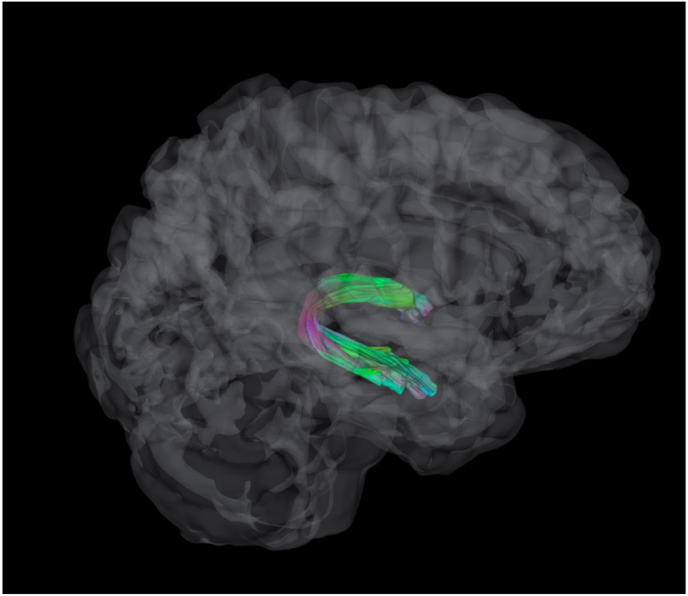
Deterministic tractography for the fornix (and whole brain control) was performed with an FA threshold of 0.13, angular threshold of 40° and step size of 1 mm (Fig. 1). Tracts with length shorter than 65 mm or longer than 280 mm were discarded. A total of 10000 tracts were calculated. For the fornix, tracts were calculated along previously defined Fornix ROIs from the Human Connectome Project HCP-1065 atlas. First, a linear registration was performed between the HCP-1065 template and each subject's anisotropy map to get a transformation matrix. Then the ROIs were nonlinearly registered to the atlas using a series discrete cosine transforms (Friston et al., 1995). Spherical regions of avoidance were placed bilaterally in the head of the caudate and anterior medial temporal lobe to prevent errant fibers extending from these regions. The resulting tracts were then used as ROIs from which diffusion metrics from each subject were extracted. We elected to focus primarily on the left fornix given hemispheric asymmetry in supporting verbal memory outcomes (Senova et al., 2020; Tucker et al., 1988; Saunders and Aggleton, 2007); however, we also examined associations with the right fornix in exploratory analyses.
Fig. 1.
Displays a sagittal view of an average fornix tract bilaterally.
FA values are the most common white matter microstructure metric; however, we also examined whether alterations in non-FA diffusion metrics were associated with GFAP and verbal episodic memory. Mean values for each of the target ROIs were calculated for mean diffusivity (MD), which reflects the overall – or average - motion of water molecules ((λ1 + λ2 + λ3)/3), axial diffusivity (DA), which reflects diffusion parallel to the fiber tracts (λ1), and radial diffusivity (RD), which reflects the magnitude of diffusion perpendicular to the fiber tract (mean of λ2 and λ3). Of note, prior reports suggest that RD values increase with myelin damage, and DA values are altered in the context of axonal damage (Barrick et al., 2010; Brickman et al., 2012).
Biomarker Ascertainment: After collection, each whole blood sample was centrifuged at 1500×g for 15 min at 22 °C and the plasma was removed. Plasma was centrifuged at 2200×g for 10 min at 4 °C to remove any residual cells, decanted and stored at − 80 °C. Blood-based biomarkers were selected based on standard and well-validated assays of AD-related pathology and vascular dysfunction. Of note, while CSF remains the gold standard for most fluid biomarkers, compelling evidence suggests that blood-based biomarkers are associated with clinical outcomes in aging adults and adults with AD (Chatterjee et al., 2022; Mila-et al., 2022; Hansson et al., 2022). Recent data also suggest that plasma GFAP is more strongly related to AD-related pathology than CSF GFAP levels (Benedet et al., 2021).
Protein analysis of GFAP and canonical biomarkers of brain amyloid (Aβ42) and neurodegeneration (NfL, total tau) were measured with the Quanterix single molecule array, (SIMOA®), SR-X Analyzer system using manufacturer-supplied antibody kits. Nominal recovery for control levels remained between 111 and 120% with a coefficient of variation (CV) < 10%. GFAP and NfL levels were available for all included study participants (n = 99). Four participants had no available Aβ42 or Total Tau values. For Aβ42, five additional participants had intra-individual CV across the two runs >20% and for total Tau, six additional participants had CVs >20%. (Final sample sizes: GFAP and NfL = 99; Aβ42 and Total Tau = 89). Given past research showing that amyloid pathology has a non-linear association with most clinical outcomes (Jack et al., 2018; Gauthier et al., 2018), we categorized participants into “low”, “medium”, and “high” Aβ42 levels to signify presumed high, medium, and low CNS levels, respectively, based on tertiles derived from the raw plasma Aβ42 measurements.
Given associations between glial dysregulation and vascular endothelial alterations, as well as considering the critical role astrocytes play in neurovascular coupling (Price et al., 2021), we also analyzed acute inflammatory and vascular adhesion markers using the Vascular Injury Panel V-PLEX kit, which included CRP, ICAM-1, SAA, and VCAM-1. Two individuals had values for ICAM-1 excluded due to intra-individual CV>20% and one individual had in intra-individual CV>20% for SAA. Thus, the final sample size for analyses with vascular markers, Aβ42, and total Tau was N = 85. Each array was scanned using a MESO QuickPlex SQ 120. Manufacturer supplied software (Discover Workbench 4.0) was used to quantify the concentration. For data reduction purposes, we created a single composite measure of vascular risk by selecting the top principal component (PC) from a principal component analysis (PCA); this variable will henceforth be referred to as the vascular PC and explained 55% of the variation in the vascular biomarkers.
Statistical Analyses: Demographic (age, sex, education), neuroimaging, and biomarker summary measures (mean, SD, median, IQR) were computed for asymptomatic and symptomatic participants (see Table 1). Distributional assumptions (normality) and linearity of associations were assessed with scatter plots. Due to the observed skewness in the distribution of plasma GFAP and vascular variables, a base-10 logarithmic transformation was employed. This transformation resulted in a linear association between the numeric predictors and the outcome variable (Gonzales et al., 2022).
Table 1.
