Abstract
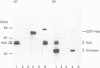
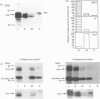
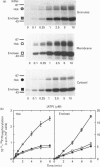
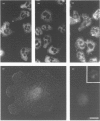
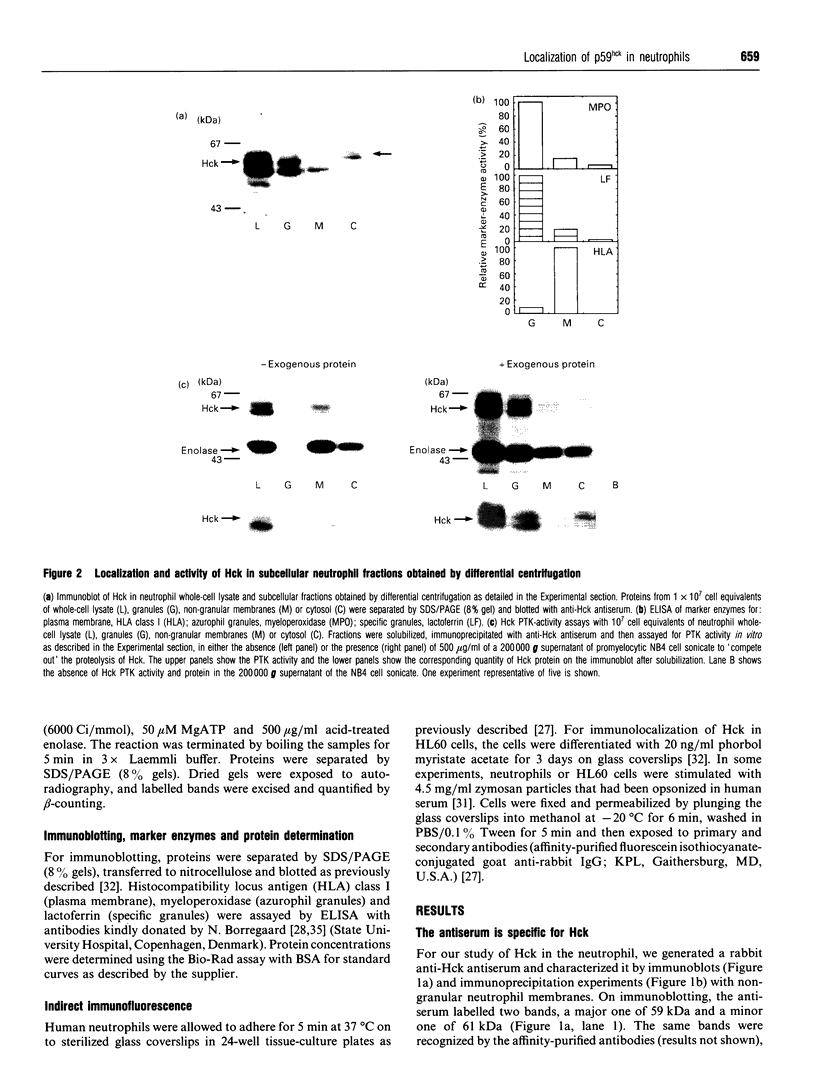
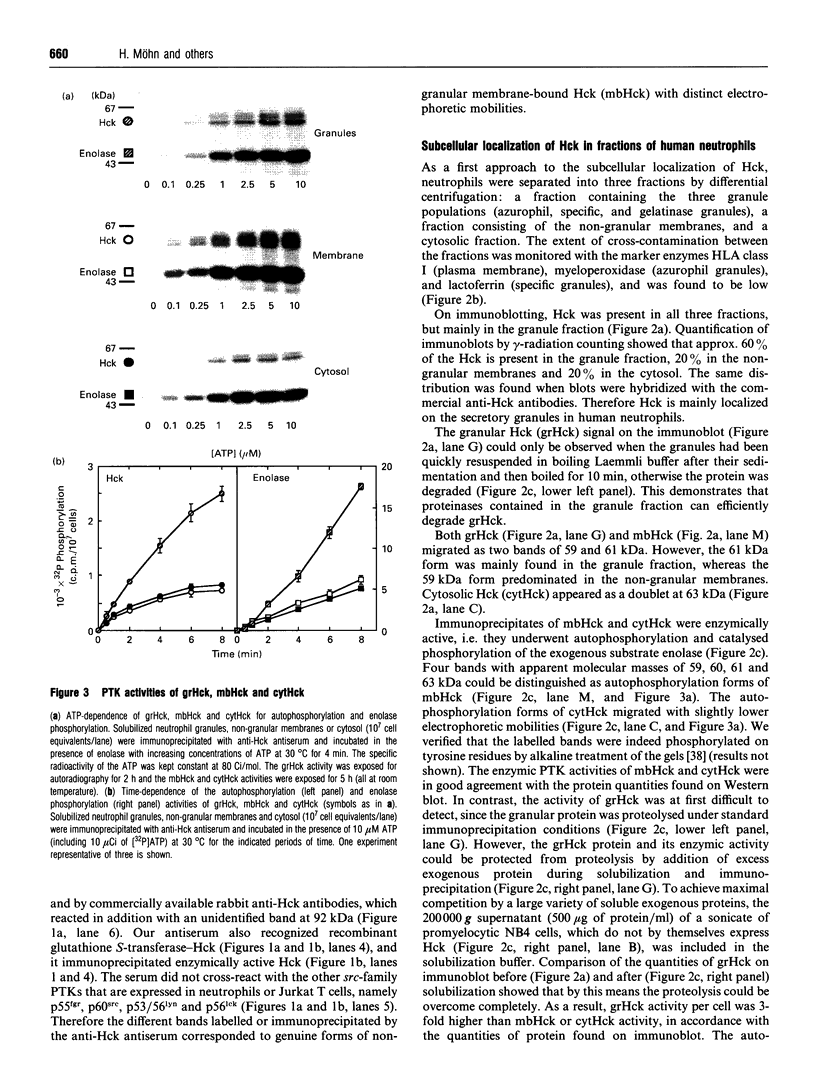
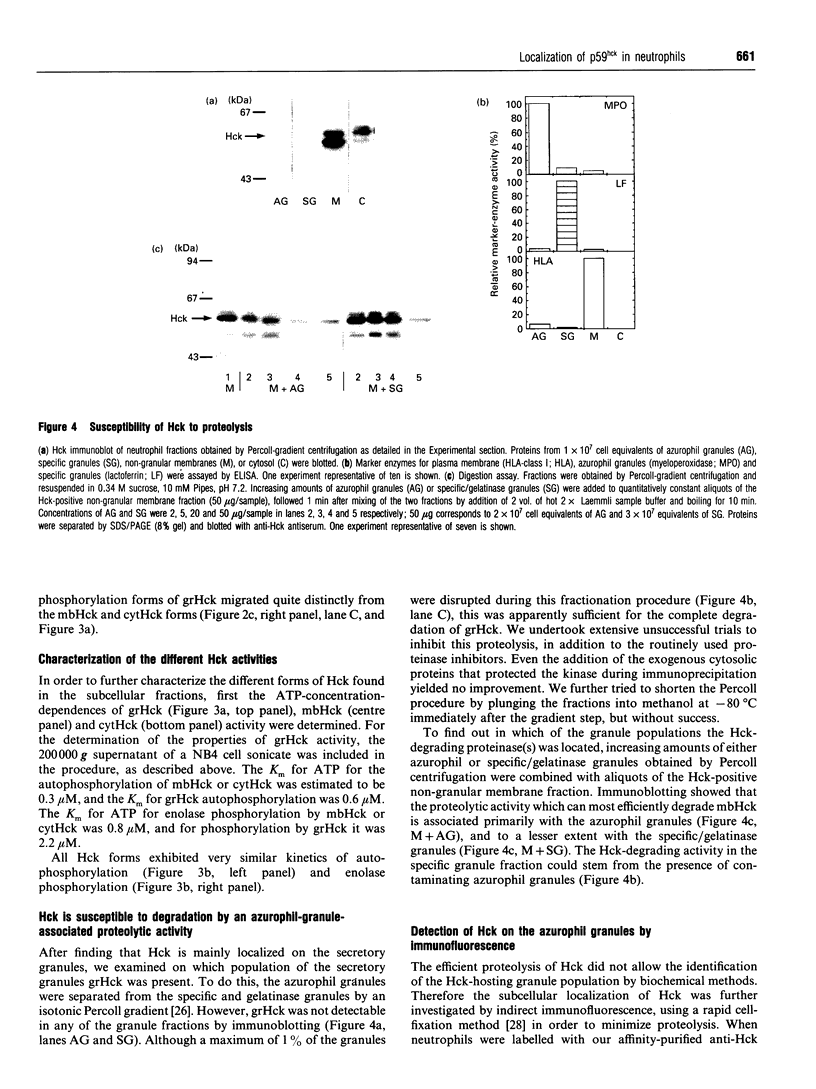
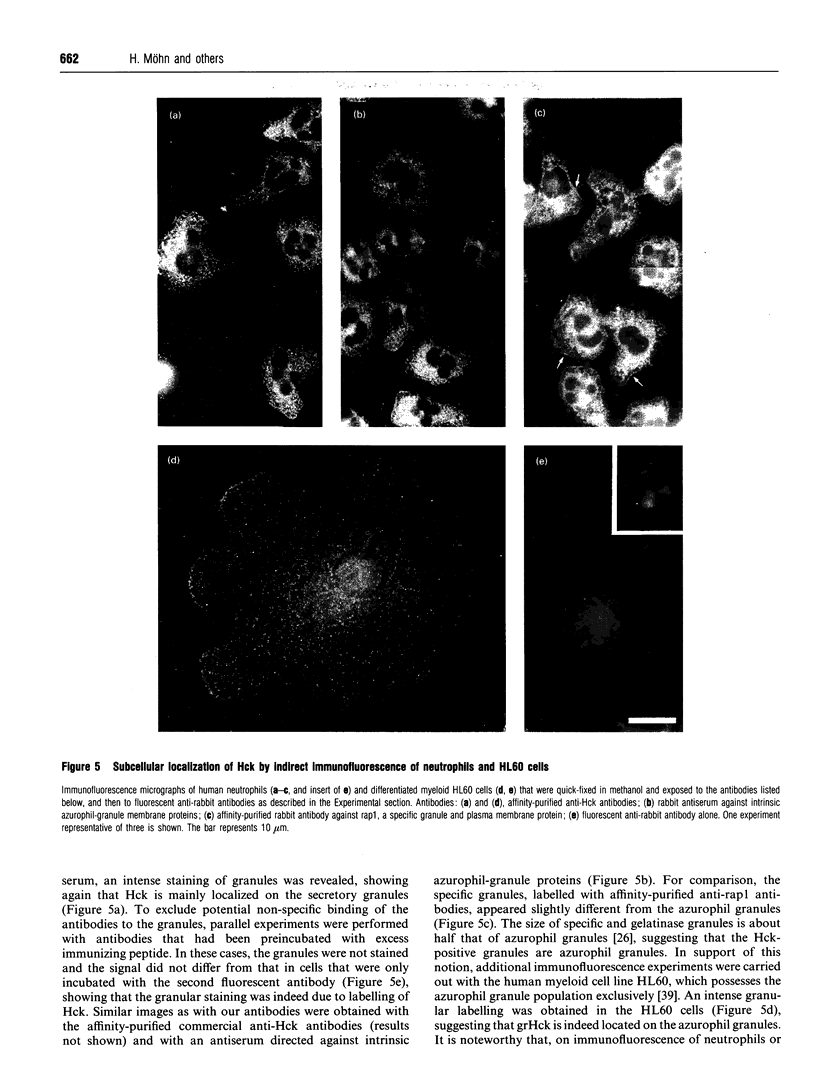
The src-family protein-tyrosine kinase p59hck is mainly expressed in neutrophils; however, its functional role in these cells is unknown. Several other src-family members are localized on secretory vesicles and have been proposed to regulate intracellular traffic. We have established here the subcellular localization of p59hck in human neutrophils. Immunoblotting of subcellular fractions showed that approx. 60% of the p59hck per cell is localized on the secretory granules; the other 40% is distributed equally between non-granular membranes and the cytosol. Immunofluorescence of neutrophils and HL60 cells suggests that the p59hck-positive granules are azurophil granules. Granular p59hck is highly susceptible to degradation by an azurophil-granule proteinase. Different forms of p59hck occur in the three subcellular compartments: a 61 kDa form is mainly found in the granules, a 59 kDa form is predominant in the non-granular membranes, whereas cytosolic p59hck migrates as a doublet at 63 kDa. During the process of phagocytosis-linked degranulation, induced by serum-opsonized zymosan in neutrophils or HL60 cells, granular p59hck translocates towards the phagosome. The subcellular localization of p59hck suggests that the enzyme could be involved in the regulation of the degranulation process.