Descriptive characteristics of participant sample.
| Asymptomatic (N = 66) | Symptomatic (N = 33) | Overall (N = 99) | |
|---|---|---|---|
| Age | |||
| Mean (SD) | 69.4 (6.46) | 71.4 (7.36) | 70.0 (6.80) |
| Sex | |||
| Female | 47 (71.2%) | 19 (57.6%) | 66 (66.7%) |
| Male | 19 (28.8%) | 14 (42.4%) | 33 (33.3%) |
| Education | |||
| Mean (SD) | 17.0 (2.17) | 16.4 (2.36) | 16.8 (2.24) |
| Memory Composite IRT | |||
| Mean (SD) | 0.92 (0.63) | −0.39 (0.82) | 0.48 (0.93) |
| E4 Allele | |||
| Absent | 43 (65.2%) | 15 (45.5%) | 58 (58.6%) |
| Present | 23 (34.8%) | 18 (54.5%) | 41 (41.4%) |
| Aβ42 | |||
| Mean (SD) | 10.3 (2.20) | 9.32 (2.11) | 9.98 (2.21) |
| Missing | 7 (10.6%) | 2 (6.1%) | 9 (9.1%) |
| Aβ42 Tertile | |||
| Low | 16 (24.2%) | 14 (42.4%) | 30 (30.3%) |
| Medium | 22 (33.3%) | 8 (24.2%) | 30 (30.3%) |
| High | 21 (31.8%) | 9 (27.3%) | 30 (30.3%) |
| Missing | 7 (10.6%) | 2 (6.1%) | 9 (9.1%) |
| Total Tau | |||
| Mean (SD) | 2.06 (0.54) | 2.46 (0.79) | 2.20 (0.66) |
| Missing | 8 (12.1%) | 2 (6.1%) | 10 (10.1%) |
| NfL | |||
| Mean (SD) | 13.7 (6.88) | 20.1 (7.31) | 15.8 (7.62) |
| GFAP | |||
| Median (IQR) | 127 (81.7) | 249 (174) | 157 (114) |
| log10(GFAP) | |||
| Mean (SD) | 2.12 (0.19) | 2.39 (0.22) | 2.21 (0.24) |
| log10(SAA) | |||
| Mean (SD) | 6.47 (0.42) | 6.45 (0.53) | 6.46 (0.46) |
| Missing | 0 (0%) | 1 (3.0%) | 1 (1.0%) |
| log10(VCAM) | |||
| Mean (SD) | 5.53 (0.11) | 5.56 (0.12) | 5.54 (0.11) |
| log10(ICAM) | |||
| Mean (SD) | 5.47 (0.085) | 5.47 (0.13) | 5.47 (0.099) |
| Missing | 1 (1.5%) | 1 (3.0%) | 2 (2.0%) |
| log10(CRP) | |||
| Mean (SD) | 6.10 (0.47) | 5.93 (0.56) | 6.05 (0.52) |
| Top PC for the vascular variables | |||
| Mean (SD) | 0.0079 (1.26) | −0.061 (1.78) | −0.015 (1.44) |
| Missing | 1 (1.5%) | 2 (6.1%) | 3 (3.0%) |
| Whole brain FA | |||
| Mean (SD) | 0.5 (0.015) | 0.49 (0.018) | 0.5 (0.016) |
| Whole brain RD | |||
| Mean (SD) | 0.43 (0.021) | 0.45 (0.026) | 0.44 (0.024) |
| Whole brain MD | |||
| Mean (SD) | 0.62 (0.023) | 0.64 (0.027) | 0.63 (0.026) |
| Whole brain DA | |||
| Mean (SD) | 0.99 (0.031) | 1.02 (0.032) | 1.0 (0.033) |
| Fornix Right FA | |||
| Mean (SD) | 0.32 (0.024) | 0.3 (0.027) | 0.31 (0.026) |
| Fornix Right RD | |||
| Mean (SD) | 0.77 (0.068) | 0.8 (0.098) | 0.78 (0.08) |
| Fornix Right MD | |||
| Mean (SD) | 0.92 (0.071) | 0.94 (0.1) | 0.92 (0.08) |
| Fornix Right DA | |||
| Mean (SD) | 1.22 (0.08) | 1.22 (0.11) | 1.22 (0.095) |
| Fornix Left FA | |||
| Mean (SD) | 0.33 (0.03) | 0.3 (0.03) | 0.32 (0.032) |
| Fornix Left RD | |||
| Mean (SD) | 0.77 (0.092) | 0.83 (0.11) | 0.79 (0.1) |
| Fornix Left MD | |||
| Mean (SD) | 0.93 (0.093) | 0.98 (0.11) | 0.95 (0.1) |
| Fornix Left DA | |||
| Mean (SD) | 1.24 (0.1) | 1.27 (0.13) | 1.25 (0.11) |
To examine Aβ42 as a moderator of the relationship between plasma GFAP and left fornix microstructure, we conducted multivariable regression analyses. We tested three models where we included the main effects of GFAP and Aβ42 tertiles, and their product interaction term as the primary predictor, and the primary outcomes were left fornix FA, MD, DA, and RD. After stratification by Aβ42 tertiles, only significant associations between GFAP and fornix were further assessed for mediation. Model 1 adjusted for demographic variables (n = 90; Model 1: fornix ∼ GFAP + Aβ42 + Aβ42 *GFAP + demographics); Model 2 additionally included the vascular PC (n = 87; Model 2: fornix ∼ GFAP + Aβ42 + Aβ42 *GFAP + demographics + vascular PC), and Model 3 further extended the adjustment by additionally including biomarkers of neurodegeneration (tau, NFL), and whole brain FA (n = 85; Model 3: fornix ∼ GFAP + Aβ42 + Aβ42 *GFAP + demographics + vascular PC + biomarker + whole brain FA). All three models were fitted using complete case analyses (no missing data in the outcomes, predictors, and covariates). In the Results section, we reported the estimated intercept and slope (coefficient of GFAP) for each Aβ42 tertile, along with their respective standard errors and p-values. Additionally, we report a p-value to assess the overall impact of the Aβ42 on the intercept and slope.
Next, to test the exploratory hypothesis that associations between higher GFAP levels (reflecting a proxy for astrogliosis/astrocyte reactivity) and worse verbal memory consolidation are mediated by alterations in the left fornix, we employed the Baron-Kenny model (Baron and Kenny, 1986) to construct a mediation pathway: GFAP → fornix → Memory. Based on our regression analysis results (see Results below), only the lowest Aβ42 tertile met the condition for testing for mediation. Multivariable regression models with complete cases were again used such that we controlled for demographic variables in Model 1 (n = 30), demographic variables and the vascular PC in Model 2 (n = 28), and demographic variables, the vascular PC, tau, NfL, and whole brain FA in Model 3 (n = 28).
To assess the significance of the mediation effect, or equivalently, the average causal mediation effect (ACME), in each model we utilized the nonparametric bootstrap method with 10000 bootstrap samples with a fixed random seed for replication purposes, implemented using the R function “mediate()” in the R package “mediation” (Imai et al., 2010; Tingley et al., 2014). Further details of the bootstrap procedure can be found elsewhere (Imai et al., 2010). We did not use the more popular and parametric Sobel's test (Sobel, 1982) due to limited sample size. We report the estimated ACME, total effect, and the proportion of the effect mediated as well as their corresponding bootstrap p-values.
3. Results
As shown in Table 1, participants (n = 99) were on average 70 years old with 16.8 years of education. The majority of individuals were female (66.7 %). Symptomatic participants had lower plasma Aβ42 (estimate = −0.976, SE = 0.49; p = 0.0496) and higher plasma total tau (estimate = 0.44; SE = 0.15; p = 0.003), NfL (estimate = 4.747, SE = 1.34; p = 0.001), and GFAP (estimate = 0.268, SE = 0.04; p < 0.001) compared to asymptomatic participants. Higher levels of GFAP (controlling for demographics) were associated with lower FA (p = 0.020), higher MD (p = 0.027), and higher RD (p = 0.015), with a trend for higher DA (p = 0.09).
Primary analyses: Aβ42 levels as a moderator of the association between plasma GFAP and left fornix microstructure (Table 2, Table 3, Table 4; Fig. 2; Full Dataset):
Table 2.