Full text
PDF

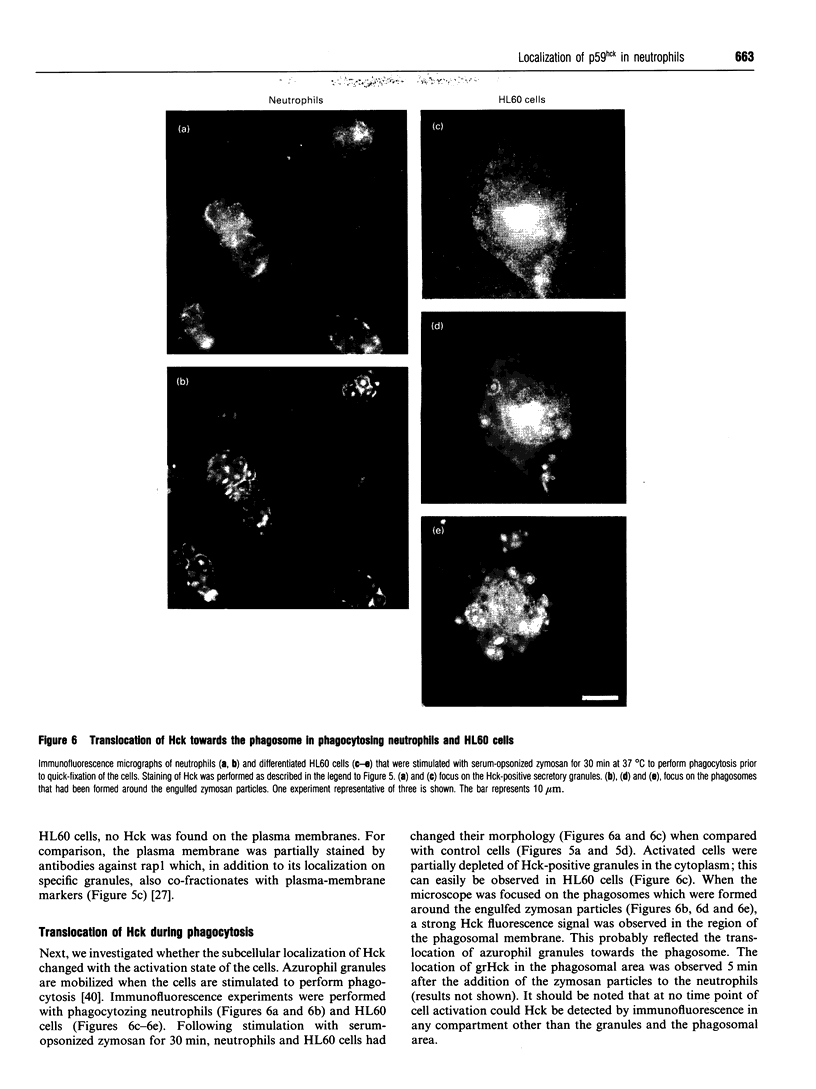


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anand R., Wilkinson J. M., Kellie S. Localisation of pp60c-src to the surface membrane of human platelets. Oncogene. 1993 Nov;8(11):3013–3020. [PubMed] [Google Scholar]
- Asahi M., Taniguchi T., Hashimoto E., Inazu T., Maeda H., Yamamura H. Activation of protein-tyrosine kinase p72syk with concanavalin A in polymorphonuclear neutrophils. J Biol Chem. 1993 Nov 5;268(31):23334–23338. [PubMed] [Google Scholar]
- Barnekow A., Jahn R., Schartl M. Synaptophysin: a substrate for the protein tyrosine kinase pp60c-src in intact synaptic vesicles. Oncogene. 1990 Jul;5(7):1019–1024. [PubMed] [Google Scholar]
- Beemon K., Hunter T. Characterization of Rous sarcoma virus src gene products synthesized in vitro. J Virol. 1978 Nov;28(2):551–566. doi: 10.1128/jvi.28.2.551-566.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjerrum O. W., Borregaard N. Mixed enzyme-linked immunosorbent assay (MELISA) for HLA class I antigen: a plasma membrane marker. Scand J Immunol. 1990 Mar;31(3):305–313. doi: 10.1111/j.1365-3083.1990.tb02773.x. [DOI] [PubMed] [Google Scholar]
- Borregaard N., Heiple J. M., Simons E. R., Clark R. A. Subcellular localization of the b-cytochrome component of the human neutrophil microbicidal oxidase: translocation during activation. J Cell Biol. 1983 Jul;97(1):52–61. doi: 10.1083/jcb.97.1.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borregaard N., Lollike K., Kjeldsen L., Sengeløv H., Bastholm L., Nielsen M. H., Bainton D. F. Human neutrophil granules and secretory vesicles. Eur J Haematol. 1993 Oct;51(4):187–198. doi: 10.1111/j.1600-0609.1993.tb00629.x. [DOI] [PubMed] [Google Scholar]
- Boulet I., Ralph S., Stanley E., Lock P., Dunn A. R., Green S. P., Phillips W. A. Lipopolysaccharide- and interferon-gamma-induced expression of hck and lyn tyrosine kinases in murine bone marrow-derived macrophages. Oncogene. 1992 Apr;7(4):703–710. [PubMed] [Google Scholar]
- Danielian S., Fagard R., Alcover A., Acuto O., Fischer S. The lymphocyte-specific protein tyrosine kinase p56lck is hyperphosphorylated on serine and tyrosine residues within minutes after activation via T cell receptor or CD2. Eur J Immunol. 1989 Dec;19(12):2183–2189. doi: 10.1002/eji.1830191202. [DOI] [PubMed] [Google Scholar]
- English B. K., Ihle J. N., Myracle A., Yi T. Hck tyrosine kinase activity modulates tumor necrosis factor production by murine macrophages. J Exp Med. 1993 Sep 1;178(3):1017–1022. doi: 10.1084/jem.178.3.1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernst M., Gearing D. P., Dunn A. R. Functional and biochemical association of Hck with the LIF/IL-6 receptor signal transducing subunit gp130 in embryonic stem cells. EMBO J. 1994 Apr 1;13(7):1574–1584. doi: 10.1002/j.1460-2075.1994.tb06420.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feder D., Bishop J. M. Purification and enzymatic characterization of pp60c-src from human platelets. J Biol Chem. 1990 May 15;265(14):8205–8211. [PubMed] [Google Scholar]
- Fittschen C., Henson P. M. Linkage of azurophil granule secretion in neutrophils to chloride ion transport and endosomal transcytosis. J Clin Invest. 1994 Jan;93(1):247–255. doi: 10.1172/JCI116952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghazizadeh S., Bolen J. B., Fleit H. B. Physical and functional association of Src-related protein tyrosine kinases with Fc gamma RII in monocytic THP-1 cells. J Biol Chem. 1994 Mar 25;269(12):8878–8884. [PubMed] [Google Scholar]
- Grandori C., Hanafusa H. p60c-src is complexed with a cellular protein in subcellular compartments involved in exocytosis. J Cell Biol. 1988 Dec;107(6 Pt 1):2125–2135. doi: 10.1083/jcb.107.6.2125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutkind J. S., Robbins K. C. Translocation of the FGR protein-tyrosine kinase as a consequence of neutrophil activation. Proc Natl Acad Sci U S A. 1989 Nov;86(22):8783–8787. doi: 10.1073/pnas.86.22.8783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris P., Ralph P. Human leukemic models of myelomonocytic development: a review of the HL-60 and U937 cell lines. J Leukoc Biol. 1985 Apr;37(4):407–422. doi: 10.1002/jlb.37.4.407. [DOI] [PubMed] [Google Scholar]
- Holtzman D. A., Cook W. D., Dunn A. R. Isolation and sequence of a cDNA corresponding to a src-related gene expressed in murine hemopoietic cells. Proc Natl Acad Sci U S A. 1987 Dec;84(23):8325–8329. doi: 10.1073/pnas.84.23.8325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan K. B., Swedlow J. R., Varmus H. E., Morgan D. O. Association of p60c-src with endosomal membranes in mammalian fibroblasts. J Cell Biol. 1992 Jul;118(2):321–333. doi: 10.1083/jcb.118.2.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Le Cabec V., Maridonneau-Parini I. Annexin 3 is associated with cytoplasmic granules in neutrophils and monocytes and translocates to the plasma membrane in activated cells. Biochem J. 1994 Oct 15;303(Pt 2):481–487. doi: 10.1042/bj3030481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Cabec V., Möhn H., Gacon G., Maridonneau-Parini I. The small GTP-binding protein rac is not recruited to the plasma membrane upon NADPH oxidase activation in human neutrophils. Biochem Biophys Res Commun. 1994 Feb 15;198(3):1216–1224. doi: 10.1006/bbrc.1994.1172. [DOI] [PubMed] [Google Scholar]