Model 1: Regression estimates (se) and p-value, controlling for demographic variables.
| FA |
MD |
RD |
DA |
|||||
|---|---|---|---|---|---|---|---|---|
| Estimate (se) | p-value | Estimate (se) | p-value | Estimate (se) | p-value | Estimate (se) | p-value | |
| GFAP coef: low blood Aβ42 | −0.055(0.02) | 0.007 | 0.24(0.062) | <0.001 | 0.239(0.062) | <0.001 | 0.242(0.067) | 0.001 |
| GFAP coef: medium blood Aβ42 | −0.031(0.023) | 0.19 | 0.063(0.072) | 0.38 | 0.079(0.071) | 0.27 | 0.032(0.077) | 0.68 |
| GFAP coef: high blood Aβ42 | 0.011(0.028) | 0.69 | −0.094(0.086) | 0.28 | −0.077(0.085) | 0.37 | −0.13(0.093) | 0.17 |
| GFAP* Aβ42: global interaction | 0.16 | 0.007 | 0.011 | 0.005 | ||||
| Sex | −0.01(0.007) | 0.15 | −0.011(0.022) | 0.61 | 0.001(0.021) | 0.95 | −0.036(0.023) | 0.12 |
Table 3.
Model 2: Regression estimates (se) and p-value, controlling for demographic and vascular blood biomarker variables.
| FA |
MD |
RD |
DA |
|||||
|---|---|---|---|---|---|---|---|---|
| Estimate (se) | p-value | Estimate (se) | p-value | Estimate (se) | p-value | Estimate (se) | p-value | |
| GFAP coef: low blood Aβ42 | −0.063(0.021) | 0.004 | 0.29(0.066) | <0.001 | 0.28(0.065) | <0.001 | 0.29(0.071) | <0.001 |
| GFAP coef: medium blood Aβ42 | −0.027(0.023) | 0.24 | 0.056(0.072) | 0.44 | 0.071(0.071) | 0.32 | 0.026(0.077) | 0.74 |
| GFAP coef: high blood Aβ42 | 0.015(0.028) | 0.59 | −0.104(0.086) | 0.23 | −0.089(0.085) | 0.303 | −0.14(0.092) | 0.14 |
| GFAP* Aβ42: global interaction | 0.083 | 0.002 | 0.003 | 0.001 | ||||
| Sex | −0.01(0.007) | 0.16 | −0.009(0.022) | 0.66 | 0.003(0.021) | 0.90 | −0.034(0.023) | 0.15 |
| Vascular PC | −0.005(0.002) | 0.032 | 0.013(0.007) | 0.057 | 0.014(0.007) | 0.04 | 0.011(0.007) | 0.12 |
Table 4.
Model 3: Regression estimates (se) and p-value, controlling for demographics, vascular blood biomarkers, and biomarkers of neurodegeneration.
| FA |
MD |
RD |
DA |
|||||
|---|---|---|---|---|---|---|---|---|
| Estimate (se) | p-value | Estimate (se) | p-value | Estimate (se) | p-value | Estimate (se) | p-value | |
| GFAP coef: low blood Aβ42 | −0.056(0.023) | 0.018 | 0.27(0.072) | <0.001 | 0.27(0.071) | <0.001 | 0.28(0.078) | 0.001 |
| GFAP coef: medium blood Aβ42 | −0.008(0.027) | 0.78 | 0.016(0.085) | 0.85 | 0.028(0.084) | 0.74 | −0.008(0.092) | 0.93 |
| GFAP coef: high blood Aβ42 | 0.022(0.031) | 0.48 | −0.11(0.096) | 0.26 | −0.096(0.095) | 0.32 | −0.14(0.104) | 0.19 |
| GFAP* Aβ42: global | 0.081 | 0.002 | 0.003 | 0.002 | ||||
| Sex | −0.01(0.008) | 0.2 | −0.006(0.024) | 0.8 | 0.006(0.023) | 0.8 | −0.03(0.026) | 0.24 |
| Vascular PC | −0.004(0.002) | 0.047 | 0.012(0.007) | 0.091 | 0.013(0.007) | 0.066 | 0.01(0.008) | 0.18 |
| Total Tau | −0.011(0.005) | 0.039 | 0.027(0.017) | 0.11 | 0.029(0.017) | 0.083 | 0.023(0.018) | 0.216 |
| NfL | -1e-04(0.001) | 0.84 | -4e-04(0.002) | 0.83 | -4e-04(0.002) | 0.84 | -5e-04(0.002) | 0.82 |
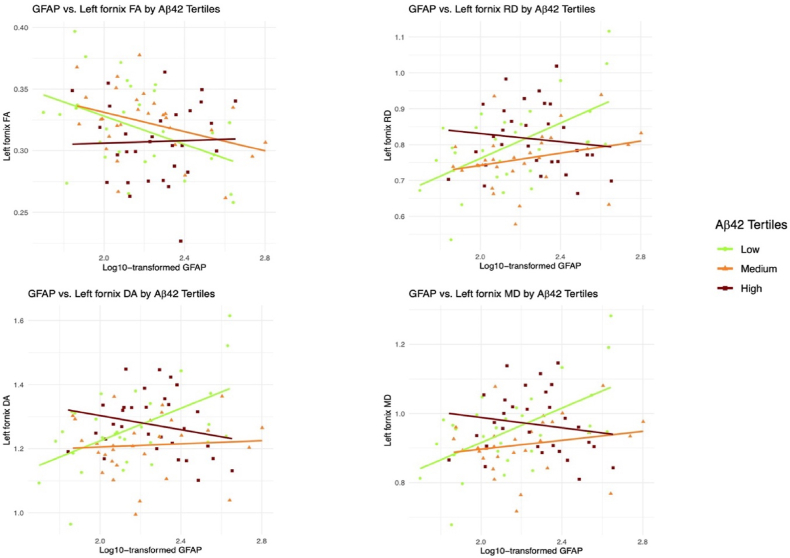
Fig. 2.
Displays the association Between blood GFAP and left fornix diffusion metrics as a function of Aβ42 levels.
We assessed the association between plasma GFAP levels and left fornix (FA, MD, RD, DA) integrity as a function of the plasma Aβ42 tertiles using three models. Fig. 2 provide a descriptive visualization of these regression results by Aβ42 tertile. Model 1 (controlling for demographics; Table 2), Model 2 (controlling for demographics and the vascular PC biomarkers; Table 3), and Model 3 (controlling for demographics, vascular PC biomarkers, neurodegeneration biomarkers [tau, NFL], and whole brain diffusion metrics; Table 4) all yielded similar results, such that among individuals in the low plasma Aβ42 tertile (representing higher amyloid burden), higher plasma GFAP was associated with lower FA (Model 1: p = 0.007; Model 2: p = 0.004; Model 3: p = 0.018), higher MD (Model 1–3: all p's < 0.001), higher RD (Model 1–3: all p's < 0.001), and higher DA (Model 1: p = 0.001; Model 2: p < 0.001; Model 3: p = 0.001) in the left fornix. For Model 1, effects were strongest for MD and RD (p < 0.001); For Model 2, effects were strongest for MD, RD, and DA (all p < 0.001); and for Model 3, the effects were strongest for MD and RD (p < 0.001). Importantly, the latter analyses demonstrate that the interactive effect of plasma GFAP and Aβ42 tertile on left fornix integrity was independent of vascular and neurodegenerative biomarkers, as well as whole brain FA. Conversely, associations between plasma GFAP and left fornix variables in individuals in the medium to high plasma Aβ42 tertiles were not found to be statistically significant in any model (p ≥ 0.14). Note that the interaction effect of GFAP and Aβ42 group on diffusion imaging outcomes was not significant for whole brain diffusion metrics (i.e., p's > 0.399 for FA, MD, RD and DA), providing additional support for specificity of the left fornix.
Exploratory analyses: Left fornix as a mediator of the association between plasma GFAP and verbal memory (Table 5; Fig. 3; Subgroup Analyses):
Table 5.
Mediation models for lowest tertile of Aβ42.
| Mediation effect: Model 1 (controlling for demographics) | ||||||
|---|---|---|---|---|---|---|
| ACME |
Direct effect |
Proportion mediated |
||||
| Estimate | p-value | Estimate | p-value | Estimate | p-value | |
| Left fornix FA | −0.603 | 0.0702 | −2.17 | 0.0024 | 0.22 | 0.07 |
| Left fornix MD | −1.06 | 0.0076 | −1.71 | 0.019 | 0.38 | 0.0086 |
| Left fornix RD | −1.06 | 0.0078 | −1.71 | 0.017 | 0.38 | 0.0074 |
| Left fornix DA | −0.99 | 0.017 | −1.79 | 0.019 | 0.36 | 0.017 |
| Mediation effect: Model 2 (controlling for demographics and vascular blood biomarkers) | ||||||
|---|---|---|---|---|---|---|
| ACME |
Direct effect |
Proportion mediated |
||||
| Estimate | p-value | Estimate | p-value | Estimate | p-value | |
| Left fornix FA | −0.57 | 0.101 | −2.52 | 0.0008 | 0.18 | 0.101 |
| Left fornix MD | −0.99 | 0.024 | −2.09 | 0.0096 | 0.32 | 0.024 |
| Left fornix RD | −0.98 | 0.031 | −2.11 | 0.0076 | 0.32 | 0.031 |
| Left fornix DA | −0.96 | 0.039 | −2.12 | 0.016 | 0.31 | 0.038 |
| Mediation effect: Model 3 (controlling for demographics, vascular blood biomarkers, and neurodegeneration biomarkers | ||||||
|---|---|---|---|---|---|---|
| ACME |
Direct effect |
Proportion mediated |
||||
| Estimate | p-value | Estimate | p-value | Estimate | p-value | |
| Left fornix FA | −0.078 | 0.96 | −2.81 | 0.056 | 0.027 | 0.92 |
| Left fornix MD | −0.42 | 0.62 | −2.47 | 0.07 | 0.14 | 0.57 |
| Left fornix RD | −0.43 | 0.62 | −2.47 | 0.073 | 0.15 | 0.57 |
| Left fornix DA | −0.37 | 0.64 | −2.52 | 0.081 | 0.13 | 0.603 |
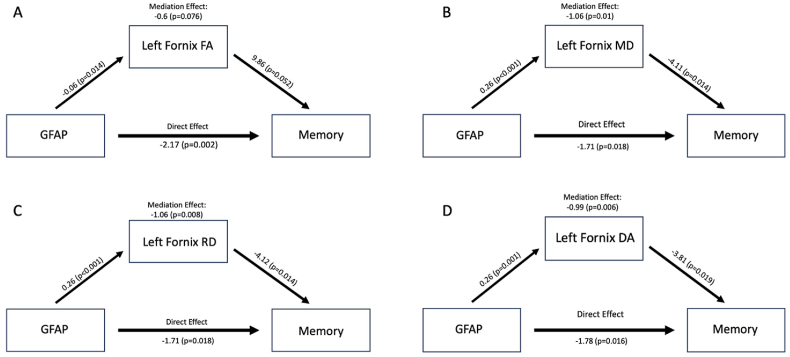
Fig. 3.
Displays direct effects, average causal mediation effect (ACME), and p-values for each diffusion metric. Note that the ACME is the product of two coefficients: one characterizing the association between GFAP and Fornix, and the other characterizing the association between Fornix and Memory while accounting for GFAP.
We have previously demonstrated a negative association between plasma GFAP levels and memory performance in the same cohort of individuals as this study. The mediation analysis here focused on the subgroup of participants with low plasma Aβ42 (reflective of higher amyloid deposition). We acknowledge that the sample size is small when restricting to this subgroup and suggest confirmatory larger studies to further investigate this interesting finding. For Models 1 and 2 (described above), we observed that the mediation pathway GFAP → Left fornix → Memory was statistically significant for MD, RD, and DA diffusion metrics, and there was a trend for FA (see Table 5; Fig. 3). The negative direct effect indicated that there was a negative direct relationship between GFAP and memory, which was independent of the fornix variables. The negative ACME indicated that the fornix variables may have amplified or exacerbated the negative effect of GFAP on memory. As an illustrative example, considering the mediation pathway GFAP → Left fornix MD → Memory in Model 1, the interpretation of the ACME is as follows: A one-unit increase in log10 GFAP (10-fold increase in GFAP) is associated with a 1.06 unit decrease in memory through the left fornix MD, accounting for approximately 38% of the total effect.
When we incorporated adjustment for additional confounding covariates (Model 3), all estimates of ACME and direct effect were consistently negative, but there was a noted increase in p-values, ultimately leading to a lack of statistical significance.
3.1. Secondary analyses
Aβ42/Aβ40 Ratio Tertiles as a Moderator of the Association Between Plasma GFAP and Left Fornix Microstructure: Considering that Aβ42/Aβ40 ratios are also standardly employed when analyzing biomarker amyloid levels, we repeated the primary analysis (Model 3) using an identical analytic approach but replacing Aβ42 tertiles with Aβ42/Aβ40 ratio tertiles. Consistent with the primary findings, associations between GFAP and fornix diffusion metrics were only significant in the low Aβ42/Aβ40 ratio groups for FA (Model 1: estimate = −0.054; SE = 0.029; p = 0.022; Model 2: estimate = −0.064, SE = 0.025, p = 0.011; Model 3: estimate = −0.058, SE = 0.026, p = 0.030), MD (Model 1: estimate = 0.183, SE = 0.076; p = 0.018; Model 2: estimate = 0.235, SE = 0.082, p = 0.005; Model 3: estimate = 0.227, SE = 0.088, p = 0.012), RD (Model 1: estimate = 0.193, SE = 0.074; p = 0.011; Model 2: estimate = 0.242, SE = 0.080, p = 0.003; Model 3: estimate = 0.233, SE = 0.086, p = 0.008); and DA (estimate = 0.162, SE = 0.082; p = 0.052; Model 2: estimate = 0.221, SE = 0.088, p = 0.014; Model 3: estimate = 0.214, SE = 0.096, p = 0.03).
Associations Between Plasma GFAP and Right Fornix: We elected to focus our primary analyses on the left fornix due to the hemispheric association with verbal memory, the latter of which was an outcome of interest and the most common method of assessing memory in late life. When evaluating plasma GFAP associations with the right fornix as a function of Aβ42 tertile, no significant global interactions between GFAP and Aβ42 (p > 0.05) were found for right fornix FA, MD, DA or RD across all models.
Associations Between Plasma GFAP and Left Fornix, Controlling for Symptom Status: All primary results were collapsed across participants, regardless of symptom status (asymptomatic, symptomatic for AD). When controlling for the symptom status variable, plasma GFAP remained significantly associated with higher MD (Model 1 estimate = 0.23, SE = 0.073, p = 0.002; Model 2 estimate = 0.26, SE = 0.075, p = 0.001; Model 3 estimate = 0.26, SE = 0.081, p = 0.002), higher RD (Model 1 estimate = 0.21, SE = 0.071, p = 0.004; Model 2 estimate = 0.25, SE = 0.074, p = 0.001; Model 3 estimate = 0.24, SE = 0.08, p = 0.003), and higher DA (Model 1 estimate = 0.26, SE = 0.78, p = 0.001; Model 2 estimate = 0.295, SE = 0.081, p < 0.001; Model 3 estimate = 0.295, SE = 0.088, p = 0.001) diffusion metrics in participants with the low plasma Aβ42 group in all three models. There remained a trend for the negative association between plasma GFAP and FA in all models (p = 0.1 to p = 0.16).