- Ley S. C., Marsh M., Bebbington C. R., Proudfoot K., Jordan P. Distinct intracellular localization of Lck and Fyn protein tyrosine kinases in human T lymphocytes. J Cell Biol. 1994 May;125(3):639–649. doi: 10.1083/jcb.125.3.639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z. H., Mahajan S., Prendergast M. M., Fargnoli J., Zhu X., Klages S., Adam D., Schieven G. L., Blake J., Bolen J. B. Cross-linking of surface immunoglobulin activates src-related tyrosine kinases in WEHI 231 cells. Biochem Biophys Res Commun. 1992 Sep 30;187(3):1536–1544. doi: 10.1016/0006-291x(92)90477-3. [DOI] [PubMed] [Google Scholar]
- Lichtenberg U., Quintrell N., Bishop J. M. Human protein-tyrosine kinase gene HCK: expression and structural analysis of the promoter region. Oncogene. 1992 May;7(5):849–858. [PubMed] [Google Scholar]
- Lock P., Ralph S., Stanley E., Boulet I., Ramsay R., Dunn A. R. Two isoforms of murine hck, generated by utilization of alternative translational initiation codons, exhibit different patterns of subcellular localization. Mol Cell Biol. 1991 Sep;11(9):4363–4370. doi: 10.1128/mcb.11.9.4363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowell C. A., Soriano P., Varmus H. E. Functional overlap in the src gene family: inactivation of hck and fgr impairs natural immunity. Genes Dev. 1994 Feb 15;8(4):387–398. doi: 10.1101/gad.8.4.387. [DOI] [PubMed] [Google Scholar]
- Maridonneau-Parini I., Tringale S. M., Tauber A. I. Identification of distinct activation pathways of the human neutrophil NADPH-oxidase. J Immunol. 1986 Nov 1;137(9):2925–2929. [PubMed] [Google Scholar]
- Maridonneau-Parini I., Yang C. Z., Bornens M., Goud B. Increase in the expression of a family of small guanosine triphosphate-binding proteins, rab proteins, during induced phagocyte differentiation. J Clin Invest. 1991 Mar;87(3):901–907. doi: 10.1172/JCI115096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maridonneau-Parini I., de Gunzburg J. Association of rap1 and rap2 proteins with the specific granules of human neutrophils. Translocation to the plasma membrane during cell activation. J Biol Chem. 1992 Mar 25;267(9):6396–6402. [PubMed] [Google Scholar]
- Marie-Cardine A., Maridonneau-Parini I., Ferrer M., Danielian S., Rothhut B., Fagard R., Dautry-Varsat A., Fischer S. The lymphocyte-specific tyrosine protein kinase p56lck is endocytosed in Jurkat cells stimulated via CD2. J Immunol. 1992 Jun 15;148(12):3879–3884. [PubMed] [Google Scholar]
- Nishibori M., Cham B., McNicol A., Shalev A., Jain N., Gerrard J. M. The protein CD63 is in platelet dense granules, is deficient in a patient with Hermansky-Pudlak syndrome, and appears identical to granulophysin. J Clin Invest. 1993 Apr;91(4):1775–1782. doi: 10.1172/JCI116388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oda A., Druker B. J., Ariyoshi H., Smith M., Salzman E. W. pp60src is an endogenous substrate for calpain in human blood platelets. J Biol Chem. 1993 Jun 15;268(17):12603–12608. [PubMed] [Google Scholar]