Role ofAPOEGene Status on GFAP-Left Fornix Associations: After controlling for the APOE status in all three models, plasma GFAP remained associated with left FA, MD, RD, and DA among individuals in the low plasma Aβ42 group, except for Model 3 (i.e., full model) where the p-value for FA is 0.051.
4. Discussion
In the setting of greater amyloid deposition in the brain, higher plasma GFAP was associated with altered fornix microstructure using tractography methodology, with strongest effects sizes for mean diffusivity (MD), radial diffusivity (RD), and axial diffusivity (DA) compared to fractional anisotropy (FA). Specifically, participants in the low plasma Aβ42 (reflective of higher brain amyloid) group showed stronger associations between plasma GFAP and left fornix integrity than people in the higher plasma Aβ42 (reflective of lower brain amyloid) group. Moreover, these associations were independent of plasma vascular risk biomarkers, neurodegeneration (i.e., NFL, total tau) biomarkers, and APOE gene status. We also extended our prior GFAP-verbal memory findings (Bettcher et al., 2021) by demonstrating that in the subset of participants in the low plasma Aβ42 group, left fornix integrity mediates the relationship between plasma GFAP and verbal memory performance. Overall, these findings suggest that astrogliosis/astrocyte reactivity, as measured by higher GFAP in this study, may play an early, pivotal role in AD pathogenesis, and further implies that the combination of astrocyte dysregulation and high brain amyloid may have a particularly negative role in forniceal-memory pathways.
The interactive effect of higher plasma GFAP and low plasma Aβ42 group on fornix structure is an important expansion on recent work that strongly links the pernicious effects of astrogliosis/astrocyte reactivity on amyloid pathology. For example, Benedet and colleagues recently demonstrated that plasma GFAP levels can discriminate between CSF-defined Aβ-positive from Aβ-negative individuals across the clinical cognitive (asymptomatic to symptomatic) spectrum (Benedet et al., 2021), suggesting that GFAP is an early indicator of AD cascades. Similarly, other groups have also shown that in the context of autosomal dominant AD (ADAD), plasma GFAP increases began 10 or more years prior to anticipated symptom onset, were related to amyloid positivity in asymptomatic ADAD, and were associated with progressive clinical severity (Chatterjee et al., 2023). Although prior studies have demonstrated a link between elevated GFAP and AD, our study indicates that the neuroanatomical effects of astrogliosis/astrocyte reactivity may be engaged by an amyloid-sensitive and potentially amyloid-specific pathway via the fornix, and exacerbated by amyloid via a potential feed-forward mechanism.
The fornix is a critical white matter structure involved in cholinergic pathways and episodic memory encoding. It is affected early in AD, and degradation of this pathway is known to impact cholinergic transmission from the basal forebrain to the hippocampus. Often overlooked in the cognitive neuroscience of early AD is that the ensuing cholinergic deficit and cognitive sequelae of this disrupted pathway have strong ties to immune dysregulation. As noted, glia not only encase cholinergic neurons (as well as all neuronal subtypes) and their cellular processes, but they also activate a7nAChRs to modulate the inflammatory response through downstream inhibition of NF-kb and activation of Nrf2 pathways. Although speculative, in the context of glial dysregulation and microstructural alterations to the fornix, it is possible that critical gating of the immune system and timely suppression of inflammation is diminished in early AD. Providing preliminary support for this pathway, our mediational analyses suggest that elevated GFAP (reflecting potential astrogliosis/astrocyte reactivity) might impact verbal episodic memory through degradation of the fornix in aging adults with greater amyloid deposition; however, additional studies are needed to clarify causal pathways and biological mechanisms of action. Specifically, in the absence of acetylcholine measurement and in the context of a cross-sectional design, results cannot address whether cholinergic transmission is altered prior to changes in GFAP or fornix microstructure, or whether longitudinal changes in strictly the asymptomatic phase recapitulates a similar pattern to the results noted with this participant sample. Similarly, large scale, longitudinal analysis of the immune proteome would be critical to understanding the temporal progression of GFAP, pro- and anti-inflammatory processes, AD pathology, and clinical outcomes. Nonetheless, our results strongly suggests that the coupling of elevated GFAP and brain amyloid levels may reflect and underlying catalyst for disrupting fornix-memory pathways across the disease spectrum.
Our findings of a synergistic effect of GFAP and Aβ42 on fornix structure were restricted to the left hemisphere. We initially focused on the left fornix due to its established contribution to verbal rather than visual memory, given that verbal memory was our primary cognitive measure in the study. It is noteworthy, however, that other studies have suggested broader asymmetry, with left > right alterations (Lubben et al., 2021) in glucose metabolism and neurite connectivity (Daianu et al., 2013), as well possibly greater vulnerability of the left hemisphere to aging and AD-related pathology (Yang et al., 2017). These findings are complex, however, as some evidence also suggests that the vulnerability of the left hemisphere to AD results in a gradual loss of asymmetry over time, although the greatest structural differences between AD and controls remained in the left hemisphere (Roe et al., 2021). The lack of interaction effect between GFAP and Aβ42 on right fornix integrity in our study suggests a possible higher vulnerability of the left fornix to elevated GFAP and Aβ42; however, future longitudinal studies would be necessary to disentangle this temporal sequence and rule out reverse causality. It is also possible that effects on the right fornix may be evident only at later stages.
Considering in-depth white matter microstructure measurements, our results suggest relatively stronger interactive effects of GFAP and Aβ42 on non-FA diffusion metrics compared to FA. FA is the most studied diffusion metric; however, it is a non-specific index of white matter organization and is calculated as a relative measure of anisotropy within a region; as such, it is difficult to attribute a single microstructural cause for low FA values, particularly with curved structures that may contain crossing fibers. Although changes, particularly increases, in DA and RD have been linked to axonal and myelin damage, respectively, there is still some caution warranted in overinterpreting underlying tissue properties with these metrics. It is also important to note that these metrics are often highly correlated with each other. Recent studies have suggested that MD metrics may be a more “robust” and reliable metric for curved structures as these measures weight diffusion along all axes of the tensor equally (Figley et al., 2021). Although interpreting whether the noted associations with FA (decreases) and DA, RD, and MD (all increases) are due to alterations in fiber coherence, myelin damage, or other structural changes are outside the scope of this paper, it is notable that there were consistent effects of GFAP x Aβ42 across all fornix metrics in the primary models and mediation models, even if the strength of effects were more pronounced in non-FA diffusion indicators.
An additional result of our study is that the moderating effect of plasma Aβ42 on GFAP and fornix pathways were not substantively impacted by blood-based vascular biomarkers. Plasma vascular biomarkers were modeled for several reasons, including the established role of astrocytes in orchestrating neurovascular coupling, as well as the known effects of vascular biology on white matter structure (Gorelick et al., 2011; Libon et al., 2004). Although plasma vascular biomarkers did not markedly diminish the effects of plasma GFAP on cognitive and neuroanatomical outcomes, the vascular factor score remained independently predictive of fornix integrity in several models. As such, there may be independent pathways by which astrocyte dysregulation and vascular dysfunction might impact fornix structure; however, further investigation is needed to determine whether astrogliosis stimulates peripheral plasma vascular biomarkers. Moreover, it is unclear whether there are specific clinical windows or pathological stages during which astrocyte and vascular dysregulation are synergistically related to early AD pathological cascades.
The study has numerous strengths, including the joint appraisal of astrogliosis, vascular, and AD-related biomarkers and the use of tractography methodology in a cohort of both asymptomatic and symptomatic older adults. In addition, the study was able to extend our prior work on GFAP-memory associations by demonstrating potential mediational pathways by which higher indicators of astrocyte dysregulation (GFAP) and greater amyloid burden might impact memory function through forniceal tracts. There are some limitations to the study, including a modest sample size. It is possible that some confounding by the adjustment factors remains, rather than limited power. In addition, the study focused strictly on plasma, thus there was no CSF or PET confirmation of clinical AD status. Although there is considerable evidence to suggest that plasma biomarkers of AD-related pathology can be used as proxies for CSF or other CNS indicators of AD status, CSF markers and amyloid PET remain the gold standard. In line with this, we did not have dichotomous positive/negative indicators for externally validated AD pathology in this study, and published thresholds cannot be readily incorporated across sites due to differences in pre-analytical factors (Verberk et al., 2022). Nonetheless, it is important to highlight that the goal of the study was to examine these biological pathways across a clinical and pathological spectrum, rather than strictly binning results into positivity/negativity, and prior studies using a range of methods have demonstrated that amyloid levels, particularly in asymptomatic older adults, are biological meaningful even when sub-threshold for positivity (Mormino et al., 2012; Bischof and Jacobs, 2019). In addition, new mass spectrometry measurements of amyloid in blood have suggested that these methodological approaches are generally superior to ultrasensitive immunoassays of Aβ42 and Aβ40 (Janelidze et al., 2021); although our results suggest clear effects of GFAP on fornix diffusion metrics in participants with low Aβ42 (or Aβ42/Aβ40 ratio), future studies should replicate results with a larger sample using recent advancements in mass spectrometry approaches and/or with validated markers of phosphorylated tau-217 (Hirtz et al., 2023; Janelidze et al., 2023). Finally, although we have discussed GFAP in the context of astrogliosis and/or astrocyte reactivity broadly, there remain many unanswered questions as to the underlying biology of this plasma-based measure in humans. Specifically, while primarily – but not entirely – derived from the CNS and strongly linked to astrogliosis, it is unclear if GFAP measures astrocyte activation, astrocyte-associated immune dysregulation or induction, astrocyte damage, or a combination of other processes. Given that glia can assume a variety of functional states, it is also important to consider that there may be dynamic changes in how plasma GFAP reflects astrocyte function.
In summary, our study demonstrates a strong, synergistic association between plasma GFAP and Aβ42 on left fornix diffusion metrics in a cohort of both asymptomatic and symptomatic participants. Importantly, in the setting of higher brain amyloid load, the association between higher GFAP and worse memory performance appears to be influenced in part by alterations in the left fornix. The growing body of literature suggests that GFAP is a critical, early indicator of amyloidogenic processes and may be integral to prognostic models on clinical outcomes. Our findings further support the idea that dysregulation in glia may exacerbate AD-related processes and yield more deleterious effects on clinical outcomes than single indicators of AD-related pathology alone.
Data availability statement
Neuroimaging and clinical data are available by data use request to corresponding author; COI: No competing interests; Ethics approval: This study was approved by the Colorado Multiple Institutional Review Board.
CRediT authorship contribution statement
Brianne M. Bettcher: Conceptualization, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Supervision, Writing – original draft, Writing – review & editing. Dan Lopez Paniagua: Data curation, Investigation, Methodology, Visualization, Writing – review & editing. Yue Wang: Data curation, Formal analysis, Visualization, Writing – review & editing. Brice V. McConnell: Conceptualization, Investigation, Writing – review & editing. Christina Coughlan: Investigation, Writing – review & editing. Tara C. Carlisle: Conceptualization, Investigation, Writing – review & editing. Ashesh A. Thaker: Investigation, Writing – review & editing. William Lippitt: Data curation, Formal analysis, Writing – review & editing. Christopher M. Filley: Conceptualization, Writing – review & editing. Victoria S. Pelak: Conceptualization, Writing – review & editing. Allison L.B. Shapiro: Conceptualization, Writing – review & editing. Kate S. Heffernan: Investigation, Writing – review & editing. Huntington Potter: Conceptualization, Funding acquisition, Writing – review & editing. Adriana Solano: Investigation, Writing – review & editing. Jada Boyd: Investigation. Nichole E. Carlson: Data curation, Formal analysis, Supervision, Writing – original draft, Writing – review & editing.
Declaration of competing interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests:
Brianne Bettcher reports financial support was provided by National Institute on Aging. Brianne M Bettcher reports a relationship with National Institute on Aging that includes: funding grants. If there are other authors, they declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This work was supported by grants from the National Institute on Aging (NIA; PI; R01 AG058772, B. Bettcher, PI) and the Department of Defense (W81XWH-19-1-0520). Research visits were also broadly supported by NIH/NCATS Colorado CTSA Grant Number UL1 TR002535 (Ronald Sokol, PI), NIH High-End Instrumentation Grant S10OD018435 (Tregellas, PI), CU Anschutz SOM Programmatic Incubator for Research (CU ASPIRE) Program, and support from the State of Colorado and many generous philanthropists.
We would like to acknowledge the participants in this study who volunteered their time for comprehensive evaluations, blood draws, and MRI scans. We would like to acknowledge and thank Dr. Tim Boyd for his assistance with setting up the biobank for storage of blood specimens and Mr. Neil Markham for overseeing APOE genotyping. Many staff and faculty at the CU Alzheimer's and Cognition Center (CUACC) assisted with the implementation of the study's design, and we are grateful for the dedication of our CUACC team.
Data availability
Data will be made available on request.