- Quintrell N., Lebo R., Varmus H., Bishop J. M., Pettenati M. J., Le Beau M. M., Diaz M. O., Rowley J. D. Identification of a human gene (HCK) that encodes a protein-tyrosine kinase and is expressed in hemopoietic cells. Mol Cell Biol. 1987 Jun;7(6):2267–2275. doi: 10.1128/mcb.7.6.2267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redmond T., Brott B. K., Jove R., Welsh M. J. Localization of the viral and cellular Src kinases to perinuclear vesicles in fibroblasts. Cell Growth Differ. 1992 Sep;3(9):567–576. [PubMed] [Google Scholar]
- Rendu F., Lebret M., Danielian S., Fagard R., Levy-Toledano S., Fischer S. High pp60c-src level in human platelet dense bodies. Blood. 1989 May 1;73(6):1545–1551. [PubMed] [Google Scholar]
- Smith D. B., Johnson K. S. Single-step purification of polypeptides expressed in Escherichia coli as fusions with glutathione S-transferase. Gene. 1988 Jul 15;67(1):31–40. doi: 10.1016/0378-1119(88)90005-4. [DOI] [PubMed] [Google Scholar]
- Smith D. E., Fisher P. A. Identification, developmental regulation, and response to heat shock of two antigenically related forms of a major nuclear envelope protein in Drosophila embryos: application of an improved method for affinity purification of antibodies using polypeptides immobilized on nitrocellulose blots. J Cell Biol. 1984 Jul;99(1 Pt 1):20–28. doi: 10.1083/jcb.99.1.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefanová I., Corcoran M. L., Horak E. M., Wahl L. M., Bolen J. B., Horak I. D. Lipopolysaccharide induces activation of CD14-associated protein tyrosine kinase p53/56lyn. J Biol Chem. 1993 Oct 5;268(28):20725–20728. [PubMed] [Google Scholar]
- Willman C. L., Stewart C. C., Longacre T. L., Head D. R., Habbersett R., Ziegler S. F., Perlmutter R. M. Expression of the c-fgr and hck protein-tyrosine kinases in acute myeloid leukemic blasts is associated with early commitment and differentiation events in the monocytic and granulocytic lineages. Blood. 1991 Feb 15;77(4):726–734. [PubMed] [Google Scholar]
- Ziegler S. F., Levin S. D., Perlmutter R. M. Transformation of NIH 3T3 fibroblasts by an activated form of p59hck. Mol Cell Biol. 1989 Jun;9(6):2724–2727. doi: 10.1128/mcb.9.6.2724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziegler S. F., Marth J. D., Lewis D. B., Perlmutter R. M. Novel protein-tyrosine kinase gene (hck) preferentially expressed in cells of hematopoietic origin. Mol Cell Biol. 1987 Jun;7(6):2276–2285. doi: 10.1128/mcb.7.6.2276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziegler S. F., Wilson C. B., Perlmutter R. M. Augmented expression of a myeloid-specific protein tyrosine kinase gene (hck) after macrophage activation. J Exp Med. 1988 Nov 1;168(5):1801–1810. doi: 10.1084/jem.168.5.1801. [DOI] [PMC free article] [PubMed] [Google Scholar]