References
- Albert M.S., DeKosky S.T., Dickson D., et al. The diagnosis of mild cognitive impairment due to Alzheimer's disease: recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement. 2011;7:270–279. doi: 10.1016/j.jalz.2011.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersson J.L.R., Sotiropoulos S.N. An integrated approach to correction for off-resonance effects and subject movement in diffusion MR imaging. Neuroimage. 2016;125:1063–1078. doi: 10.1016/j.neuroimage.2015.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baron R.M., Kenny D.A. The moderator-mediator variable distinction in social psychological research: conceptual, strategic, and statistical considerations. J. Pers. Soc. Psychol. 1986;51:1173–1182. doi: 10.1037//0022-3514.51.6.1173. [DOI] [PubMed] [Google Scholar]
- Barrick T.R., Charlton R.A., Clark C.A., Markus H.S. White matter structural decline in normal ageing: a prospective longitudinal study using tract-based spatial statistics. Neuroimage. 2010;51:565–577. doi: 10.1016/j.neuroimage.2010.02.033. [DOI] [PubMed] [Google Scholar]
- Bateman J.R., Filley C.M., Kaplan R.I., Heffernan K.S., Bettcher B.M. Lifetime surgical exposure, episodic memory, and forniceal microstructure in older adults. J. Clin. Exp. Neuropsychol. 2019;41:1048–1059. doi: 10.1080/13803395.2019.1647151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benear S.L., Ngo C.T., Olson I.R. Dissecting the fornix in basic memory processes and neuropsychiatric disease: a review. Brain Connect. 2020;10:331–354. doi: 10.1089/brain.2020.0749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benedet A.L., Mila-Aloma M., Vrillon A., et al. Differences between plasma and cerebrospinal fluid glial fibrillary acidic protein levels across the alzheimer disease continuum. JAMA Neurol. 2021;78:1471–1483. doi: 10.1001/jamaneurol.2021.3671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bettcher B.M., Walsh C.M., Watson C., et al. Body mass and white matter integrity: the influence of vascular and inflammatory markers. PLoS One. 2013;8 doi: 10.1371/journal.pone.0077741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bettcher B.M., Olson K.E., Carlson N.E., et al. Astrogliosis and episodic memory in late life: higher GFAP is related to worse memory and white matter microstructure in healthy aging and Alzheimer's disease. Neurobiol. Aging. 2021;103:68–77. doi: 10.1016/j.neurobiolaging.2021.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beyer L., Stocker H., Rujescu D., et al. Amyloid-beta misfolding and GFAP predict risk of clinical Alzheimer’s disease diagnosis within 17 years. Alzheimer’s Dement. 2023;19:1020–1028. doi: 10.1002/alz.12745. [DOI] [PubMed] [Google Scholar]
- Bischof G.N., Jacobs H.I.L. Subthreshold amyloid and its biological and clinical meaning: long way ahead. Neurology. 2019;93:72–79. doi: 10.1212/WNL.0000000000007747. [DOI] [PubMed] [Google Scholar]
- Boyle P.A., Wang T., Yu L., et al. To what degree is late life cognitive decline driven by age-related neuropathologies? Brain. 2021;144:2166–2175. doi: 10.1093/brain/awab092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brickman A.M., Meier I.B., Korgaonkar M.S., et al. Testing the white matter retrogenesis hypothesis of cognitive aging. Neurobiol. Aging. 2012;33:1699–1715. doi: 10.1016/j.neurobiolaging.2011.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatterjee P., Pedrini S., Ashton N.J., et al. Diagnostic and prognostic plasma biomarkers for preclinical Alzheimer's disease. Alzheimers Dement. 2022;18:1141–1154. doi: 10.1002/alz.12447. [DOI] [PubMed] [Google Scholar]
- Chatterjee P., Vermunt L., Gordon B.A., et al. Plasma glial fibrillary acidic protein in autosomal dominant Alzheimer's disease: associations with Abeta-PET, neurodegeneration, and cognition. Alzheimers Dement. 2023;19:2790–2804. doi: 10.1002/alz.12879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daianu M., Jahanshad N., Nir T.M., et al. Breakdown of brain connectivity between normal aging and Alzheimer's disease: a structural k-core network analysis. Brain Connect. 2013;3:407–422. doi: 10.1089/brain.2012.0137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ewers M., Walsh C., Trojanowski J.Q., et al. Prediction of conversion from mild cognitive impairment to Alzheimer's disease dementia based upon biomarkers and neuropsychological test performance. Neurobiol. Aging. 2010;2010/12/17 doi: 10.1016/j.neurobiolaging.2010.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Figley C.R., Uddin M.N., Wong K., Kornelsen J., Puig J., Figley T.D. Potential pitfalls of using fractional anisotropy, axial diffusivity, and radial diffusivity as biomarkers of cerebral white matter microstructure. Front. Neurosci. 2021;15 doi: 10.3389/fnins.2021.799576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fletcher E., Carmichael O., Pasternak O., Maier-Hein K.H., DeCarli C. Early brain loss in circuits affected by Alzheimer's disease is predicted by fornix microstructure but may be independent of gray matter. Front. Aging Neurosci. 2014;6:106. doi: 10.3389/fnagi.2014.00106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franceschi C., Garagnani P., Vitale G., Capri M., Salvioli S. Inflammaging and 'garb-aging'. Trends Endocrinol. Metabol. 2017;28:199–212. doi: 10.1016/j.tem.2016.09.005. [DOI] [PubMed] [Google Scholar]
- Friston K.J., Ashburner J., Frith C.D., Poline J.B., Heather J.D., Frackowiak R.S. Spatial registration and normalization of images. Hum. Brain Mapp. 1995;3:165–189. [Google Scholar]
- Gamage R., Wagnon I., Rossetti I., et al. Cholinergic modulation of glial function during aging and chronic neuroinflammation. Front. Cell. Neurosci. 2020;14 doi: 10.3389/fncel.2020.577912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gauthier S., Zhang H., Ng K.P., Pascoal T.A., Rosa-Neto P. Impact of the biological definition of Alzheimer's disease using amyloid, tau and neurodegeneration (ATN): what about the role of vascular changes, inflammation, Lewy body pathology? Transl. Neurodegener. 2018;7:12. doi: 10.1186/s40035-018-0117-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzales M.M., Wiedner C., Wang C.P., et al. A population-based meta-analysis of circulating GFAP for cognition and dementia risk. Ann Clin Transl Neurol. 2022;9:1574–1585. doi: 10.1002/acn3.51652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorelick P.B., Scuteri A., Black S.E., et al. Vascular contributions to cognitive impairment and dementia: a statement for healthcare professionals from the american heart association/american stroke association. Stroke; a journal of cerebral circulation. 2011;42:2672–2713. doi: 10.1161/STR.0b013e3182299496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansson O., Edelmayer R.M., Boxer A.L., et al. The Alzheimer’s Association appropriate use recommendations for blood biomarkers in Alzheimer’s disease. Alzheimer’s Dement. 2022;18:2669–2686. doi: 10.1002/alz.12756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris P.A., Taylor R., Thielke R., Payne J., Gonzalez N., Conde J.G. Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. J. Biomed. Inf. 2009;42:377–381. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirtz C., Busto G.U., Bennys K., et al. Comparison of ultrasensitive and mass spectrometry quantification of blood-based amyloid biomarkers for Alzheimer's disease diagnosis in a memory clinic cohort. Alzheimer's Res. Ther. 2023;15:34. doi: 10.1186/s13195-023-01188-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imai K., Keele L., Tingley D. A general approach to causal mediation analysis. Psychol. Methods. 2010;15:309–334. doi: 10.1037/a0020761. [DOI] [PubMed] [Google Scholar]
- Imai K., Keele L., Yamamoto T. Identification, inference and sensitivity analysis for causal mediation effects. Statist. Sci. 2010;25(1):51–71. [Google Scholar]
- Jack C.R., Jr., Bennett D.A., Blennow K., et al. NIA-AA Research Framework: toward a biological definition of Alzheimer's disease. Alzheimers Dement. 2018;14:535–562. doi: 10.1016/j.jalz.2018.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jack, C.R., Jr., Andrews, J.S., Beach, T.G., et al., 2024. Revised criteria for diagnosis and staging of Alzheimer’s disease: Alzheimer’s Association Workgroup. Online, ahead of print. Alzheimer's Dement. 1-27. [DOI] [PMC free article] [PubMed]
- Janelidze S., Teunissen C.E., Zetterberg H., et al. Head-to-Head comparison of 8 plasma amyloid-beta 42/40 assays in alzheimer disease. JAMA Neurol. 2021;78:1375–1382. doi: 10.1001/jamaneurol.2021.3180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janelidze S., Bali D., Ashton N.J., et al. Head-to-head comparison of 10 plasma phospho-tau assays in prodromal Alzheimer's disease. Brain. 2023;146:1592–1601. doi: 10.1093/brain/awac333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar A., Fontana I.C., Nordberg A. Reactive astrogliosis: a friend or foe in the pathogenesis of Alzheimer’s disease. J. Neurochem. 2023;164(3):309–324. doi: 10.1111/jnc.15565. [DOI] [PubMed] [Google Scholar]
- Lee D.Y., Fletcher E., Carmichael O.T., et al. Sub-regional hippocampal injury is associated with fornix degeneration in Alzheimer's disease. Front. Aging Neurosci. 2012;4:1. doi: 10.3389/fnagi.2012.00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Libon D.J., Price C.C., Davis Garrett K., Giovannetti T. From Binswanger's disease to leuokoaraiosis: what we have learned about subcortical vascular dementia. Clin. Neuropsychol. 2004;18:83–100. doi: 10.1080/13854040490507181. [DOI] [PubMed] [Google Scholar]
- Lubben N., Ensink E., Coetzee G.A., Labrie V. The enigma and implications of brain hemispheric asymmetry in neurodegenerative diseases. Brain Commun. 2021;3 doi: 10.1093/braincomms/fcab211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKhann G.M., Knopman D.S., Chertkow H., et al. The diagnosis of dementia due to Alzheimer's disease: recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimer's Dementia : the journal of the Alzheimer's Association. 2011;7:263–269. doi: 10.1016/j.jalz.2011.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mila-Aloma M., Ashton N.J., Shekari M., et al. Plasma p-tau231 and p-tau217 as state markers of amyloid-beta pathology in preclinical Alzheimer's disease. Nat. Med. 2022;28:1797–1801. doi: 10.1038/s41591-022-01925-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mormino E.C., Brandel M.G., Madison C.M., et al. Not quite PIB-positive, not quite PIB-negative: slight PIB elevations in elderly normal control subjects are biologically relevant. Neuroimage. 2012;59:1152–1160. doi: 10.1016/j.neuroimage.2011.07.098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mungas D., Reed B.R., Marshall S.C., Gonzalez H.M. Development of psychometrically matched English and Spanish language neuropsychological tests for older persons. Neuropsychology. 2000;14:209–223. doi: 10.1037//0894-4105.14.2.209. [DOI] [PubMed] [Google Scholar]
- Mungas D., Reed B.R., Crane P.K., Haan M.N., Gonzalez H. Spanish and English Neuropsychological Assessment Scales (SENAS): further development and psychometric characteristics. Psychol. Assess. 2004;16:347–359. doi: 10.1037/1040-3590.16.4.347. [DOI] [PubMed] [Google Scholar]
- Mungas D., Reed B.R., Haan M.N., Gonzalez H. Spanish and English neuropsychological assessment scales: relationship to demographics, language, cognition, and independent function. Neuropsychology. 2005;19:466–475. doi: 10.1037/0894-4105.19.4.466. [DOI] [PubMed] [Google Scholar]
- Mungas D., Widaman K.F., Reed B.R., Tomaszewski Farias S. Measurement invariance of neuropsychological tests in diverse older persons. Neuropsychology. 2011;25:260–269. doi: 10.1037/a0021090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nasreddine Z.S., Phillips N.A., Bedirian V., et al. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J. Am. Geriatr. Soc. 2005;53:695–699. doi: 10.1111/j.1532-5415.2005.53221.x. [DOI] [PubMed] [Google Scholar]
- Oeckl P., Halbgebauer S., Anderl-Straub S., et al. Glial fibrillary acidic protein in serum is increased in Alzheimer's disease and correlates with cognitive impairment. J Alzheimers Dis. 2019;67:481–488. doi: 10.3233/JAD-180325. [DOI] [PubMed] [Google Scholar]
- Pereira J.B., Janelidze S., Smith R., et al. Plasma GFAP is an early marker of amyloid-beta but not tau pathology in Alzheimer's disease. Brain. 2021;144:3505–3516. doi: 10.1093/brain/awab223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price B.R., Johnson L.A., Norris C.M. Reactive astrocytes: the nexus of pathological and clinical hallmarks of Alzheimer's disease. Ageing Res. Rev. 2021;68 doi: 10.1016/j.arr.2021.101335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabin J.S., Perea R.D., Buckley R.F., Johnson K.A., Sperling R.A., Hedden T. Synergism between fornix microstructure and beta amyloid accelerates memory decline in clinically normal older adults. Neurobiol. Aging. 2019;81:38–46. doi: 10.1016/j.neurobiolaging.2019.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roe J.M., Vidal-Pineiro D., Sorensen O., et al. Asymmetric thinning of the cerebral cortex across the adult lifespan is accelerated in Alzheimer's disease. Nat. Commun. 2021;12:721. doi: 10.1038/s41467-021-21057-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunders R.C., Aggleton J.P. Origin and topography of fibers contributing to the fornix in macaque monkeys. Hippocampus. 2007;17:396–411. doi: 10.1002/hipo.20276. [DOI] [PubMed] [Google Scholar]
- Senova S., Fomenko A., Gondard E., Lozano A.M. Anatomy and function of the fornix in the context of its potential as a therapeutic target. J. Neurol. Neurosurg. Psychiatry. 2020;91:547–559. doi: 10.1136/jnnp-2019-322375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith S.M., Jenkinson M., Woolrich M.W., et al. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage. 2004;23(Suppl. 1):S208–S219. doi: 10.1016/j.neuroimage.2004.07.051. [DOI] [PubMed] [Google Scholar]
- Sobel M.E. Asymptotic confidence intervals for indirect effects in structural equation models. Socio. Methodol. 1982;13:290–312. [Google Scholar]
- Teles-Grilo Ruivo L.M., Mellor J.R. Cholinergic modulation of hippocampal network function. Front. Synaptic Neurosci. 2013;5:2. doi: 10.3389/fnsyn.2013.00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tingley D., Yamamoto T., Hirose K., Keele L., Imai K. Mediation: R package for causal mediation analysis. J. Stat. Software. 2014;59:1–38. [Google Scholar]
- Tucker D.M., Roeltgen D.P., Tully R., Hartmann J., Boxell C. Memory dysfunction following unilateral transection of the fornix: a hippocampal disconnection syndrome. Cortex. 1988;24:465–472. doi: 10.1016/s0010-9452(88)80010-8. [DOI] [PubMed] [Google Scholar]
- Verberk I.M.W., Misdorp E.O., Koelewijn J., et al. Characterization of pre-analytical sample handling effects on a panel of Alzheimer's disease-related blood-based biomarkers: results from the Standardization of Alzheimer's Blood Biomarkers (SABB) working group. Alzheimers Dement. 2022;18:1484–1497. doi: 10.1002/alz.12510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker K.A., Ficek B.N., Westbrook R. Understanding the role of systemic inflammation in Alzheimer's disease. ACS Chem. Neurosci. 2019;10:3340–3342. doi: 10.1021/acschemneuro.9b00333. [DOI] [PubMed] [Google Scholar]
- Yang C., Zhong S., Zhou X., Wei L., Wang L., Nie S. The abnormality of topological asymmetry between hemispheric brain white matter networks in Alzheimer's disease and mild cognitive impairment. Front. Aging Neurosci. 2017;9:261. doi: 10.3389/fnagi.2017.00261. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Neuroimaging and clinical data are available by data use request to corresponding author; COI: No competing interests; Ethics approval: This study was approved by the Colorado Multiple Institutional Review Board.
Data will be made available on request.